Abstract
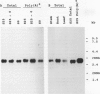
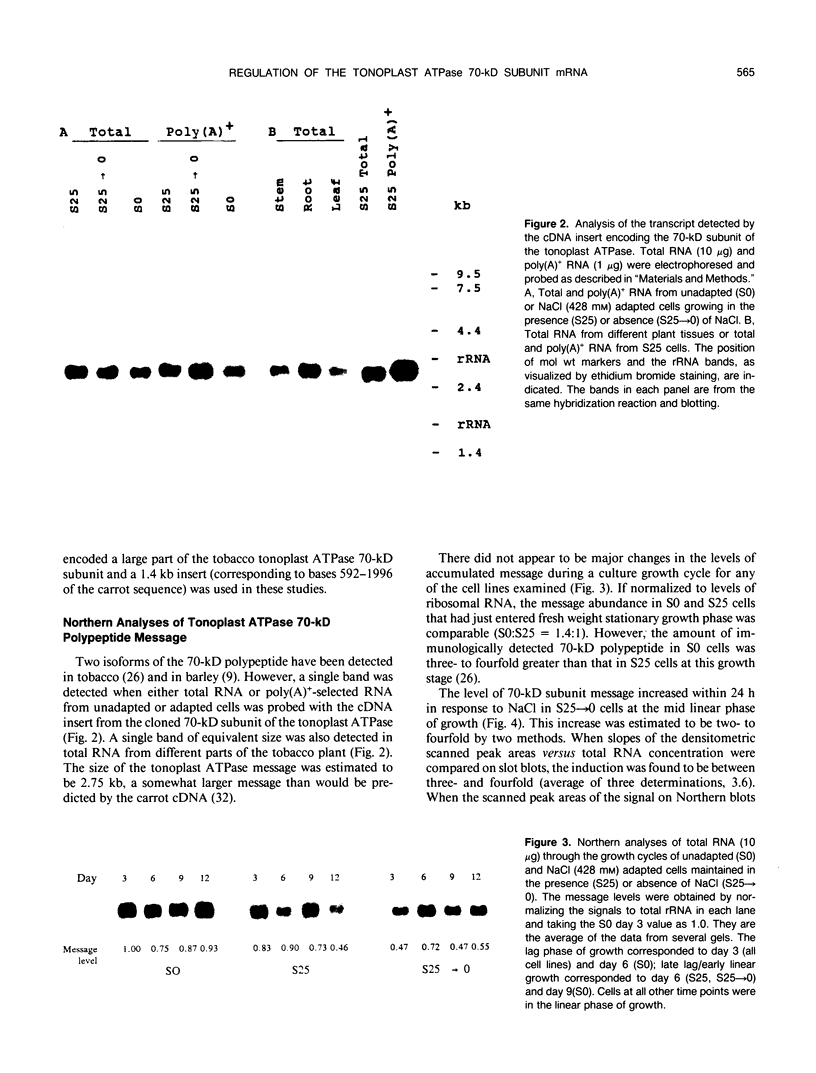
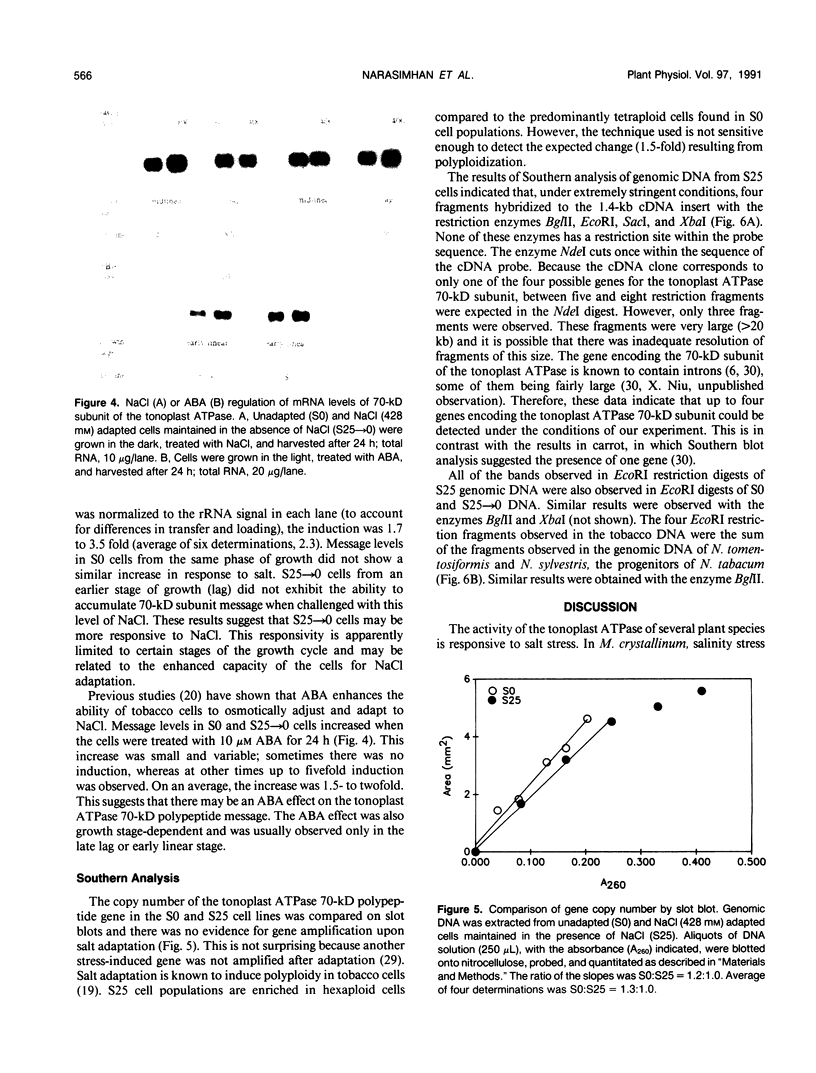
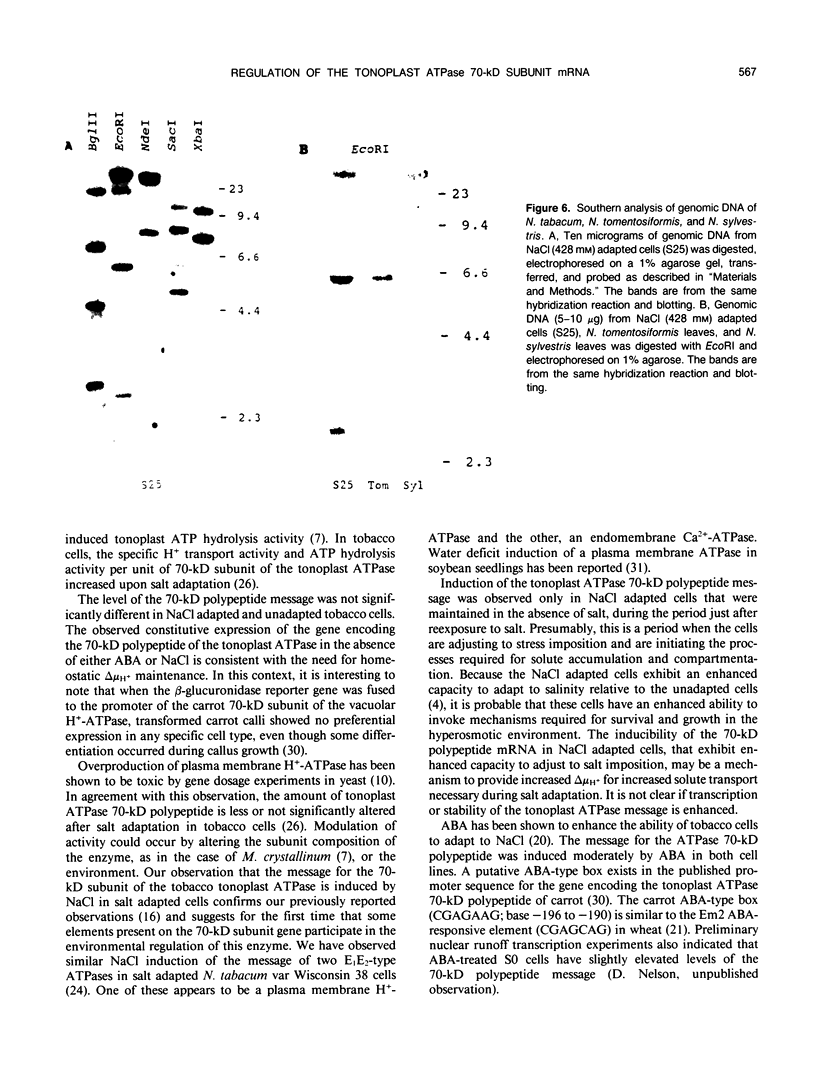
A cDNA clone encoding the 70-kilodalton subunit of the tobacco (Nicotiana tabacum var Wisconsin 38) tonoplast ATPase has been isolated. The 1.656 kilobase insert contains only open reading frame that represents more than 80% of the carrot cDNA coding region. The deduced amino acid sequence has greater than 95% sequence identity with the homologous carrot sequence. A transcript of approximately 2.7 kilobase was detected on Northern blots of tobacco poly(A)+ selected or total RNA using labeled probe produced from this clone. The gene was expressed throughout the growth cycle in unadapted and 428 millimolar NaCl adapted cells. Transcription of the 70-kilodalton subunit gene or mRNA stability was induced by short-term NaCl treatment in NaCl adapted cells or by abscisic acid treatment in both adapted and unadapted cells. Southern analysis indicated the presence of up to four genes encoding the 70-kilodalton subunit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binzel M. L., Hasegawa P. M., Handa A. K., Bressan R. A. Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):118–125. doi: 10.1104/pp.79.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binzel M. L., Hasegawa P. M., Rhodes D., Handa S., Handa A. K., Bressan R. A. Solute Accumulation in Tobacco Cells Adapted to NaCl. Plant Physiol. 1987 Aug;84(4):1408–1415. doi: 10.1104/pp.84.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Allen R., Wechser M. A., Bowman E. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-2 encoding the 57-kDa polypeptide and comparison to vma-1. J Biol Chem. 1988 Oct 5;263(28):14002–14007. [PubMed] [Google Scholar]
- Bowman E. J., Tenney K., Bowman B. J. Isolation of genes encoding the Neurospora vacuolar ATPase. Analysis of vma-1 encoding the 67-kDa subunit reveals homology to other ATPases. J Biol Chem. 1988 Oct 5;263(28):13994–14001. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Dupont F. M., Tanaka C. K., Hurkman W. J. separation and Immunological Characterization of Membrane Fractions from Barley Roots. Plant Physiol. 1988 Mar;86(3):717–724. doi: 10.1104/pp.86.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eraso P., Cid A., Serrano R. Tight control of the amount of yeast plasma membrane ATPase during changes in growth conditions and gene dosage. FEBS Lett. 1987 Nov 16;224(1):193–197. doi: 10.1016/0014-5793(87)80446-5. [DOI] [PubMed] [Google Scholar]
- Gogarten J. P., Kibak H., Dittrich P., Taiz L., Bowman E. J., Bowman B. J., Manolson M. F., Poole R. J., Date T., Oshima T. Evolution of the vacuolar H+-ATPase: implications for the origin of eukaryotes. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6661–6665. doi: 10.1073/pnas.86.17.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsbrough P. B., Cullis C. A. Characterisation of the genes for ribosomal RNA in flax. Nucleic Acids Res. 1981 Mar 25;9(6):1301–1309. doi: 10.1093/nar/9.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Kurkdjian A., Guern J., Flügge U. I. Comparative studies on the electrical properties of the H+ translocating ATPase and pyrophosphatase of the vacuolar-lysosomal compartment. EMBO J. 1989 Oct;8(10):2835–2841. doi: 10.1002/j.1460-2075.1989.tb08430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane P. M., Yamashiro C. T., Wolczyk D. F., Neff N., Goebl M., Stevens T. H. Protein splicing converts the yeast TFP1 gene product to the 69-kD subunit of the vacuolar H(+)-adenosine triphosphatase. Science. 1990 Nov 2;250(4981):651–657. doi: 10.1126/science.2146742. [DOI] [PubMed] [Google Scholar]
- Larosa P. C., Hasegawa P. M., Rhodes D., Clithero J. M., Watad A. E., Bressan R. A. Abscisic Acid Stimulated Osmotic Adjustment and Its Involvement in Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1987 Sep;85(1):174–181. doi: 10.1104/pp.85.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte W. R., Jr, Russell S. H., Quatrano R. S. Abscisic acid-responsive sequences from the em gene of wheat. Plant Cell. 1989 Oct;1(10):969–976. doi: 10.1105/tpc.1.10.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry R. V., Turner J. C., Rea P. A. High purity preparations of higher plant vacuolar H+-ATPase reveal additional subunits. Revised subunit composition. J Biol Chem. 1989 Nov 25;264(33):20025–20032. [PubMed] [Google Scholar]
- Reuveni M., Bennett A. B., Bressan R. A., Hasegawa P. M. Enhanced H Transport Capacity and ATP Hydrolysis Activity of the Tonoplast H-ATPase after NaCl Adaptation. Plant Physiol. 1990 Oct;94(2):524–530. doi: 10.1104/pp.94.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih C. K., Wagner R., Feinstein S., Kanik-Ennulat C., Neff N. A dominant trifluoperazine resistance gene from Saccharomyces cerevisiae has homology with F0F1 ATP synthase and confers calcium-sensitive growth. Mol Cell Biol. 1988 Aug;8(8):3094–3103. doi: 10.1128/mcb.8.8.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Nelson D. E., Kuhn D., Hasegawa P. M., Bressan R. A. Molecular Cloning of Osmotin and Regulation of Its Expression by ABA and Adaptation to Low Water Potential. Plant Physiol. 1989 Jul;90(3):1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struve I., Rausch T., Bernasconi P., Taiz L. Structure and function of the promoter of the carrot V-type H(+)-ATPase catalytic subunit gene. J Biol Chem. 1990 May 15;265(14):7927–7932. [PubMed] [Google Scholar]
- Surowy T. K., Boyer J. S. Low water potentials affect expression of genes encoding vegetative storage proteins and plasma membrane proton ATPase in soybean. Plant Mol Biol. 1991 Feb;16(2):251–262. doi: 10.1007/BF00020556. [DOI] [PubMed] [Google Scholar]
- Zimniak L., Dittrich P., Gogarten J. P., Kibak H., Taiz L. The cDNA sequence of the 69-kDa subunit of the carrot vacuolar H+-ATPase. Homology to the beta-chain of F0F1-ATPases. J Biol Chem. 1988 Jul 5;263(19):9102–9112. [PubMed] [Google Scholar]