Abstract
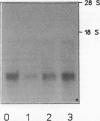
The postanoxic injury, also known as reperfusion injury, is associated with the returning of anoxic tissues to normal atmosphere. Using tetrazolium chloride staining, ATP content, and seedling growth rate as indicators, we found that postanoxic injuries in soybean (Glycine max) seedlings were more severe after 1 and 2 hours of anoxia than after longer anoxic durations (3 to 5 hours). Anaerobic incubation of root tips in the presence of 100 mm ascorbate, an antioxidant and free radical-scavenging compound, alleviated the postanoxic injury associated with the short durations of anoxia. Extracts from soybean seedling roots returned to air from 1 hour of anoxia had an elevated capacity to produce superoxide radicals over extracts from postanoxic roots stressed for 3 or 5 hours. Activity of superoxide dismutase in soybean roots returned to air from 1 and 2 hours of anoxia was 30 to 50% lower than activities in roots returned to air from 5 hours of anoxia. Superoxide dismutase-specific transcripts were also lower in postanoxic roots stressed for 1 hour than in roots stressed for longer anoxic durations. The evidence suggested that the postanoxic injury of soybean roots after a short anoxic stress was associated with an increased superoxide radicals production capacity coupled with a reduced superoxide dismutase activity. Periods of anoxia of at least 3 hours were necessary for soybean seedlings to develop the ability to cope with postanoxic stress.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asada K., Urano M., Takahashi M. Subcellular location of superoxide dismutase in spinach leaves and preparation and properties of crystalline spinach superoxide dismutase. Eur J Biochem. 1973 Jul 2;36(1):257–266. doi: 10.1111/j.1432-1033.1973.tb02908.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Del Río L. A., Fernández V. M., Rupérez F. L., Sandalio L. M., Palma J. M. NADH Induces the Generation of Superoxide Radicals in Leaf Peroxisomes. Plant Physiol. 1989 Mar;89(3):728–731. doi: 10.1104/pp.89.3.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer S. E., Dryer R. L., Autor A. P. Enhancement of mitochondrial, cyanide-resistant superoxide dismutase in the livers of rats treated with 2,4-dinitrophenol. J Biol Chem. 1980 Feb 10;255(3):1054–1057. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hoffman N. E., Bent A. F., Hanson A. D. Induction of lactate dehydrogenase isozymes by oxygen deficit in barley root tissue. Plant Physiol. 1986 Nov;82(3):658–663. doi: 10.1104/pp.82.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk L. S., Fagerstedt K. V., Crawford R. M. Superoxide Dismutase as an Anaerobic Polypeptide : A Key Factor in Recovery from Oxygen Deprivation in Iris pseudacorus? Plant Physiol. 1987 Dec;85(4):1016–1020. doi: 10.1104/pp.85.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITTER W. Die Bildung von Vitaminen durch Oospora lactis. Schweiz Z Pathol Bakteriol. 1950;13(5):547–553. [PubMed] [Google Scholar]
- Roberts J. K., Andrade F. H., Anderson I. C. Further Evidence that Cytoplasmic Acidosis Is a Determinant of Flooding Intolerance in Plants. Plant Physiol. 1985 Feb;77(2):492–494. doi: 10.1104/pp.77.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandalio L. M., Fernández V. M., Rupérez F. L., Del Río L. A. Superoxide free radicals are produced in glyoxysomes. Plant Physiol. 1988 May;87(1):1–4. doi: 10.1104/pp.87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom K., Crapo J. D. Structural and biochemical adaptive changes in rat lungs after exposure to hypoxia. Lab Invest. 1983 Jan;48(1):68–79. [PubMed] [Google Scholar]