Abstract
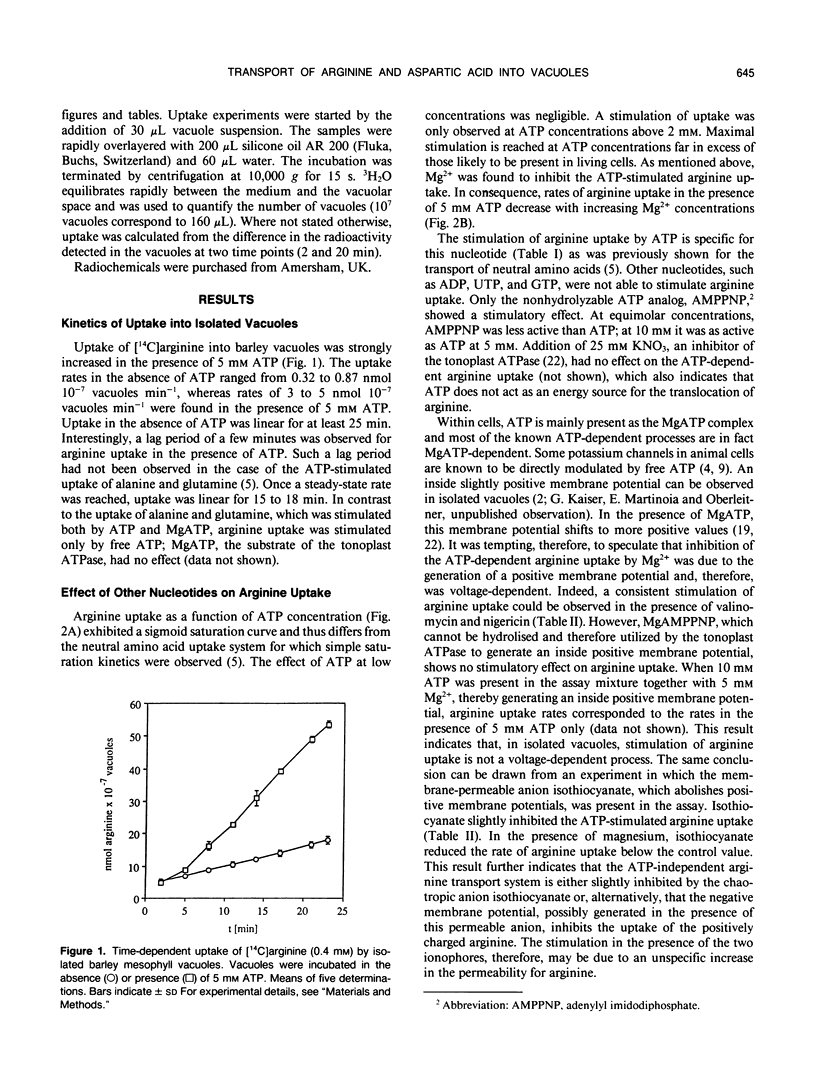
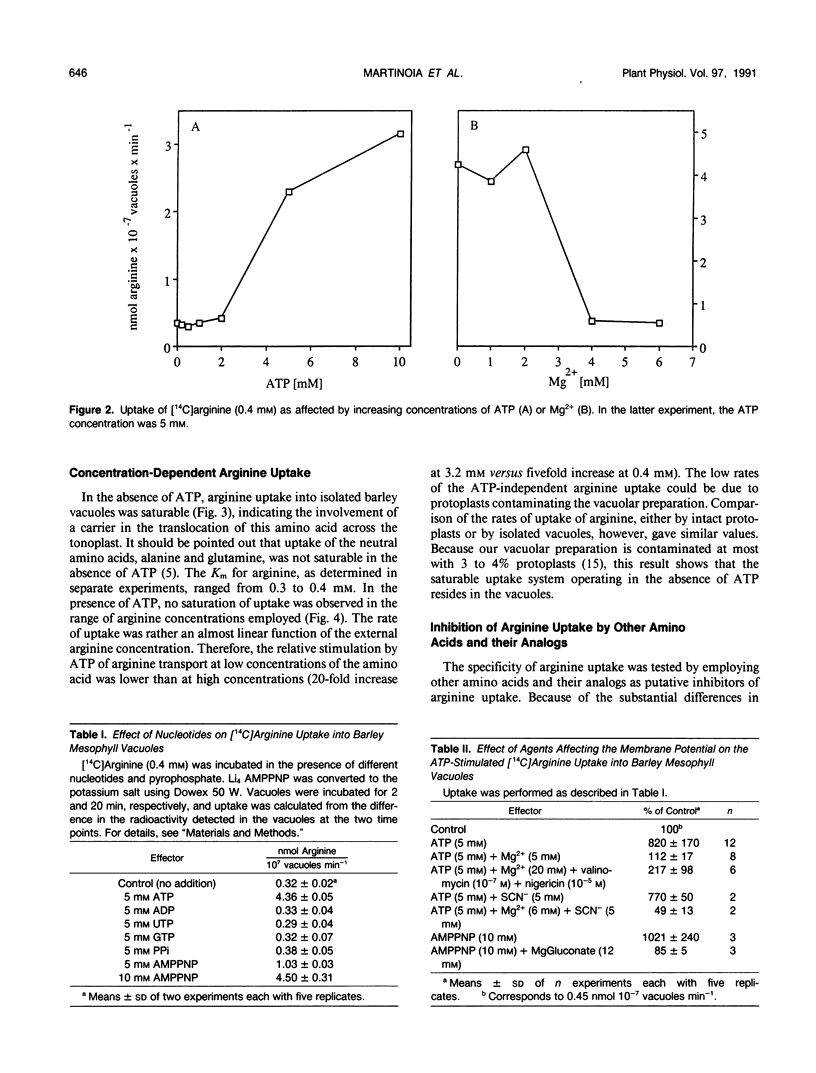
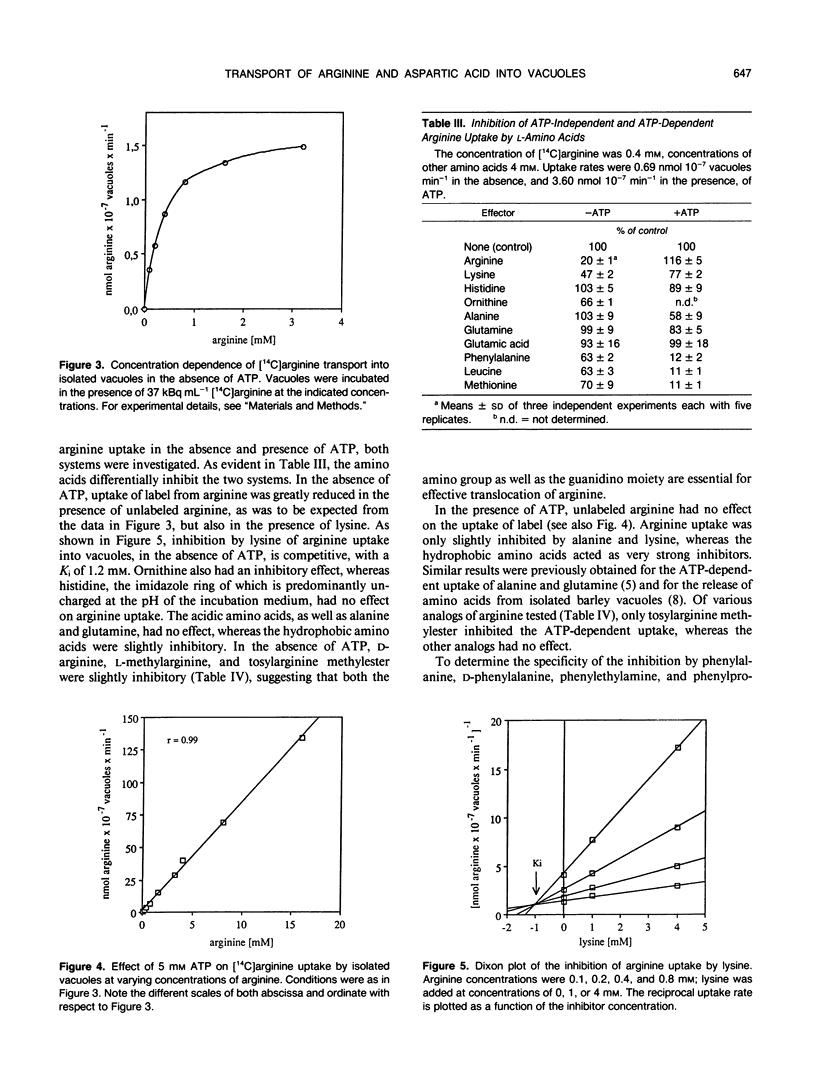
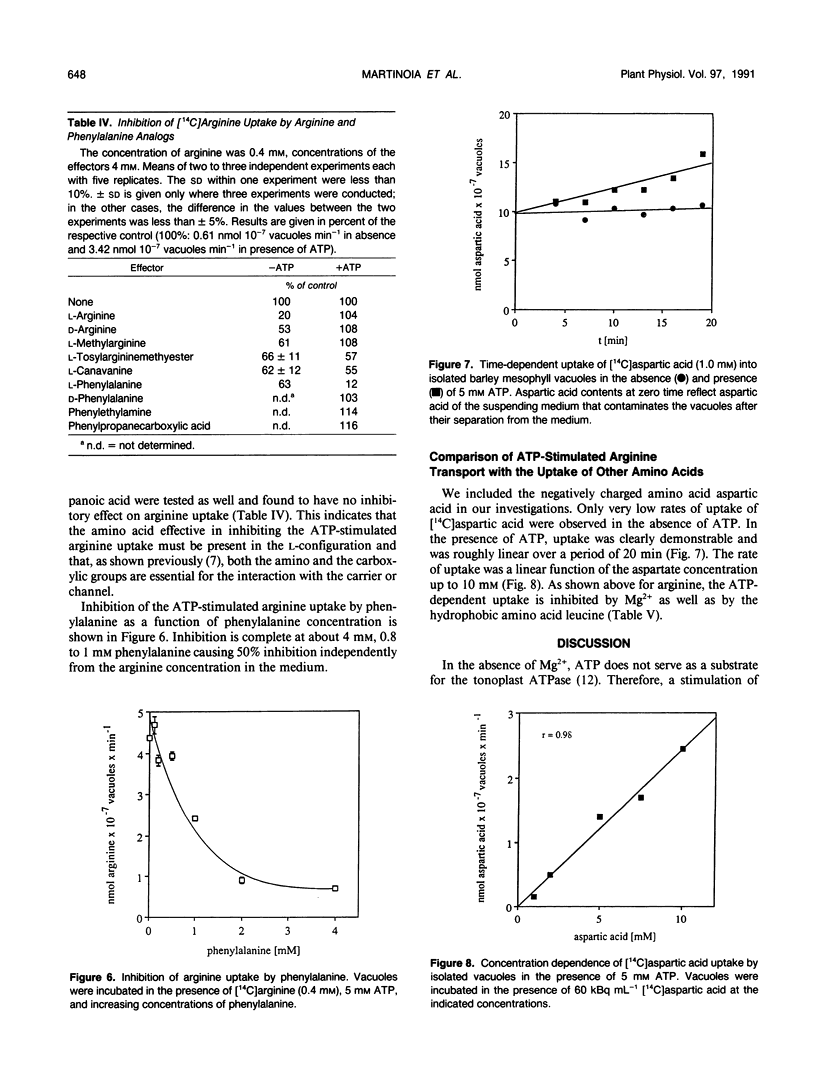
The transport of arginine into isolated barley (Hordeum vulgare L.) mesophyll vacuoles was investigated. In the absence of ATP, arginine uptake was saturable with a Km of 0.3 to 0.4 millimolar. Positively charged amino acids inhibited arginine uptake, lysine being most potent with a Ki of 1.2 millimolar. In the presence of free ATP, but not of its Mg-complex, uptake of arginine was drastically enhanced and a linear function of its concentration up to 16 millimolar. The nonhydrolyzable adenylyl imidodiphosphate, but no other nucleotide tested, could substitute for ATP. Therefore, it is suggested that this process does not require energy and does not involve the tonoplast ATPase. The ATP-dependent arginine uptake was strongly inhibited by p-chloromercuriphenylsulfonic acid. Furthermore, hydrophobic amino acids were inhibitory (I50 phenylalanine 1 millimolar). Similar characteristics were observed for the uptake of aspartic acid. However, rates of ATP-stimulated aspartic acid transport were 10-fold lower as compared to arginine transport. Uptake of aspartate in the absence of ATP was negligible.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boller T., Dürr M., Wiemken A. Characterization of a specific transport system for arginine in isolated yeast vacuoles. Eur J Biochem. 1975 May;54(1):81–91. doi: 10.1111/j.1432-1033.1975.tb04116.x. [DOI] [PubMed] [Google Scholar]
- Cook D. L., Hales C. N. Intracellular ATP directly blocks K+ channels in pancreatic B-cells. Nature. 1984 Sep 20;311(5983):271–273. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Dietz K. J., Jäger R., Kaiser G., Martinoia E. Amino Acid Transport across the Tonoplast of Vacuoles Isolated from Barley Mesophyll Protoplasts : Uptake of Alanine, Leucine, and Glutamine. Plant Physiol. 1990 Jan;92(1):123–129. doi: 10.1104/pp.92.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K. J., Lang M., Schönrock M., Zink C. ATP dependence of anion uptake by isolated vacuoles: requirement for excess Mg2+. Biochim Biophys Acta. 1990 May 24;1024(2):318–322. doi: 10.1016/0005-2736(90)90360-z. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., West-Jordan J. A., Abraham R. J., Edwards R. H., Petersen O. H. The gating of nucleotide-sensitive K+ channels in insulin-secreting cells can be modulated by changes in the ratio ATP4-/ADP3- and by nonhydrolyzable derivatives of both ATP and ADP. J Membr Biol. 1988 Sep;104(2):165–177. doi: 10.1007/BF01870928. [DOI] [PubMed] [Google Scholar]
- Homeyer U., Litek K., Huchzermeyer B., Schultz G. Uptake of Phenylalanine into Isolated Barley Vacuoles Is Driven by Both Tonoplast Adenosine Triphosphatase and Pyrophosphatase : Evidence for a Hydrophobic l-Amino Acid Carrier System. Plant Physiol. 1989 Apr;89(4):1388–1393. doi: 10.1104/pp.89.4.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner K. H., Sze H. Potential-dependent anion transport in tonoplast vesicles from oat roots. Plant Physiol. 1987 Mar;83(3):483–489. doi: 10.1104/pp.83.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinoia E., Schramm M. J., Kaiser G., Kaiser W. M., Heber U. Transport of anions in isolated barley vacuoles : I. Permeability to anions and evidence for a cl-uptake system. Plant Physiol. 1986 Apr;80(4):895–901. doi: 10.1104/pp.80.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek Y. L., Weiss R. L. Identification of an arginine carrier in the vacuolar membrane of Neurospora crassa. J Biol Chem. 1989 May 5;264(13):7285–7290. [PubMed] [Google Scholar]
- Sato T., Ohsumi Y., Anraku Y. Substrate specificities of active transport systems for amino acids in vacuolar-membrane vesicles of Saccharomyces cerevisiae. Evidence of seven independent proton/amino acid antiport systems. J Biol Chem. 1984 Sep 25;259(18):11505–11508. [PubMed] [Google Scholar]
- Zerez C. R., Weiss R. L., Franklin C., Bowman B. J. The properties of arginine transport in vacuolar membrane vesicles of Neurospora crassa. J Biol Chem. 1986 Jul 5;261(19):8877–8882. [PubMed] [Google Scholar]


