Abstract
Enterohemorrhagic Escherichia coli (EHEC) infection is associated with watery diarrhea and can lead to complications, including hemorrhagic colitis and the hemolytic-uremic syndrome. The mechanisms by which these organisms produce diarrheal disease remain to be elucidated. Changes in T84 epithelial cell electrophysiology were examined following EHEC infection. T84 cell monolayers infected with EHEC O157:H7 displayed a time-dependent decrease in transepithelial resistance. Increases in the transepithelial flux of both [3H]mannitol and 51Cr-EDTA accompanied the EHEC-induced decreases in T84 resistance. Altered barrier function induced by EHEC occurred at the level of the tight junction since immunofluorescent staining of the tight-junction-associated protein ZO-1 was disrupted when examined by confocal microscopy. Decreased resistance induced by EHEC involved a protein kinase C (PKC)-dependent pathway as the highly specific PKC inhibitor, CGP41251, abrogated the EHEC-induced drop in resistance. PKC activity was also increased in T84 cells infected with EHEC. Calmodulin and myosin light chain kinase played a role in EHEC-induced resistance changes as inhibition of these effector molecules partially reversed the effects of EHEC on barrier function. These studies demonstrate that intracellular signal transduction pathways activated following EHEC infection link the increases in T84 epithelial permeability induced by this pathogen.
Enterohemorrhagic Escherichia coli (EHEC), also referred to as verotoxin-producing E. coli and Shiga toxin-producing E. coli, is a pathogenic bacterium that causes diarrhea and hemorrhagic colitis and that may lead to life-threatening systemic effects including the hemolytic-uremic syndrome and thrombotic thrombocytopenic purpura (23). The burden of EHEC infections is considerable. At present, there is no vaccine for EHEC and the only established treatment is supportive therapy (1). The mechanisms by which EHEC produces diarrheal disease remain to be elucidated. EHEC organisms do not produce classical enterotoxins nor do they actively invade the intestinal epithelium (52). Although EHEC organisms produce Shiga toxins (Stxs; also referred to as verotoxins), which inhibit cell protein synthesis (52), these toxins do not appear to play a role in the diarrheal illness induced by EHEC (27, 53).
EHEC and the related enteropathogenic E. coli (EPEC) adhere to epithelial cells in a morphologically distinct pattern known as attaching and effacing (A/E) adhesion (34). A/E adhesion is characterized by the loss of microvilli with intimate contact between the bacteria and the apical plasma membrane. Induction of A/E lesion formation by EHEC is coincident with a number of events within the infected host cell. The phosphatidyl inositol pathway is stimulated during infection, resulting in increased levels of Ca2+ and inositol triphosphate within the cytoplasm of infected cells (20). EHEC infection also causes the rearrangement of cytoskeletal proteins, including filamentous actin (F-actin) and the actin cross-linking protein α-actinin such that these proteins accumulate in pedestals below adherent bacteria (21, 26).
To date, an ideal animal model for EHEC infection has not been developed. Although rabbits have been used in a number of studies, this model may not be suitable for studying EHEC-induced diarrhea. Unlike the human intestine (18), the rabbit intestine possesses globotriaosylceramide (42), which is the receptor for Stx1 and Stx2 (28). This complicates the conclusions made from studies employing this model since the effects of the toxin cannot be separated from the effects of the bacteria alone. To resolve this problem, monolayers of the human intestinal cell line T84 were infected with EHEC and the resulting pathophysiology was examined. Similar to native intestinal epithelial cells, T84 cells do not express detectable globotriaosylceramide (39), thereby providing an appropriate model for studying EHEC-induced changes in intestinal epithelial cell function. This cell line is also practical for studies of the pathogenesis of EHEC infection since these cells resemble human colonic epithelial cells, which are the in vivo target cells for EHEC infection (23).
In the present study, we found that EHEC of serotype O157:H7 altered T84 barrier function in a time-dependent fashion, as demonstrated by both a decrease in transepithelial monolayer resistance and an increase in intercellular permeability by radiolabeled probes. The underlying mechanisms by which bacterial infection altered epithelial permeability were then examined. The intracellular effector molecules protein kinase C (PKC), calmodulin (CaM), and myosin light chain kinase (MLCK) were found to play a role in the EHEC-induced drop in T84 barrier function. Specific inhibitors of these signaling proteins partially corrected the T84 permeability defect induced following EHEC infection.
(This work was presented in part at the Annual Meeting of the American Gastroenterological Association, Washington, D.C., May 1997.)
MATERIALS AND METHODS
Bacterial strains and toxin.
The bacterial strains used in this study are the laboratory control strain E. coli HB101 (serotype O:rough) and the EHEC strain CL56 (serotype O157:H7) that produces both Stx1 and Stx2 (46). Bacterial strains were pelleted and resuspended to a density of 109 CFU per ml of antibiotic-free medium and added to T84 monolayers at various concentrations between 5 × 102 and 5 × 107 CFU. Viable counts of bacteria were obtained by serial 10-fold dilutions that were plated onto bile salt agar plates.
Cell culture.
T84 cells (passages 32 to 55) were grown on permeable filter supports for 7 days postseeding. The tissue culture medium contained a 1:1 mixture of Dulbecco’s modified Eagle medium and F-12 medium (Gibco, Grand Island, N.Y.) supplemented with 10% (vol/vol) fetal calf serum (Cansera, Rexdale, Ontario, Canada) and 2% (vol/vol) penicillin-streptomycin (Gibco), and the cells were grown at 37°C in 5% CO2 (7). Under these conditions, high transepithelial resistances (>1,000 Ω · cm2) were consistently obtained. Prior to infection with the various E. coli strains, the medium was changed to medium without supplementary antibiotics.
Bacterial adhesion to T84 cells.
T84 cells grown on filter supports were inoculated with bacterial strains and incubated at 37°C in 5% CO2 for up to 15 h. Nonadherent bacteria were rinsed off the monolayers, and then the monolayers were fixed with 2% glutaraldehyde in 0.1 M phosphate buffer and processed for transmission electron microscopy, as previously described (47). Briefly, samples were postfixed in 2% osmium tetroxide and dehydrated through a series of graded acetone washes. Samples were embedded in Epon, and ultrathin sections were placed onto either 300-μm-pore-size mesh copper grids or microscope slides. For light microscopy, sections were stained with toluidine blue and viewed under bright-field microscopy. Copper grids were stained with uranyl acetate and lead salts for transmission electron microscopy and examined with a Philips 300 transmission electron microscope at an accelerating voltage of 60 kV.
Electrophysiological parameters.
Cocultures of bacteria and T84 cells were incubated for various time periods (3 to 18 h), and the monolayers were then mounted into Ussing chambers to measure electrophysiological changes induced by EHEC infection. Oxygenated Kreb’s buffer (115 mM NaCl, 8 mM KCl, 1.2 mM CaCl2, 2 mM KH2PO4, 25 mM NaHCO3, 10 mM glucose [pH 7.3, 37°C]) bathed the cells in the chambers (24). The spontaneous potential difference was short-circuited except at 10-min intervals when the potential difference was measured. The barrier function of the epithelium was assessed by calculating transepithelial resistance from the potential difference and the imposed current by applying Ohm’s law.
In additional experiments, uninfected and EHEC-infected T84 cells were coincubated in the presence of the PKC inhibitor CGP41251 (Ciba-Geigy, Basel, Switzerland) or an inactive analog, CGP42700, before electrophysiological parameters were examined. Preliminary experiments examining the dose-response relationships to CGP41251 indicated that a concentration of 200 nM maximally inhibited EHEC-induced resistance changes in T84 cells. This concentration is similar to the concentration shown to inhibit purified PKC in vitro (33).
ML-9, an inhibitor of MLCK, and W7, a CaM antagonist, were also used in separate experiments (both from Sigma, St. Louis, Mo.). Final concentrations of 20 μM (for ML-9) and 50 μM (for W7) were used in these studies based on other reports describing the use of these inhibitors (17, 54). Preliminary dose-response curves also showed maximal responses at these concentrations. Inhibitors were added to both the inner (apical) and outer (basolateral) compartments of the Transwell units.
Flux experiments.
Changes in T84 intercellular permeability following infection were assessed by measuring the mucosal-to-serosal fluxes of two radiolabeled probes, [3H]mannitol (hydrodynamic diameter, 6.7 Å; Sigma) and 51Cr-EDTA (11.5 Å; Radiopharmacy, McMaster-Chedoke Hospital, Hamilton, Ontario, Canada). After the monolayers had established stable baseline Isc, [3H]mannitol and 51Cr-EDTA were added to the luminal side of the Ussing chamber to give final concentrations of 6.5 and 2.4 μCi/ml, respectively (40). After a 30-min equilibration period, samples were taken from the basolateral compartment for two 30-min flux periods. Radioactive counts in each sample were measured by liquid scintillation spectrometry to determine [3H]mannitol counts and by use of a gamma counter to determine 51Cr-EDTA. Rates of flux for these two probes were then calculated by using standard formulae (24).
Examination of ZO-1 and E-cadherin.
Distributions of the tight junction protein ZO-1 and the cell adhesion molecule E-cadherin were examined by using immunocytochemistry and confocal microscopy (2, 40). Briefly, uninfected, HB101-infected, and EHEC-infected monolayers grown on filter supports were washed and then fixed with 100% cold methanol for 10 min. The cells were incubated with 1% bovine serum albumin in phosphate-buffered saline (PBS) for 5 min, and then anti-ZO-1 or anti-E-cadherin antibodies were added at a 1:100 dilution (both antibodies from Zymed Laboratories, San Francisco, Calif.). Monolayers were incubated for 45 min at 37°C, washed, and then treated with a 1:100 dilution of fluorescein-conjugated anti-immunoglobulin G for 45 min at 37°C. Following washes in PBS, monolayers were excised from the filter supports, mounted onto slides, and examined with a confocal scanning laser microscope (Zeiss, Frankfurt, Germany).
PKC activity.
Confluent monolayers of T84 cells grown in flasks were infected with E. coli strains for 4 h or were treated with the phorbol ester phorbol 12-myristate 13-acetate (PMA; Sigma) for 2 h. Membrane and cytosolic fractions were then prepared by the procedure of Matthews et al. (32) with some modifications. Following washes in PBS, cells were scraped and disrupted by sonication in buffer containing 20 mM Tris (pH 7.4), 0.5 mM EDTA, 0.5 mM EGTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 25 μg each of aprotinin and leupeptin per ml. Homogenates were centrifuged in a Beckman ultracentrifuge with a TLA 100.3 rotor at 70,000 × g for 60 min to separate cell lysates into cytosolic (supernatant) and membrane (pellet) fractions. Membrane fractions were resuspended in 0.8 ml of the buffer described above with 0.5% Triton X-100, sheared with a 23-gauge needle, and then centrifuged at 15,000 × g for 15 min. Supernatants were then partially purified over a DEAE-cellulose (Whatman DE52) anion-exchange column, washed with a buffer containing 20 mM Tris (pH 7.5), 0.5 mM EDTA, and 0.5 mM EGTA, and eluted with a mixture of 20 mM Tris (pH 7.5), 0.5 mM EDTA, 0.5 mM EGTA, 10 mM dithiothreitol, and 200 mM NaCl.
PKC activity in cytosolic and membrane fractions was then determined by using a colorimetric PKC assay system (Pierce, Rockford, Ill.). This assay for kinase activity is based on the phosphorylation of dye-labeled pseudosubstrate and the affinity of phosphorylated proteins for Spinzyme affinity membranes. Phosphorylated pseudosubstrate was eluted from the membranes, and absorbance was then determined spectrophotometrically at 570 nm. The results are expressed as membrane-associated PKC activity as a percentage of total levels of cellular PKC.
Cell viability.
To assess viability of the T84 cells following infection with the various E. coli strains, lactate dehydrogenase (LDH) release assays were performed as previously described (40). Toluidine blue-stained sections of uninfected and infected T84 monolayers were also examined by light microscopy to ensure integrity of the monolayers.
Statistical analyses.
Results are either presented as means ± standard errors of the means (SEM) or normalized to control values and then expressed as the percent change from the value for uninfected T84 cells run in parallel. To test statistical significance among multiple groups, one-way analysis of variance, followed by post hoc comparisons with the Newman-Keuls test, was applied (SAS statistical package; SAS Institute, Cary, N.C.).
RESULTS
EHEC infection reduces T84 cell barrier function.
By transmission electron microscopy, it was found that EHEC CL56 produced A/E lesions on filter-grown T84 cells (data not shown), similar to our previous findings of EHEC-infected flask-grown T84 cells (20). To examine the effect of EHEC infection on barrier function of the epithelium, T84 cells infected with 5 × 107 CFU of EHEC CL56 per ml (a multiplicity of infection of approximately 10 bacteria per cell) were mounted into Ussing chambers. CL56 induced a time-dependent decrease in T84 transepithelial resistance after 12 h of infection, with maximum decreases in resistance observed following 15 h of infection (48% ± 7% of baseline resistance values) (Fig. 1A). Infection of T84 cells for 15 h with the control E. coli strain, HB101, did not result in any change in monolayer resistance in comparison to that of uninfected cells (Fig. 1B).
FIG. 1.
T84 transmonolayer resistance following E. coli infection. (A) Time course of EHEC-induced resistance decreases in T84 cells. In comparison to that of uninfected cells (▪), the monolayer resistance of EHEC CL56-infected cells (•) first showed a decrease 12 h after infection. Monolayer resistance was maximally decreased 15 h postinfection with EHEC. Each point represents the mean ± SEM (n = 3 to 12). ∗, P < 0.05. (B) Monolayer resistance of uninfected T84 cells and cells infected for 15 h with the EHEC strain CL56 and the nonadherent control E. coli strain, HB101. CL56 induced a significant decrease in monolayer resistance compared to that of both uninfected and HB101-infected cells. In comparison to that of uninfected cells, the monolayer resistance of HB101-infected cells was unchanged (P < 0.05, n = 13 to 15). Error bars indicate standard deviations. ∗, P < 0.05.
Bacterial dose-response relationships were examined to determine the inoculum size that resulted in significant decreases in T84 monolayer resistance. An inoculum size of 5 × 107 CFU/ml was found to consistently induce maximal decreases (45% ± 4% of baseline values) in T84 monolayer resistance following a 15-h incubation period (Table 1). This inoculum of CL56 was then used for all subsequent experiments.
TABLE 1.
T84 monolayer resistance following 15 h of infection with different-size inocula of EHEC O157:H7 CL56
| Inoculum size (CFU) | Monolayer resistance (% of control value)a |
|---|---|
| 5 × 102 | 113 ± 11 |
| 5 × 104 | 94 ± 2 |
| 5 × 106 | 64 ± 3b |
| 5 × 107 | 45 ± 7b |
Compared to that of uninfected T84 monolayers (resistance of uninfected T84 monolayers, 2,346 ± 97 Ω · cm2). Values represent means ± SEM (n = 3).
Significantly different from value for uninfected cells at P of <0.05.
Live and metabolically active bacteria were required to induce resistance changes in T84 monolayers. Bacteria that were gamma irradiated (200,000 rads) or treated with kanamycin (2 mg/ml) did not mediate resistance changes in T84 cells (104% ± 2% and 108% ± 8% of baseline resistance values for T84 cells receiving irradiated CL56 bacteria and kanamycin-treated bacteria, respectively; n = 2). The possibility that soluble factors released from the bacteria or the infected cells were inducing the changes in resistance was also examined. Filtered medium from CL56-infected T84 monolayers incubated for 15 h (conditioned medium) was added to the apical and basolateral compartments of uninfected cells for an additional 15 h. Conditioned medium was confirmed to possess Stx cytotoxic activity by use of the Vero cell cytotoxicity assay (42) (data not shown). Decreases in monolayer resistance were not observed (113% ± 3% of baseline values; n = 3).
The time dependency of the EHEC-induced resistance changes in T84 cells could indicate that eukaryotic protein synthesis was required in order to observe the EHEC-mediated drop in barrier function. To investigate this possibility, cycloheximide was added to T84 cells at a concentration of 5 μM (13) and the cells were then infected with CL56 for 15 h. In these cells, resistance decreased relative to that of uninfected cells (34% ± 5% of baseline; n = 3), indicating that host cell protein synthesis is not necessary for the induction of EHEC-mediated barrier defects in T84 cells.
Accompanying the EHEC-induced loss in T84 electrical resistance was an increase in intercellular permeability by the labeled probes, [3H]mannitol and 51Cr-EDTA. As shown in Fig. 2A, CL56-infected T84 monolayers exhibited a 3.5-fold increase in permeability by the 3H-mannitol probe relative to uninfected monolayers (138 ± 13 nmol/h/cm2 versus 39 ± 4 nmol/h/cm2 for CL56-infected and uninfected cells, respectively, P < 0.05). Although HB101 infection of T84 cells did not result in a decrease in transepithelial resistance (Fig. 1B), T84 permeability by [3H]mannitol increased (68 ± 7 nmol/h/cm2) in comparison to that of uninfected cells. This increase was not as marked, however, as the increase in flux observed in EHEC-infected cells.
FIG. 2.
T84 monolayer permeability assessed by the intercellular flux of radiolabeled probes. (A) Flux of [3H]mannitol across uninfected T84 monolayers and monolayers infected with either the control strain HB101 or EHEC CL56. Infection with EHEC for 15 h led to a significant increase in the flux of [3H]mannitol compared to that of either uninfected cells or cells infected with HB101. ∗, P < 0.05. Although no difference in electrical resistance between HB101-infected cells and uninfected cells was measured, a small increase in [3H]mannitol flux in the HB101-infected cells was detected. This increase, however, was not as marked as that induced by EHEC infection. †, P < 0.05. (B) 51Cr-EDTA flux across uninfected and E. coli-infected T84 monolayers. Following 15 h of infection with EHEC CL56, the permeability of T84 monolayers by 51Cr-EDTA was increased compared to that of uninfected or HB101-infected cells. ∗, P < 0.05 (n = 8 to 12).
CL56 infection of T84 monolayers also increased the epithelial permeability by 51Cr-EDTA (Fig. 2B). The flux of this probe across CL56-infected monolayers was increased (4.2 ± 0.7 nmol/h/cm2) in comparison to that across monolayers of both uninfected cells (1.5 ± 0.2 nmol/h/cm2) and cells infected with HB101 (1.6 ± 0.4 nmol/h/cm2, P < 0.05).
ZO-1 staining is altered in the tight junctions of EHEC-infected T84 cells.
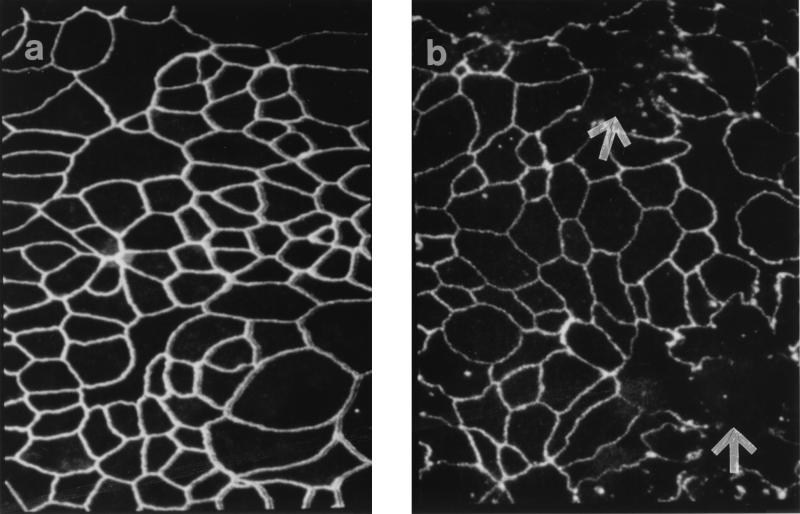
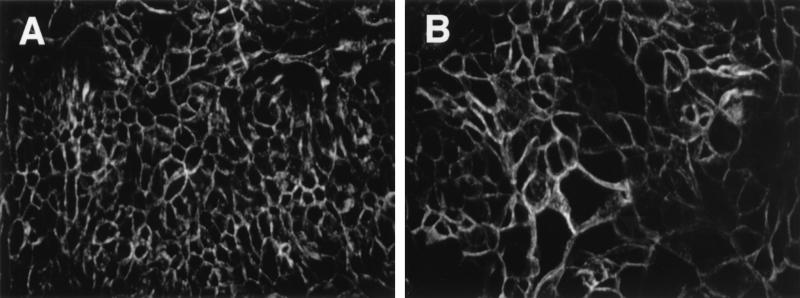
Figure 3 shows confocal micrographs of T84 monolayers stained for the tight junction protein ZO-1. Following infection with EHEC CL56 for 15 h, a disruption in staining was observed (Fig. 3b) relative to that of uninfected cells (Fig. 3a). The overall intensity of the stain appeared diminished in CL56-infected cells. In addition, a disruption of ZO-1 belts was observed. HB101-infected T84 monolayers that had reacted with anti-ZO-1 antibodies resulted in a pattern of staining that resembled the pattern obtained for uninfected cells (data not shown). In contrast, the adherens junction protein E-cadherin was not affected by EHEC infection (Fig. 4). The intensities of E-cadherin staining were comparable in uninfected cells and CL56-infected cells.
FIG. 3.
Representative confocal laser scanning micrographs (Z-series, overlay) of T84 cell monolayers immunostained for ZO-1 with antibodies to this protein followed by secondary antibodies conjugated to fluorescein-isothiocyanate. (a) Uninfected T84 cells show a normal distribution of this tight junction-associated protein outlining the perimeter of the cells. (b) T84 cells infected with EHEC CL56 for 15 h. The intensity of the stain was decreased compared to that observed for uninfected cells. In addition, areas where the ZO-1 belts were disrupted were present (arrows). Images were collected in 1-μm increments beginning at the apical aspect of the monolayers and optically sectioning to the basolateral membrane. Original magnification, ×2,400.
FIG. 4.
Representative confocal images of T84 monolayers treated with antibodies to the adherence junction protein E-cadherin. (A) Uninfected T84 cells; (B) monolayers infected with EHEC CL56 for 15 h. E-cadherin staining patterns were similar for uninfected and EHEC-infected monolayers. Images were collected as described in the legend to Fig. 3. Original magnification, ×2,400.
Activation of PKC is involved in EHEC-induced resistance decreases in T84 cells.
EHEC infection of T84 cells resulted in an increase in PKC activity in infected cells. An increase in membrane-associated PKC activity was observed following infection of T84 cells with EHEC CL56 (62% ± 4% membrane-associated PKC activity as a percentage of total cellular PKC compared to 44% ± 5% for uninfected T84 cells; n = 5; P < 0.05). This increase was comparable to that induced by the positive control, the phorbol ester PMA (71% ± 4%). Infection of T84 cells with a nonpathogenic E. coli strain, HB101, resulted in levels of membrane PKC activity that were comparable to uninfected values (45% ± 1%; n = 2).
To examine the possibility that PKC mediates the EHEC-induced decrease in T84 barrier function, an inhibitor of this enzyme was then used. The effect on cellular responses to EHEC infection of a highly selective staurosporine analog, CGP41251 (33), was examined. Treatment of T84 cells with CGP41251, at a concentration of 200 nM, corrected the EHEC-induced decrease in resistance to 70% of the resistance uninfected control monolayers (Table 2). Incubation of T84 cells with a 200 nM concentration of the inactive analog, CGP42700 (33), did not affect the ability of CL56 to lower monolayer resistance after 15 h of infection (Table 2).
TABLE 2.
Effects of the PKC inhibitor CGP41251 and its inactive analog CGP42700, the CaM antagonist W7, and the MLCK inhibitor ML-9 on EHEC-induced resistance in T84 cells
The PKC inhibitor did not affect resistance values of uninfected T84 cells (104% ± 11% of baseline value; n = 2) or the viability of CL56 (4 × 108 CFU/ml following 15 h of incubation in the presence of CGP41251 compared to 1 × 108 CFU/ml in the presence of vehicle alone).
A CaM antagonist and an inhibitor of MLCK inhibit the EHEC-induced drop in T84 cell resistance.
W7, a CaM antagonist, and ML-9, an inhibitor of MLCK, both partially reversed the EHEC-induced decrease in T84 barrier function. Concentrations of 50 μM W7 (17) and 20 μM ML-9 (54) maximally inhibited EHEC-induced resistance decreases in T84 monolayers (Table 2).
Effect of EHEC infection on cell viability and the integrity of the monolayer.
T84 monolayers grown on filter supports and infected for 15 h with EHEC CL56 remained confluent, as assessed by light microscopic evaluation of toluidine blue-stained sections (data not shown). By transmission electron microscopy, CL56-infected T84 cells appeared morphologically normal despite apically adherent bacteria and the loss of microvilli. Separation of tight junctions between neighboring cells was not evident (data not shown).
Assays to detect the release of cytosolic LDH were performed as an indirect measure of cell viability. The level of LDH released into the medium from EHEC-infected cells (8.8% ± 0.2% of total cellular LDH) was not different from that released from uninfected cells (6.0% ± 0.8%) or from that released from cells infected with the control strain, HB101 (6.3% ± 0.1%; n = 3 to 6, P > 0.05).
DISCUSSION
In this report, the pathogenic mechanisms of EHEC O157:H7 infection were examined by using T84 cells as a model system to delineate the effects of bacterial infection on intestinal epithelial cell function. EHEC infection of these cells directly altered barrier function of the monolayer. Both a decrease in transmonolayer resistance and an increase in intercellular permeability were detected in T84 monolayers following EHEC infection. By examining proteins involved in cell-cell adhesion, it was found that infection by EHEC altered the distribution of ZO-1, a tight junction-associated protein. Since the tight junction is the primary barrier to intercellular diffusion across polarized epithelium (41), EHEC-mediated disruption of ZO-1 in the tight junction provides a mechanism underlying permeability increases in T84 cells infected with EHEC O157:H7. The roles of PKC, CaM, and MLCK were examined as possible mediators of these EHEC-induced changes in tight junction permeability. Inhibitors of these effector molecules partially blocked the EHEC-induced disruption of T84 barrier function.
EHEC infection of T84 intestinal cell monolayers led to a time-dependent decrease in barrier function. Following 12 h of infection, decreases in transmonolayer resistance to EHEC infection were first observed. Maximal effects of EHEC on T84 barrier function were seen at 15 h postinfection, when resistance values dropped to less than 50% of uninfected control values. At this time point, the permeability of EHEC-infected T84 monolayers was also increased. Flux rates of two radiolabeled probes, [3H]mannitol and 51Cr-EDTA, were increased across T84 monolayers infected with EHEC. However, as shown in a recent study by our group, the intercellular passage of large proteins such as Stx is restricted despite EHEC-induced decreases in monolayer resistance (39). These effects on T84 barrier function induced by EHEC were specific for the bacteria since filtered conditioned medium obtained from EHEC-infected T84 cells did not alter transmonolayer resistance when added to naive monolayers. These data also support our recent studies showing that purified Stx does not have an impact on barrier function of T84 cell monolayers (39).
Altered barrier function of the epithelium in response to EHEC infection led us to examine the possible dysregulation of tight junction permeability in infected cells. Using an antibody to ZO-1, a component of the tight junction complex, and confocal microscopy, we demonstrated that EHEC infection specifically affects the distribution of this protein in T84 cells. Immunostaining of ZO-1 appeared less intense in T84 cells infected with EHEC than in uninfected cells, and there were areas in which the ZO-1 belts were disrupted. In contrast, the distribution of E-cadherin, which is a component of the adherens junction, was not altered by EHEC infection. These results support morphological assessments indicating that T84 cells maintain polarity and monolayer integrity following bacterial infection.
This is not the first report of bacterial infection inducing permeability defects in epithelia by influencing the tight junctions between adjacent cells. EPEC (5, 40, 48) as well as a number of bacterial toxins (9, 13, 15) alters the distribution of proteins associated with the tight junction and thereby affects intercellular permeability. This mechanism may well be a common theme by which enteropathogens induce or contribute to the diarrheal process in vivo. Although to date other A/E pathogens have not been tested in this system, at least in terms of EPEC and EHEC, A/E lesion formation or the specific products secreted by these enteropathogens may be responsible for these pathophysiological changes in T84 cells. These possibilities are currently being pursued in our laboratories.
The pathophysiological consequences of bacterium-induced increases in epithelial permeability in vivo could be either an alteration in electrochemical gradients in the intestinal epithelium resulting in diarrhea (12) or the initiation of an inflammatory response (38). Intestinal inflammation is a characteristic of EHEC-associated disease since marked neutrophilic infiltration is observed in histological sections of EHEC-infected colonic mucosa (27). Moreover, antibodies to the leukocyte adhesion molecule CD18 attenuate the severity of diarrhea in an animal model of EHEC infection (8).
A previous report from our laboratory has shown that EHEC infection of epithelial cells results in the activation of the phosphatidyl inositol pathway, leading to the release of host second messenger molecules, including Ca2+ and inositol triphosphate (20). Findings presented in this paper indicate that EHEC-induced signaling responses in epithelial cells also include the activation of PKC, a downstream effector molecule of the phosphatidyl signal transduction cascade (4). T84 cells infected with EHEC demonstrated increased PKC activity associated with the membrane fraction relative to the cytosol, an indication of PKC activation (36). The level of PKC activity induced by EHEC infection was comparable to that induced by the phorbol ester PMA. These data are similar to recent findings demonstrating EPEC-induced PKC activation (6). The possible link between the activation of PKC and the changes in T84 epithelial function induced by EHEC was then examined. By using a specific inhibitor of PKC, the EHEC-induced decreases in T84 monolayer resistance were abrogated. These data indicate that PKC activated by EHEC is also involved in the dysregulation of tight junction permeability following EHEC infection.
Regulation of tight junction permeability occurs at many sites within the epithelial cell. Several studies demonstrate that tight junction permeability is regulated by mechanisms that involve the phosphorylation of tight junction proteins (49, 51). ZO-1 in the tight junction protein complex is a target of both tyrosine phosphorylation (49) and serine or threonine phosphorylation through the action of PKC (51). Activation of PKC through the addition of phorbol esters increases intercellular permeability in a variety of cultured epithelial cell lines (16, 29, 37, 50). During tight junction assembly, PKC is required for formation of tight junctions in epithelial cells. Moreover, in vitro experiments show that ZO-1 is a direct target of this enzyme (51). The role of PKC-specific phosphorylation of ZO-1 in cells with preformed tight junctions and the potential role of phosphorylated ZO-1 in the regulation of intercellular permeability, however, are not known. Future studies will examine PKC-induced phosphorylation patterns of ZO-1 in T84 cells infected with EHEC as a possible mechanism involved in the dysregulation of tight junction permeability during infection.
PKC activation can also lead to reorganization of the actin cytoskeleton and may lead to reduced tethering of cells to each other and to the extracellular matrix (43). The resulting increase in centripetal forces at the perijunctional ring has been hypothesized to account for increases in intercellular permeability induced by PKC in the absence of changes in myosin light chain (MLC) phosphorylation (see below) in both endothelial (10) and epithelial cells (44). PKC activation in T84 cells during EHEC infection could lead to a similar cytoskeletal reorganization that may contribute to increases in intercellular permeability. The possibility that PKC is also involved in the cytoskeletal alterations during A/E lesion formation by EHEC has not been investigated thus far.
In this study, it was demonstrated that inhibition of MLCK with ML-9 partially blocked EHEC-induced decreases in T84 monolayer resistance. Since MLCK is Ca2+ and CaM dependent, the CaM antagonist W7 was also able to partially inhibit the effect of EHEC infection on T84 barrier function. Since phosphorylation of the 20-kDa MLC is an important determinant of contractile tension in both smooth-muscle and nonmuscle cells (11, 25, 45), intercellular permeability can be regulated through MLC-mediated contraction of the actomyosin ring that wraps epithelial cells at the level of the tight junction (14, 30). Tension generated within the actomyosin ring separates adjacent epithelial cells at the apical region and thereby increases intercellular permeability (30). Data presented in this paper support a role for MLC phosphorylation and the subsequent generation of tension at the actomyosin ring in the T84 permeability increases following infection with EHEC. Although phosphorylation patterns of MLC following EHEC infection were not examined in this study, a related bacterial pathogen, EPEC, induces MLC phosphorylation in epithelial cells (3, 31). Moreover, another report demonstrates the involvement of MLCK in the EPEC-induced drop in barrier function of T84 cells (54).
This study demonstrates an important role for both PKC and MLCK in EHEC-induced resistance changes in T84 monolayers. These two signaling molecules likely act at different sites within the cytosol of epithelial cells to cause the same pathophysiological outcome of increased epithelial permeability observed following EHEC infection. It is also possible that PKC regulates the activity of MLCK. In fact, PKC phosphorylation of smooth-muscle MLCK has been demonstrated previously (19, 35). It has also been suggested that PKC can indirectly regulate MLCK by modulating the availability of CaM (10). PKC phosphorylation of myristoylated alanine-rich C kinase substrate (MARCKS), which is the major substrate of PKC in most cells (22), releases CaM bound to MARCKS so that it is available for CaM-dependent enzymes such as MLCK (10). Future studies will focus on the possible interaction of these regulatory molecules and target proteins like MARCKS during EHEC infection and their effects on T84 barrier function. Understanding the complex interplay of these signal transduction responses and their role in mediating the changes in epithelial function induced by EHEC will provide the basis for the development of new strategies for the treatment of EHEC infections of humans.
ACKNOWLEDGMENTS
We thank Julia Hwang and Lois Lines of the Immunopathology Department at the Hospital for Sick Children for processing samples for electron microscopy. We thank Pam Singh for technical assistance. The PKC inhibitor CGP41251 and the inactive analog CGP42700 were kindly supplied by Ciba-Geigy.
D.J.P. was supported by a Medical Research Council of Canada studentship award and a postdoctoral fellowship award from the Canadian Association of Gastroenterology/Glaxo Wellcome. W.M. was supported by a summer studentship award from Canadian Crohn’s and Colitis Foundation. This work was supported by grants from the Medical Research Council of Canada, an A. C. Finkelstein award, and a Hospital for Sick Children Foundation Grant to M.H.P.
REFERENCES
- 1.Anonymous. Consensus Conference Statement: Escherichia coli O157:H7 infections—an emerging national health crisis, July 11–13, 1994. Gastroenterology. 1995;108:1923–1934. [PubMed] [Google Scholar]
- 2.Azghani A O, Gray L D, Johnson A R. A bacterial protease perturbs the paracellular barrier function of transporting epithelial monolayers in culture. Infect Immun. 1993;61:2681–2686. doi: 10.1128/iai.61.6.2681-2686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin T J, Brooks S F, Knutton S, Manjarrez-Hernandez H A, Aitken A, Williams P H. Protein phosphorylation by protein kinase C in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect Immun. 1990;58:761–765. doi: 10.1128/iai.58.3.761-765.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge M J. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 5.Canil C, Rosenshine I, Ruschkowski S, Donnenberg M S, Kaper J B, Finlay B B. Enteropathogenic Escherichia coli decreases the transepithelial electrical resistance of polarized epithelial monolayers. Infect Immun. 1993;61:2755–2762. doi: 10.1128/iai.61.7.2755-2762.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crane J K, Oh J S. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect Immun. 1997;65:3277–3285. doi: 10.1128/iai.65.8.3277-3285.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dharmsathaphorn K, McRoberts J A, Mandel K G, Tisdale L D, Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984;246:G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- 8.Elliot E, Li Z, Bell C, Stiel D, Buret A, Wallace J, Brzuszczak I, O’Loughlin E. Modulation of host response to Escherichia coli O157:H7 infection by anti-CD18 antibody in rabbits. Gastroenterology. 1994;106:1554–1561. doi: 10.1016/0016-5085(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 9.Fasano A, Baudry B, Pumplin D W, Wasserman S S, Tall B D, Ketley J M, Kaper J B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci USA. 1991;88:5242–5246. doi: 10.1073/pnas.88.12.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia J G N, Davis H W, Patterson C E. Regulation of endothelial cell gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J Cell Physiol. 1995;163:510–522. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- 11.Hai C, Murphy R. Sr2+ activates cross-bridge phosphorylation and latch state in smooth muscle. Am J Physiol. 1988;255:C401–C407. doi: 10.1152/ajpcell.1988.255.3.C401. [DOI] [PubMed] [Google Scholar]
- 12.Hecht G. Bugs and barriers: enteric pathogens exploit yet another epithelial function. News Physiol Sci. 1995;10:160–166. [Google Scholar]
- 13.Hecht G, Koutsouris A, Pothoulakis C, LaMont J T, Madara J L. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992;102:416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- 14.Hecht G, Pestic L, Nikcevic G, Koutsouris A, Tripuraneni J, Lorimar D D, Nowak G, Guerriero V, Elson E L, de Lanerolle P. Expression of the catalytic domain of myosin light chain kinase increases paracellular permeability. Am J Physiol. 1996;271:C1678–C1684. doi: 10.1152/ajpcell.1996.271.5.C1678. [DOI] [PubMed] [Google Scholar]
- 15.Hecht G, Pothoulakis C, LaMont T J, Madara J L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hecht G, Robinson B, Koutsouris A. Reversible disassembly of an intestinal epithelial monolayer by prolonged exposure to phorbol esters. Am J Physiol. 1994;266:G214–G221. doi: 10.1152/ajpgi.1994.266.2.G214. [DOI] [PubMed] [Google Scholar]
- 17.Hidaka H, Tanaka T. Naphthalenesulfonamides as calmodulin antagonists. Methods Enzymol. 1983;102:185–194. doi: 10.1016/s0076-6879(83)02019-4. [DOI] [PubMed] [Google Scholar]
- 18.Holgersson J, Jovall P A, Breimer M E. Glycosphingolipids of human large intestine: detailed structural characterization with special reference to blood group compounds and bacterial receptor structures. J Biochem. 1991;110:120–131. doi: 10.1093/oxfordjournals.jbchem.a123530. [DOI] [PubMed] [Google Scholar]
- 19.Ikebe M, Inagaki M, Kanamaru K, Hidaka H. Phosphorylation of smooth muscle light chain kinase by Ca2+-activated, phospholipid-dependent protein kinase. J Biol Chem. 1985;260:4547–4550. [PubMed] [Google Scholar]
- 20.Ismaili A, Philpott D J, Dytoc M T, Sherman P M. Signal transduction responses following adhesion of verocytotoxin-producing Escherichia coli. Infect Immun. 1995;63:3316–3326. doi: 10.1128/iai.63.9.3316-3326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ismaili A, Philpott D J, Dytoc M T, Soni R, Ratnam S, Sherman P M. Alpha-actinin accumulation in epithelial cells infected with attaching and effacing gastrointestinal pathogens. J Infect Dis. 1995;172:1393–1396. doi: 10.1093/infdis/172.5.1393. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson B C, Pober J S, Fenton J W, Ewenstein B M. Thrombin and histamine rapidly stimulate the phosphorylation of the myristoylated alanine-rich C-kinase substrate in human umbilical vein endothelial cells: evidence for distinct patterns of protein kinase activation. J Cell Physiol. 1992;152:166–176. doi: 10.1002/jcp.1041520121. [DOI] [PubMed] [Google Scholar]
- 23.Karmali M A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989;2:15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karnaky K J. Electrophysiology assessment of epithelia. In: Stevenson B R, Gallin W J, Paul D L, editors. Cell-cell interactions. Oxford, United Kingdom: IRL Press at Oxford University Press; 1992. pp. 257–274. [Google Scholar]
- 25.Kawamoto S, Bengur A, Sellers J, Adelstein R. In situ phosphorylation of human platelet myosin heavy and light chains by protein kinase C. J Biol Chem. 1989;264:2258–2265. [PubMed] [Google Scholar]
- 26.Knutton S, Baldwin T, Williams P H, McNeish A S. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect Immun. 1989;57:1290–1298. doi: 10.1128/iai.57.4.1290-1298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z, Bell C, Buret A, Robins-Browne R M, Stiel D, O’Loughlin E. The effect of Escherichia coli O157:H7 on intestinal structure and solute transport in rabbits. Gastroenterology. 1993;104:467–474. doi: 10.1016/0016-5085(93)90415-9. [DOI] [PubMed] [Google Scholar]
- 28.Lingwood C A, Law H, Richardson S E, Petric M, Brunton J L, de Grandis S, Karmali M A. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem. 1987;262:8834–8839. [PubMed] [Google Scholar]
- 29.Lynch J J, Ferro T J, Blumenstock F A, Brockenauer A M, Malik A B. Increased endothelial albumin permeability mediated by protein kinase C activation. J Clin Invest. 1990;85:1991–1998. doi: 10.1172/JCI114663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madara J L. Loosening tight junctions. Lessons from the intestine. J Clin Invest. 1989;83:1089–1094. doi: 10.1172/JCI113987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manjarrez-Hernandez H A, Baldwin T J, Williams P H, Haigh R, Knutton S, Aitken A. Phosphorylation of myosin light chain at distinct sites and its association with the cytoskeleton during enteropathogenic Escherichia coli infection. Infect Immun. 1996;64:2368–2370. doi: 10.1128/iai.64.6.2368-2370.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthews J B, Awtrey C S, Hecht G, Tally K J, Thompson R S, Madara J L. Phorbol ester sequentially downregulates cAMP-regulated basolateral and apical Cl− transport pathways in T84 cells. Am J Physiol. 1993;265:C1109–C1117. doi: 10.1152/ajpcell.1993.265.4.C1109. [DOI] [PubMed] [Google Scholar]
- 33.Meyer T, Regenass U, Fabbro D, Alteri E, Rosel J, Muller M, Caravatti G, Matter A. A derivative of staurosporine ( CGP41251) shows selectivity for protein kinase C inhibition and in vitro anti-proliferative as well as in vivo anti-tumor activity. Int J Cancer. 1989;43:851–856. doi: 10.1002/ijc.2910430519. [DOI] [PubMed] [Google Scholar]
- 34.Moon H W, Whipp S C, Argenzio R A, Levine M M, Giannella R A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishikawa M, Shirakawa S, Adelstein R S. Phosphorylation of smooth muscle light chain kinase by protein kinase C. J Biol Chem. 1985;260:8978–8983. [PubMed] [Google Scholar]
- 36.Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour-promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- 37.Ojakian G K. Tumor promoter-induced changes in the permeability of epithelial cell tight junctions. Cell. 1981;23:95–103. doi: 10.1016/0092-8674(81)90274-9. [DOI] [PubMed] [Google Scholar]
- 38.Perdue M H, McKay D M. Integrative immunophysiology in the intestinal mucosa. Am J Physiol. 1994;267:G151–G165. doi: 10.1152/ajpgi.1994.267.2.G151. [DOI] [PubMed] [Google Scholar]
- 39.Philpott D J, Ackerely C A, Kiliaan A J, Karmali M A, Perdue M H, Sherman P M. Translocation of verotoxin-1 across T84 intestinal cell monolayers: mechanism of bacterial toxin penetration of the epithelium. Am J Physiol. 1997;273:G1349–G1358. doi: 10.1152/ajpgi.1997.273.6.G1349. [DOI] [PubMed] [Google Scholar]
- 40.Philpott D J, McKay D M, Sherman P M, Perdue M H. Infection of T84 intestinal epithelial cells with enteropathogenic Escherichia coli alters barrier and transport functions. Am J Physiol. 1996;270:G634–G645. doi: 10.1152/ajpgi.1996.270.4.G634. [DOI] [PubMed] [Google Scholar]
- 41.Powell D W. Barrier function of epithelia. Am J Physiol. 1981;241:G275–G288. doi: 10.1152/ajpgi.1981.241.4.G275. [DOI] [PubMed] [Google Scholar]
- 42.Richardson S E, Rotman T A, Jay V, Smith C R, Becker L E, Petric M, Olivieri N, Karmali M A. Experimental verocytotoxemia in rabbits. Infect Immun. 1992;60:4154–4167. doi: 10.1128/iai.60.10.4154-4167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schliwa M T, Nakamura K R, Porter K R, Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984;99:1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shasby D M, Kamath J M, Moy A B, Shasby S S. Ionomycin and PDBU increase MDCK monolayer permeability independently of myosin light chain phosphorylation. Am J Physiol. 1995;269:L144–L150. doi: 10.1152/ajplung.1995.269.2.L144. [DOI] [PubMed] [Google Scholar]
- 45.Sheldon R, Moy A, Lindsley K, Shasby S, Shasby D M. Role of myosin light chain phosphorylation in endothelial cell retraction. Am J Physiol. 1993;265:L606–L612. doi: 10.1152/ajplung.1993.265.6.L606. [DOI] [PubMed] [Google Scholar]
- 46.Sherman P, Soni R, Petric M, Karmali M. Surface properties of verocytotoxin-producing Escherichia coli O157:H7. Infect Immun. 1987;55:1824–1829. doi: 10.1128/iai.55.8.1824-1829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherman P M, Fleming N, Forstner J, Roomi N, Forstner G. Bacteria and the mucus blanket in experimental small bowel bacterial overgrowth. Am J Pathol. 1987;126:527–534. [PMC free article] [PubMed] [Google Scholar]
- 48.Spitz J, Yuhan R, Koutsouris A, Blatt C, Alverdy J, Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995;268:G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- 49.Staddon J M, Herrenknecht K, Smales C, Rubin L L. Evidence that tyrosine phosphorylation may increase tight junction permeability. J Cell Sci. 1995;108:609–619. doi: 10.1242/jcs.108.2.609. [DOI] [PubMed] [Google Scholar]
- 50.Stenson W F, Easom R A, Riehl T E, Turk J. Regulation of paracellular permeability in Caco-2 cell monolayers by protein kinase C. Am J Physiol. 1993;265:G955–G962. doi: 10.1152/ajpgi.1993.265.5.G955. [DOI] [PubMed] [Google Scholar]
- 51.Stuart R O, Nigam S K. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci USA. 1995;92:6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tesh V L, O’Brien A D. Adherence and colonization mechanisms of enteropathogenic and enterohemorrhagic Escherichia coli. Microb Pathog. 1992;12:245–254. doi: 10.1016/0882-4010(92)90043-n. [DOI] [PubMed] [Google Scholar]
- 53.Tzipori S, Karch H, Wachsmuth K I, Robins-Browne R M, O’Brien A D, Lior H, Cohen M L, Smithers J, Levine M M. Role of a 60-megadalton plasmid and Shiga-like toxins in the pathogenesis of infection caused by enterohemorrhagic Escherichia coli O157:H7 in gnotobiotic piglets. Infect Immun. 1987;55:3117–3125. doi: 10.1128/iai.55.12.3117-3125.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuhan R, Koutouris A, Savkovic S D, Hecht G. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology. 1997;138:1873–1882. doi: 10.1016/s0016-5085(97)70006-4. [DOI] [PubMed] [Google Scholar]