Abstract
Biological sex contributes to phenotypic sex effects through genetic (sex chromosomal) and hormonal (gonadal) mechanisms. There are profound sex differences in the prevalence and progression of age-related brain diseases, including neurodegenerative diseases. Inflammation of neural tissue is one of the most consistent age-related phenotypes seen with healthy aging and disease. The pro-inflammatory environment of the aging brain has primarily been attributed to microglial reactivity and adoption of heterogeneous reactive states dependent upon intrinsic (i.e., sex) and extrinsic (i.e., age, disease state) factors. Here, we review sex effects in microglia across the lifespan, explore potential genetic and hormonal molecular mechanisms of microglial sex effects, and discuss currently available models and methods to study sex effects in the aging brain. Despite recent attention to this area, significant further research is needed to mechanistically understand the regulation of microglial sex effects across the lifespan, which may open new avenues for sex informed prevention and treatment strategies.
1. Introduction
1.1. Why study sex effects in neuroimmunity in the aging brain?
Protecting the nervous system from injury and infection is essential for organismal survival. As a result, complex neuro-immune interactions have evolved across taxa [1]. Sex effects in peripheral immunity have been widely studied (as reviewed here [2]). Of note, sex effects in both innate and adaptive peripheral immunity exist across the lifespan but change at critical periods of development and hormonal transition (i.e., puberty, menopause/andropause) [2]. This suggests that both genes and hormones contribute to sex differences in peripheral immunity. How sex influences neuroimmunity has been historically less studied than peripheral sex effects [3]. With a welcomed emphasis to include sex as a biological variable in all scientific studies, there has been a wave of reports suggesting sex is an important factor in the development of age-related inflammation of neural tissue [4–8]. Microglia are brain-resident macrophages that originate from yolk-sac progenitors [9] and represent the only parenchymal CNS immune cell [10]. In contrast, meningeal, choroid plexus, and perivascular macrophages are derived from bone-marrow hematopoietic stem cells and occupy non-parenchymal spaces in the brain [10]. This review will focus on the regulation of microglial sex effects in the aging brain.
Although microglia are principally referred to as immune cells, they serve diverse CNS functions across the lifespan (Figure 1A). For example, microglia contribute to learning and memory by synapse remodeling, neuroprotection, and trophic support [11–14]. During sex differentiation of the rodent brain, male-specific hormonal signaling increases microglial phagocytic capacity in the medial amygdala which leads to engulfment of newborn astrocytes. The decreased astrocytic population is necessary for the development of male-specific play behavior [15]. Additionally, microglia react when exposed to inflammatory stimuli (i.e., pathogens) and assume classical immune functions [16]. In the absence of infection, microglia adopt reactive phenotypes with aging [17], a ‘sterile neuroinflammation’, which can contribute to the breakdown of the blood-brain barrier (BBB) [18] and neurodegeneration [19]. ‘Sterile neuroinflammation’ and concomitant microglial reactivity rely on detection of contents released from damaged or dying cells (i.e., nucleic acids, proteins, lipids) that can be broadly classified as Damage Associated Molecular Patterns (DAMPs) [20]. DAMPs signal through pattern recognition receptors, such as toll-like receptors (TLRs) and NOD-like receptors (NLRs), which are broadly expressed by CNS cells, including microglia [21]. While which DAMPs occur with aging and their sources is still an area of investigation, microglial reactivity with aging is conserved across species and is one of the most consistent phenotypes of brain aging [5, 16, 17, 22, 23]. Through constant environmental surveillance, microglia can quickly detect and adapt to changes in their microenvironment. Microglial functional heterogeneity relies upon the cell’s ability to react to diverse stimuli to alter: motility, phagocytosis, transcriptional programming, cell surface marker expression, and release of soluble factors [24].
Figure 1. Microglial aging and potential mechanisms of sex effect regulation.
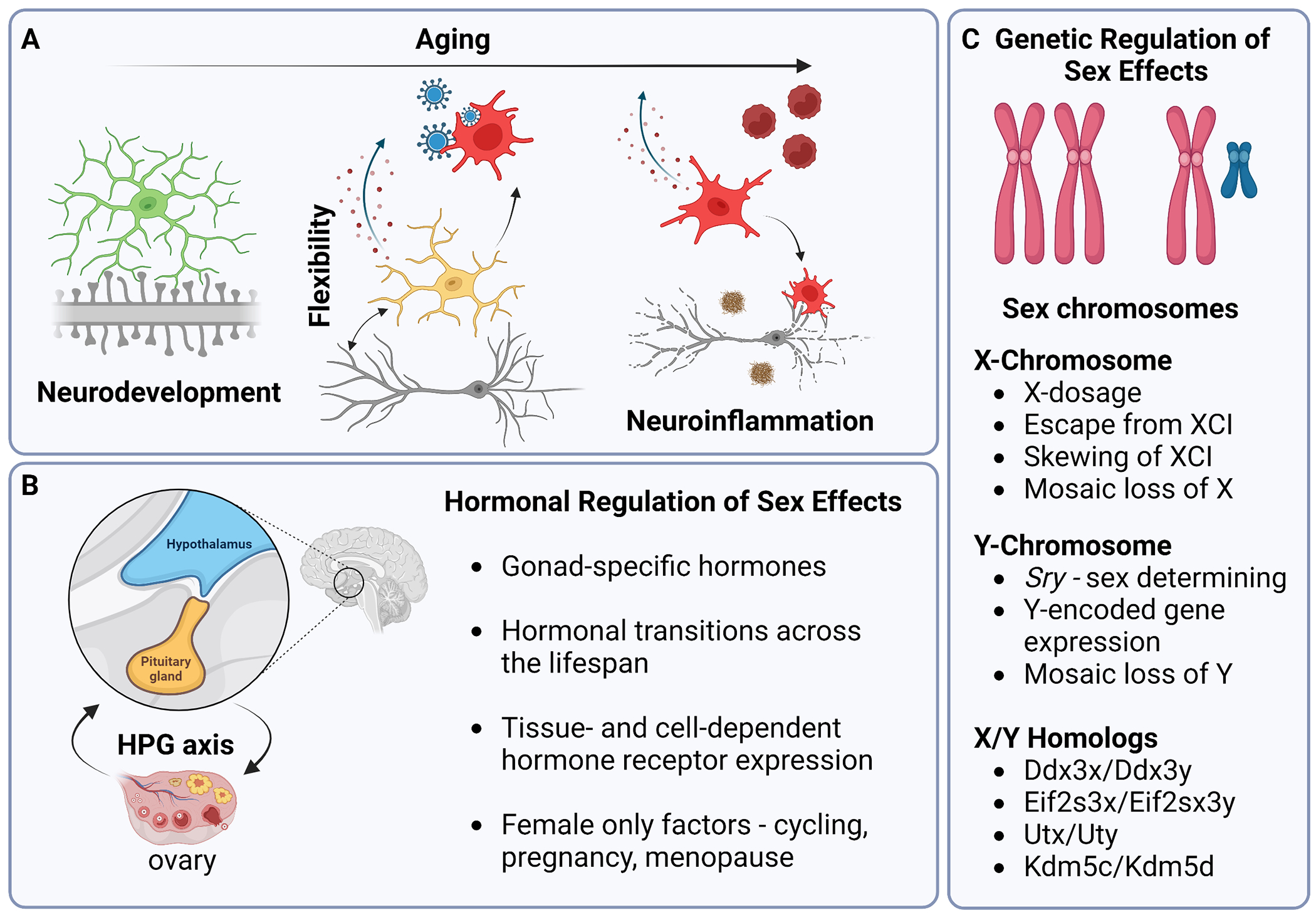
A) During development microglia perform important CNS functions, including synaptic pruning in the establishment of neural circuits. Adult microglia continue to support neuronal function through synaptic remodeling and cross-cellular communication. In response to inflammatory stimuli (i.e., infection) microglia react and adopt diverse phenotypic states. With aging microglia adopt a chronic inflammatory phenotype that is characterized by upregulation of pro-inflammatory cytokines/chemokines, decreased motility, and dysfunctional phagocytosis. Aged microglia release factors that alter the integrity of the blood-brain-barrier (BBB) and recruit circulating immune cells to invade the brain parenchyma. Pathological states (i.e., Aβ plaque formation in AD) can interact with microglial aging phenotypes and contribute to neurodegeneration. B) The Hypothalamus-Pituitary-Gonadal (HPG) axis controls the release of gonadal sex hormones (i.e., estrogens) from the ovaries or testes. Males and females produce distinct gonadal hormones that alter in profile at key transitionary periods across the lifespan and interact with specific cell types through hormone receptors. C) Sex chromosomes (XX v. XY) can regulate sex effects through many mechanisms, including escape from XCI and mosaic LOY.
Microglial reactivity is also implicated in age-related neurodegenerative diseases [25], such as Alzheimer’s disease (AD) [26–29], Parkinson’s disease (PD) [30–32], and Multiple Sclerosis (MS) [33], all of which have sex biases in presentation and progression [34–43]. Thus, it has been posited that sex effects seen in age-related neurodegenerative diseases stem from differences in microglial reactivity (as reviewed here [44]). The goal of this review is to explore the molecular mechanisms, whether genetically or hormonally driven, that may contribute to sex effects seen in microglia during brain aging and discuss currently available models and methods to study sex effects in the aging brain (Figure 1B–C).
1.2. Categorizing sex effects: determination, differentiation, dimorphism, difference, & divergence/convergence
Sex effects is a general term to describe sex “differences” without considering the nature of the difference in the context of development, aging, or disease. McCarthy et al. [45] operationally categorized types of sex effects into three groups: 1) sex dimorphisms, 2) sex differences, and 3) sex convergences or divergences (Figure 2). Sex dimorphisms are defined by having two forms, one in males and another in females. For example, expression of Y-chromosome encoded genes (such as Ddx3y) is sexually dimorphic because its expression is limited to males. A sex difference occurs when the measured endpoint is a continuous variable, and the average is different between males and females. Human height is a sex difference because, on average, men are taller than women. A sex divergence or convergence is a difference that becomes more different or similar, respectively, in response to another variable (i.e., time, response to stress, disease state). An example of a sex divergence is the induction of microglial-mediated inflammatory signaling in the mouse hippocampus with aging [5], which shows no difference between males and females at a young age and higher induction in old females than old males.
Figure 2. Classification of sex effects.
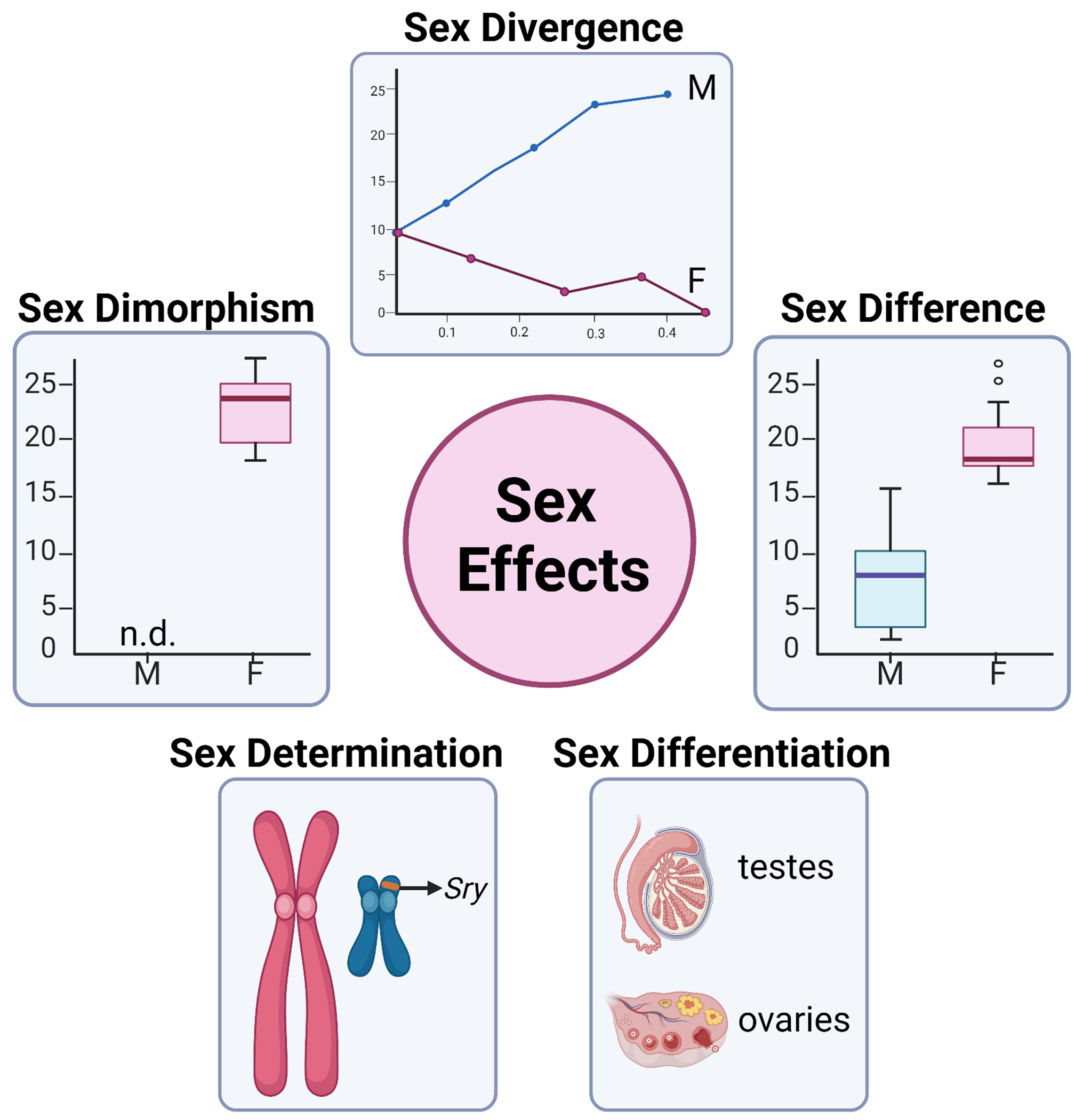
A) Sex effects can be classified operationally into five categories, sex: 1) determination, 2) differentiation, 3) dimorphism, 4) difference, and 5) divergence/convergence.
In addition to the three categories proposed by McCarthy et al. [45], there are also two cases of sex effects during development: 1) sex determination and 2) sex differentiation (Figure 2). Sex determination is the commitment of the bipotential gonad during early development to become either testes or ovaries [46]. In mammals, sex determination occurs in response to the presence or absence of the sex-determining region of Y (Sry) gene, encoded on the short arm of the Y-chromosome [47]. As such, the Sry gene is considered the “master switch” in mammalian sex determination that triggers sex differentiation. During mammalian embryogenesis, bipotential gonads form from the genital ridge. Expression of Sry upregulates SRY box containing gene 9 (Sox9) leads to the differentiation of Sertoli cells, the formation of testes, and the suppression of the female sex-determining pathway [48]. In the absence of Sry, female-specific gene programming (including Wnt4, Foxl2, Fst) cause the differentiation of the bipotential gonad into ovaries [48]. Using these terms provides additional information into the nature of the sex effect, especially when considering the outside influence of variables such as disease and age.
2. Sex effects in neuroimmunity in the aging brain
2.1. Immunity in the brain
Neuroinflammatory signaling processes encompass several pathways and cell types, from phenotype switching in microglia and astrocytes to increased circulating cytokine levels. Conventionally, the brain was considered ‘immune-privileged’ due to the lack of or limited ability to develop immune responses to foreign antigens in the CNS [49, 50]. The brain does lack conventional dendritic cells that are required to mount an adaptive immune responses in most organ systems [51, 52]. However, in response to infection or other CNS pathologies, the CNS can recruit leukocytes to mount an adaptive immune response [53]. This difference between the CNS and other organ systems often led to the presumption that many of the immune-related proteins expressed in other organ systems had no role in CNS function (except during viral infection and some pathological states where the BBB was highly compromised). This has proven to be a mistaken assumption, as newer molecular biological and biochemical tools have identified several immune-related genes expressed by CNS cells (i.e., class I major histocompatibility complex (MHC-I) [54], complement components [55, 56], and toll-like receptors (TLRs) [57]). The initial characterization of these molecules (i.e., MHC-I, TLRs) as ‘immunological’ is a by-product of their discovery in the immune system. Rapidly accumulating evidence suggests that many of these ‘immune’ genes are functionally pleiotropic, with different functions in different cellular milieus [58]. For example, mice deficient in complement components C1q, C3, or CR3 display defects in CNS synapse elimination [59, 60]. In addition, microglial phagocytosis of presynaptic inputs during retinogeniculate pruning is dependent on both neural activity and microglial signaling through CR3 [60]. Similarly, deficiency of the MHC-I molecule β2m leads to higher cortical synapse density in the mouse brain [61]. Together, these results demonstrate that ‘immunological’ molecules in the CNS are better classified as signaling molecules that work in both cis (cell-autonomous signaling within one cell) and trans (signaling between two cells, such as across synapses) manners to different functional ends. This has led the field to a more complete and nuanced view of immune privilege [62, 63]. Given that immune-related processes occur in the homeostatic brain [59–61] and that sex has profound effects on peripheral immunity [2], it stands to reason that neuroimmune processes are also affected by sex.
Due to the separation imposed by the BBB, differentiating sex effects between brain-resident and circulating immune processes is crucial; however, this bifurcation should not be considered absolute. Infiltration of circulating immune cells into the brain parenchyma can contribute to the pathogenesis of neurodegenerative diseases, as in MS [64]. However, circulating immune cells must pass through the BBB to gain access to the brain. Endothelial transport into the brain is regulated by interactions between astrocytes, pericytes, microglia, and the basement membrane [65]. The integrity of the BBB is crucial to confer homeostasis to the CNS and to limit the entry of blood-borne molecules and circulating leukocytes [66, 67]. During disease, a ‘leaky’ BBB facilitates the infiltration of circulating blood-derived molecules, potentially neurotoxic substances, and immune cells [68, 69]. Aging contributes to the degradation of the BBB, which promotes inflammation and neurotoxicity [70] and may contribute to brain diseases. On the other hand, increased BBB permeability observed in age-related diseases (i.e., stroke, AD, PD, MS) [71, 72] is exacerbated by underlying pathologies, microglial reactivity, and genetic factors, among others. Whether BBB breakdown is causal or a by-product of neurodegeneration is a cause of debate. However, increased BBB permeability leads to neurotoxin and leukocyte infiltration into the brain, initiating an immune response and propagating cell death [70, 73].
Although microglia are rarely considered a key member of BBB [74], they are in constant bi-directional communication with endothelial cells and help regulate the influx of blood-derived molecules into the brain [69]. In response to inflammatory stimuli (i.e., systemic infection, brain disease), reactive microglia can contribute to a leaky phenotype through several mechanisms that degrade the BBB, including the: 1) downregulation of paracellular tight-junction proteins [18, 72, 75], 2) secretion of neurotoxic molecules (i.e., reactive oxygen species, nitric oxide, quinolinic acid), and 3) upregulation of chemokines (MCP-1, CXCL-1, MIP-1α) and inflammatory cytokines (IL-6, TNF-α, IL1β) [71, 76, 77]. Taken together, a nuanced view of microglial reactivity and sex effects encompasses both brain intrinsic processes and the impact of the circulating factors on the brain, both of which may alter the BBB permeability to change the communication between the brain and periphery.
2.2. The contributions of microglia to inflammaging in the brain
‘Inflammaging’ is a term used to describe the sterile, chronic inflammation that occurs with aging [78, 79] and is hypothesized to result from accumulating cell debris, epigenetic changes, genome instability, and oxidative stress [78, 80, 81]. Inflammation of neural tissue is consistently described as a byproduct of aging [82–85] and is associated with injury [86–90] and disease [19, 25–27, 33, 91, 92]. In the context of healthy brain aging, human inflammaging is associated with decreased cognitive function [93, 94]. As such, targeting microglial reactivity to treat age-related brain diseases has become a promising avenue for therapeutic development. However, strong immune suppression is not without consequences. For example, the interferon-modulating MS treatment natalizumab increases the risk of a viral brain disease that causes progressive multifocal leukoencephalopathy [95]. Thus, it is imperative to understand the nuances of microglial reactivity in the context of specific diseases, as well as factors such as sex which may guide future therapeutic development.
As previously mentioned, microglia serve essential immune and non-immune functions in the brain [96], with the flexibility to adapt to changes in their microenvironment across the lifespan. In line with phenotypic descriptions of other macrophages, microglia were originally categorized into pro-inflammatory ‘M1’ or anti-inflammatory ‘M2’ phenotypes [97]. However, with the increased granularity of single-cell approaches, the archetype has shifted to include diverse microglial phenotypes based on their transcriptomic profiles [98]. As a result, several reports have identified new microglial phenotypes [27, 35, 99–104] described in the context of specific disease states and/or locations within the CNS. A summary of microglial phenotypic states is given in Table 1.
Table 1.
Recently identified microglial phenotypic states identified in single cell RNA-seq studies.
| Microglial phenotypic state | Acronym | Sex Effect? | Description | Citation |
|---|---|---|---|---|
| Disease-associated microglia | DAM | N/A | AD risk genes, associate with Aβ plaques | Keren-Shaul et al., 2017 [100] |
| Microglial neurodegenerative phenotype | MGnD | N/A | Clec7a, Lgals3, Gpnmb, Itgax, Spp1, Ccl2, Fabp5 | Kraseman et al., 2017 [320] |
| Activated Response Microglia | ARM | Female microglia have faster progression to ARM in APPNL-G-F mice | MHCII-high, tissue repair & AD risk genes | Sala Frigerio et al., 2019 [35] |
| Interferon-Response Microglia | IRM | No sex difference found | Innate immune & interferon type I pathways | Sala Frigerio et al., 2019 [35] |
| Axon Tract-Associated Microglia | ATM | No sex difference found | Localized to axon tract at restricted developmental window, SPP1, IGF1, lysosomal genes | Hammond et al., 2019 [99] |
| Proliferative-region-associated microglia | PAM | N/A | DAM markers, metabolically active, phagocytose oligodendrocytes | Li et al., 2019 [103] |
| Human AD microglia | HAM | Mostly restricted to sex chromosomal genes | Distinct from DAM, upregulate APOE, aging signature | Srinivasan et al., 2020 [27] |
| Lipid-droplet-accumulating microglia in aging mice and humans | LDAM | N/A | Production of nitric oxide and ROS, lysosomal genes, lipid metabolism | Marschallinger et al., 2020 [101] |
| White matter-associated microglia | WAM | Did not notice any sex effects, no presented analysis of sex effects | TREM2, APOE, as well as DAM markers | Safaiyan et al., 2021 [104] |
| Microglia inflamed in multiple sclerosis | MIMS | Sex matching applied, no presented analysis of sex effects | C1q, as well as DAM markers | Absinta et al., 2022 [102] |
Recently, a group of leading microglial biologists wrote a white paper on microglial nomenclature to guide the classification of microglial phenotypic states [24]. They concluded that, unlike terminally differentiated neurons, microglia are difficult to classify due to their incredible plasticity. The authors suggest that the ‘M1/M2’ classification system is too generic. Instead, microglial classification should be based on intrinsic (species, ontogeny, sex, genetic background) and extrinsic (age, spatial location, environment) determinants inferred from multiple lines of evidence (morphology, transcriptomics, proteomics, epigenomics). Ultimately, microglia exist on a phenotypic gradient and current work is being done to establish the conditions that evoke certain phenotypic states.
Even in the absence of injury or disease, aged microglia adopt a dysfunctional state that is characterized by six hallmarks: 1) impaired phagocytosis, 2) senescence, 3) dystrophy, 4) impaired motility, 5) altered signaling, and 6) impaired proteostasis [17]. Cellular senescence is defined as an irreversible cell-cycle arrest with various forms of cell damage, dysregulated metabolism, and production of the senescence-associated secretory phenotype (SASP) [105]. Microglial senescence with aging has been identified in mice [106] and humans [22] and likely contributes to the ‘inflammaging’ phenotype seen in the healthy aging brain. Since microglia are implicated in the pathogenesis of many sexually divergent age-related brain diseases (i.e., AD, PD, MS), there is an increased focus on the nature and regulation of microglial sex differences across the lifespan [44]. However, the nuances of microglial sex effects across the lifespan have not yet been fully elucidated.
2.3. Sex effects in microglial-mediated neuroimmunity in the aging brain
Understanding microglial sex effects during brain aging and their underlying mechanisms are essential to identifying pathways that confer risk and resilience to age-related brain diseases and will help guide sex-informed therapeutic intervention. Generalizing microglial sex effects in the aging brain is difficult due to the plastic nature of microglia in response to intrinsic and extrinsic determinants [24], such as disease state and brain region. In addition, the inconsistent inclusion of both sexes in aging studies has made it more difficult to define sex effects. Several recent reviews have highlighted sex effects in microglia [44, 107–111], each implying that microglia likely contribute broadly to sex discrepancies in neurological diseases and disorders.
Microglia display sex differences in the unique ranges of transcriptomic signatures and morphological phenotypes required to perform their diverse functions. In rodents, no sex differences in the number or morphology of microglia are observed at embryonic day 17 (E17) [112]. Although rodent gonadal determination is established by E13.5 [113], testosterone surges necessary for finalizing gonadal differentiation do not occur until E18-19 [114]. As early as E18.5, microglial transcriptomic signatures reveal female-biased induction of pathways involved in inflammatory response and apoptotic processes, among others [115]. Sex divergences in microglia continue to be detected immediately after birth, as postnatal day 4 (P4) male rodents have more amoeboid microglia [116, 117]. On the other hand, female microglia showed higher expression of several cytokines and chemokines, including Il10, Cxcl9, Ccl22, and Ccr4, which varied between P0 and P60 [116]. A study by Lenz et al. [117] showed that neonatal (P0) microglia play critical roles in sexual differentiation and organization of the brain architecture. Importantly, a neonatal testosterone surge at P0 is required for the masculinization of the brain and the development of male-typical sexual behaviors [118]. This highlights the initial role of gonadal hormones in establishing sex differences in the brain through microglial-mediated mechanisms early in life. However, it is believed that hormonal contributions continue later in life with changes in the circulating gonadal hormone levels contributing to microglia diversity in healthy aging.
Microglia show different regional distributions by sex in the rodent brain as well that varies with aging [107, 116, 117]. Differences in phagocytic capacity [6] and receptor expression [119] have also been noted between the sexes. Transcriptomic sequencing of sorted microglia from 3-month-old mouse brains identified several differentially expressed genes between male and female microglia [7]. The differentially expressed genes showed an inflammatory phenotype in male microglia. In the same study, female microglia transplanted into the male brain retained their sex identity and female microglia were protective against ischemic injury [7]. These results suggest that some permanent organizational effects of hormones may contribute to sex differences seen in microglial phenotypes.
Still, other transcriptomic analyses of microglial sex effects highlight that brain region, disease state, and age interact with sex to generate diverse sex effects across the lifespan [120–123]. For example, female-biased induction of microglial-mediated neuroinflammatory pathways occurs only in the old mouse hippocampus and not the young or adult [4, 5]. In a mouse model of AD, female microglia proceed more quickly to the activated response microglia (ARM) phenotype, with overexpression of MHC II and enrichment in AD risk genes [35]. Of note, sex effects were not examined in many of the recent studies that identified diverse microglial phenotypes (Table 1). An examination of microglial heterogeneity across the lifespan did not identify sex differences in microglial diversity or distributions of cells in each subpopulation at E14.5, P4/5, or P100. There were minor differences observed in a small sub-cluster that was present only at P4/5 [99]. However, this study did not interrogate microglial sex effects in the context of specific brain regions, with aging, or in a disease model.
Sex-specific differences in microglial function need to be elucidated across aging, brain region, and disease state. In addition, the regulation of microglial sex effects by sex chromosomes and/or gonadal hormones will assist in identifying new targets for sex-specific intervention.
3. Potential mechanistic regulators of sex effects in neuroimmunity
3.1. Interaction between sex hormones and the brain across development
Sex steroids are mainly produced in the gonads following stimulation by the gonadotropins follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which are secreted by the anterior pituitary in response to gonadotropin-releasing hormone (GnRH) pulses [124]. In the ovaries, 17β-estradiol (E2) and progesterone (P) are the primary sex steroids produced [125], whereas the testes primarily produce testosterone (T) [126]. The neuroendocrine system connecting the CNS and gonads is called the hypothalamic-pituitary-gonadal (HPG) axis [127], which exerts control over the timing of the sex steroid production as well as sex differentiation of the brain. GnRH pulsatility initially occurs during fetal development, as FSH and LH are crucial to neuronal and gonadal development [128, 129]. At this point, differences are noticed in FSH and LH concentrations according to the sex of the fetus [130].
In a seminal paper, Phoenix et al. [118] described the organizational-activational theory of sexual differentiation in which hormones have an organizational effect on neural tissue during early development, modifying circuitry that mediates mating behaviors. During adulthood, once the organizational framework is established, activational effects transiently occur in the presence of hormones. Sex differentiation begins with the development of male or female gonads and continues to the development of male or female genitalia and sexualization of various tissues (including the brain).
Following gonadal development, hormonal secretions influence the organism’s sexual phenotype. Organizational effects of hormonal secretions cause irreversible sex differentiation while activational effects are temporary and can occur at any stage of life [131]. There are two critical periods during life in which the organizational framework is adjusted by hormones: 1) fetal development and 2) puberty [132]. The hormonal changes associated with pregnancy and menopause are often classified as activational effects, however, there are impacts of hormones that likely impart irreversible changes across body systems. A summary of important hormonal transitionary periods in both females and males is summarized in Figure 2.
During fetal development, in addition to guiding the development of sex-specific tissue (including genitalia and breast tissue), gonadal hormones (especially androgens produced by the testes) play important roles in establishing sex differences in the developing brain [133]. In males, testes development triggers the production of T and Anti-Müllerian hormone (AMH), which encourages the development of the Wolffian duct and male external genitalia. In females, the ovaries also secrete sex hormones but not until puberty [133]. In humans, activation of the HPG axis for a short period after birth is also important for further gonadal development. Hence, pubertal hormone levels are observed in babies up to six months after birth, in what is called minipuberty [134]. After this period, however, HPG activity decreases and remains relatively quiescent until puberty. At puberty, the pulsatile release of GnRH and the secretion of gonadotropins in the pituitary cells are restored. After the onset of puberty, final sexual maturation is achieved (reviewed by [135]).
Female puberty marks the beginning of estrous (rodent) or menstrual (human) cycling, which regulates ovulation and fertility through intricately timed hormonal release under control of the ovarian follicle and HPG-axis [136]. The rodent estrous cycle can be divided in four phases (diestrous, proestrous, estrous and metestrous) [137], whereas the menstrual cycle is two phases (follicular, luteal) [138]. Each phase of the estrous/menstrual cycle is distinguished by particular levels of hormones released from the hypothalamus (GnRH), pituitary (FSH, LH), and ovaries (E2, P) [139]. Therefore, the brain (especially the hypothalamus and pituitary) must be responsive to ovarian hormones to provide feedback regulation within the HPG axis. Behavioral changes according to estrous cycle have been repeatedly reported (reviewed by [140]), as well as different responses to stress (reviewed by [141]). Rodent studies have shown that estrous-cycling induces changes in the brain transcriptome in a region-dependent fashion with the highest number of changes occurring in the hippocampus [142] and that changes in the transcriptome occur simultaneously with changes in chromatin state [143]. Changes in the brain with estrous or menstrual cycle are examples of activational effects as they are transient and reversible changes.
Pregnancy is another female-specific hormonal transition that requires the strict regulation of human chorionic gonadotropin (hCG), P, E2, and other protein hormones (i.e., prolactin) [144]. In the brain, hCG decreases spatial memory and alters Aβ levels in rodents [145] and prolactin provides neuroprotective effects through neuroinflammatory modulation [146]. Ovarian steroid hormones (P, E2) are lipophilic and can readily pass through the BBB and alter gene programming [147]. Early studies in rodents showed that E2-concentrating neurons were localized to the hypothalamus [148–150] and that actions of E2 were exerted primarily through estrogen receptors alpha or beta (ERα/ERβ) [151]. ERα and ERβ have different localization in the rodent brain, with ERα being the predominant estrogen receptor of the hippocampus, hypothalamus, and pre-optic area (POA) and ERβ found primarily in the olfactory bulb, cerebral cortex, and amygdala (among other regions) [152]. In addition to regulating ovulation, estrogens exert profound effects on memory, mood, mental state, and neurodegeneration, as further discussed in Section 3.2 [153–159]. Similarly, progesterone exerts non-reproductive functions on the brain, including neuroprotection, neuroplasticity, and mood [160, 161]. Steroid levels have also been associated with maintenance of healthspan in animal models and humans. E2 is known to exert neuroprotective effects (reviewed by [162–164]) and metabolic modulations [165, 166].
With advancing age, sex steroid levels decline, especially in females. As the ovarian follicular reserve depletes, there is a decline in E2 production that results in the cessation of cycling and ultimately results in the initiation of menopause [167, 168]. Lasting several years, the peri-menopausal transitionary period is characterized by irregular cyclicity and dysregulated hormone production [169]. Menopause is associated with an increased risk for chronic, age-related diseases, including heart disease, osteoporosis, and neurodegeneration [157, 170–174]. Cognitive decline is also observed with E2 deficiency, and post-menopausal women have an increased risk of dementia [175, 176]. Hormonal replacement therapies have been prescribed to women to decrease the effects of the lack of endogenous E2 production. Early evidence supported the use of these therapies [177, 178] although some more recent studies show less marked effects [171, 179]. In addition, potential adverse outcomes, such as breast and uterine cancers [180], have diminished enthusiasm for hormone replacement therapy (HRT) treatment approaches. Recent results from the European Prevention of Alzheimer’s Disease (EPAD) cohort indicate that HRT was associated with improved memory and larger entorhinal and amygdala volumes, but only in APOE4 carriers [181]. These results suggest that understanding the interaction between genetic and hormonal factors is essential to treating neurodegenerative diseases and supports the use of precision medicine.
Early studies focused on the role of sex hormones (principally T) in developing sex-specific behaviors related to mating. Studies in rodents showed that early-life ovariectomy followed by pubertal T treatment could induce male-typical sex behavior concurrent with an increase in the size of the sexually dimorphic nucleus of the POA [182]. The POA has important roles in guiding male copulatory behavior and controls releases of gonadotropin from the anterior pituitary [183, 184]. Interestingly, T is converted to E2 by aromatase in the brain and E2 is essential for the masculinization of the brain [185]. Microglia play a vital role in the masculinization of the male brain. For example, microglial inhibition during sex differentiation prevents: 1) sex differences in microglia, 2) male-specific dendritic spine density, and 3) adult copulatory behavior [186]. A recent study indicated that feminization of the POA is not simply a default, but requires active suppression of masculinization via DNA methylation [187].
In males, T is associated with protective effects against frailty and cognitive and metabolic declines (reviewed by [188]). In males, declines in steroid levels associated with aging are less abrupt and occur later in life which garners less attention to the matter. However, men are known to undergo andropause, which is characterized by hypogonadism and a decline in T levels around the age of 70 [189]. At this point, erectile dysfunction, diminished libido, and muscle/bone mass loss are observed (reviewed by [190, 191]). The decreases in T levels during andropause are also associated with the cognitive declines observed at this age [192, 193].
3.2. Impact of gonadal sex hormones on microglial-mediated neuroimmunity
It is clear that gonadal sex hormones (i.e., E2, P, T) cross the BBB to interact with cells in the parenchyma and mediate sex-specific actions. However, due to the difficulty in controlling sex hormones experimentally (especially in the case of cycling females), sex effects often go unexplored or are masked by high levels of variability.
Microglia express sex steroid hormone receptors, primarily ERα [194], ERβ [195], and progesterone receptors (PR) [196, 197]. Since sex hormone receptors are distributed throughout the brain in a region-dependent manner [152], it is likely that microglia sex hormone receptors differ by region and are altered during critical hormonal transitions. Although androgen receptors (AR) are not expressed by microglia under basal conditions, conversion of T to E2 by aromatase in the brain [198, 199] allows T to potentiate direct effects on microglia through ERα.
Actions of E2 through estrogen receptors (ERs) exert anti-inflammatory actions on microglia in response to acute and chronic brain injury (as reviewed [162, 200]). Of note, in vitro treatment with E2 inhibits inflammation in BV-2 microglia in response to hypoxia [201] and Aβ [202]. In rodent models, E2 attenuates microglial reactivity in response to the following stimuli: global ischemia [203], penetrating brain injury [204], and intracerebroventricular (icv) injection of lipopolysaccharide (LPS) [205]. Additionally, icv infusion of Aβ induced greater memory impairment, amyloidogenesis, and microglial reactivity in ovariectomized (OVX) mice when compared to sham control [202]. Together these studies suggest that E2 is acting directly on microglia to mediate differential responses to neuroinflammatory stimuli.
Progesterone also exerts anti-inflammatory actions on microglia. For example, in vitro treatment of BV-2 microglial cells with progesterone decreased LPS-induced inflammation [196]. Additionally, progesterone therapy led to decreased microglial reactivity in rodent models of cuprizone-induced demyelination [206] and intracerebral hemorrhage [207]. Similarly, T has been shown to decrease reactive microgliosis in rats following stab wound injury to the brain in orchiectomized (OCX) rats [208]. However, whether T is acting directly through AR or through ERs following aromatase conversion to E2 is unknown.
In summary, gonadal sex steroids mediate primarily anti-inflammatory actions on microglia. As such, critical periods of hormonal transition in both males and females (Figure 3) likely impact microglial phenotypes. Of particular interest in the aging field is how the decline of sex steroid hormones associated with andropause and menopause influences the sex-biased development of age-related brain diseases.
Figure 3. Critical periods of sex hormonal transition during the lifespan.
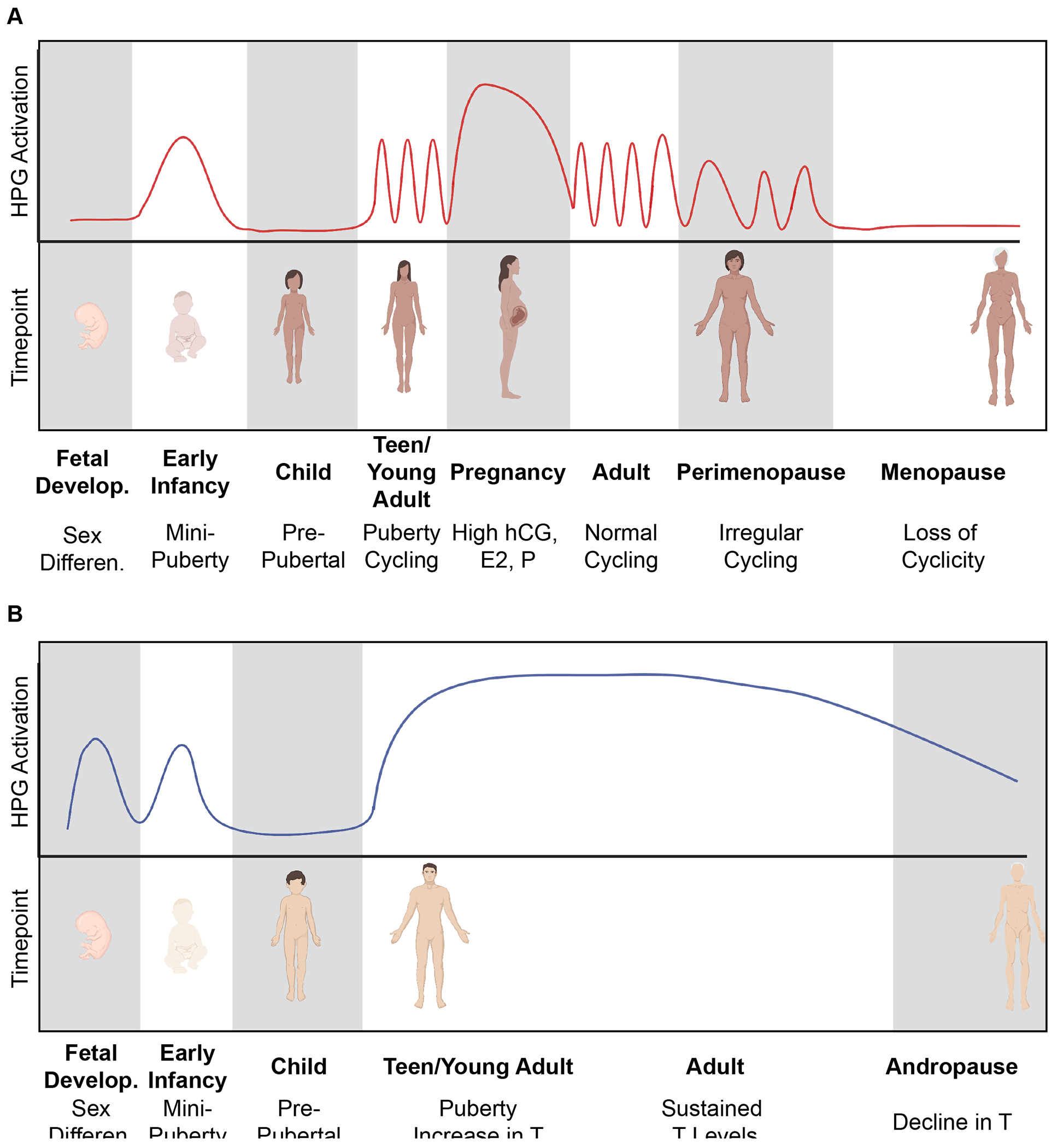
A) Females undergo many important hormonal transitions throughout the lifespan. During fetal development, the absence of male sex hormones causes sex differentiation of female secondary sex characteristics. During early infancy, a “minipuberty” period solidifies the HPG axis and further develops the ovaries and female genitalia. The pre-pubertal window of childhood has low HPG axis activity until the onset of puberty. Female puberty begins the menstrual cycle and hormone levels within the HPG axis are constantly changing. Pregnancy causes cycling to pause and hormones (hCG, E2, P, protein hormones) stay elevated to maintain the healthy pregnancy. Parturition is associated with a sharp decline in pregnancy hormones and a return to normal cyclicity. During peri-menopause, cyclicity becomes irregular as the ovarian follicular reserve is depleted. In the absence of follicles, ovarian hormones (E2, P) are not produced and cycling ceases completely to enter the menopausal period (~50 years old). B) In males, sex differentiation is determined by the production of T and AMH during fetal development. Males also undergo a “minipuberty” during early infancy with activation of the HPG axis which causes the continued development of the testes and external male genitalia. During pre-pubertal childhood, there is relative quiescence of the HPG axis until the onset of puberty. HPG axis activation during puberty results in the increase in T under the control of LH. In addition, FSH triggers the production of sperm by Sertoli cells. T levels remain relatively steady across the lifespan (with small decreases with aging), until ~70 years old when T declines during andropause. Created with Biorender.com.
3.3. Regulation of neuroimmune sex effects by sex chromosomes
As previously mentioned, the contributors to sex effects are: (1) activational effects of sex hormones, (2) organizational effects of sex hormones, and (3) sex chromosome effects [209]. In the previous sections, we discussed the impact of sex hormones on sex effects in microglia without considering the distinct impact of sex chromosomes.
In mammals, the first impact of sex chromosomes occurs during sex determination and expression of Sry. Generally, in mammals, XX genotypes develop into females and XY genotypes develop into males. Since gonadal sex is initially determined by the sex chromosomes, it is difficult to separate the contributions of sex chromosomes and gonadal sex hormones in establishing phenotypic sex effects. As such, there is an incomplete understanding of the role that sex chromosomes play in regulating sex effects. In fact, sex chromosomes are often removed from genome-wide association studies (GWAS) [210], even when specifically interrogating sex effects [211].
However, observations from naturally occurring sex chromosome aneuploidies in humans indicate that sex chromosomes play an important role in neural development and immune functions. For example, Turner syndrome (XO), Triple X Syndrome (XXX), and Klinefelter syndrome (XXY) display diverse neurological symptoms and immune alterations [212–214]. Females with Turner syndrome, or X-chromosome monosomy, are at a greater risk for autoimmune diseases and neurodevelopment disorders (i.e., autism, ADHD) [212]. Females with Triple X syndrome, or Trisomy X, have widely variable symptoms that can include delayed speech and language skills, learning disabilities, increased prevalence of ADHD/autism, and increased anxiety and depression [215]. Males with Klinefelter syndrome have higher rates of anxiety/depression, ADHD, and autism, as well as developing autoimmune diseases at higher rates more similar to XX females [216]. In a human condition called androgen insensitivity syndrome, an XY genotypic male is unable to respond to androgens and although testes develop, they never descend and secondary female external sex characteristics develop without uterine development [217].
Sex chromosomes contribute to sex effects in the brain through a variety of mechanisms (Figure 1C). The difference in X-chromosome dosage between males (1X) and females (2X) leads to sex effects through: 1) escape from X-chromosome inactivation (XCI) [218], 2) increasing bias in paternal vs maternal X-inactivation [219], or 3) X-chromosome aneuploidy [220]. In terms of the Y-chromosome, sex effects are generated by: 1) sex determination by Sry [48], 2) male-only expression of Y-chromosome encoded genes [221], or 3) mosaic loss of Y [222]. In addition, there are X-chromosomal genes (Ddx3x, Eif2s3x, Utx, Kdm5c) that have Y-chromosomal homologs (Ddx3y, Eif2s3y, Uty, Kdm5d, respectively) [223, 224]. DDX3X syndrome is a female-biased neurodevelopmental disorder caused by a mutation in the DEAD-box helicase 3 X-linked (Ddx3x) gene [225, 226]. X-chromosome encoded histone lysine demethylase 6A (Utx/Kdm6a) was associated with neuronal resilience to AD pathology in mice [227]. As such, sex chromosomally regulated effects may be partially driven by epigenomic modification of histones.
The human Y-chromosome is relatively gene poor, containing 568 genes (71 protein-coding) [221]. As previously mentioned, sex determination occurs depending on Sry expression. Sry expression in midbrain dopamine neurons plays a role in catecholamine synthesis and metabolism in the male brain [228] and is upregulated in nigral dopamine neurons in animal models of Parkinson’s disease [229]. These findings suggest that Sry expression contributes to sex effects beyond sex determination and differentiation. The Y-chromosome houses several genes expressed specifically in the testes that are important in germ cell development and male fertility. In contrast, some genes on the Y-chromosome are more widely expressed between tissues and have X-homologs that escape XCI [230]. Whereas autosomal chromosomes are homologous to their pair along the entire chromosome, the X- and Y-chromosomes share only short homologous segments called pseudo-autosomal regions (PAR) at the distal ends of the short and long arms [231]. Mosaic loss of Y (LOY) is a sex chromosome aneuploidy acquired with aging in which the Y-chromosome is completely absent from some cells [232]. Mosaic LOY has been linked to increases in many age-related diseases, including cancer [233–235], AD [236], autoimmune diseases [237], schizophrenia [238], and age-related macular degeneration [239]. A single-cell study of the aged human brain noted that LOY was enriched in microglia compared to other cell types, increased with aging, and was more likely in cases of AD [222]. In addition, microglial LOY was associated with differential expression of ~200 genes, spread across the autosomes, X-chromosome, and the PAR. Together, these studies suggest that the Y-chromosome may play important roles in modulating microglial-specific sex effects, outside of sex steroid hormones. Similarly, mosaic loss of the X-chromosome in females may also play a role in age-related brain disease [220].
In comparison, the X-chromosome is more gene dense than the Y-chromosome, housing between 900 and 1500 genes. However, X-chromosome encoded gene expression is complicated by the inactivation of one X-chromosome, termed XCI, to compensate for the extra dosage of X in females [240, 241]. X inactive-specific transcript (Xist) is a long non-coding RNA (lncRNA) that is almost exclusively expressed in females (with XX genotypes) and plays an important role in XCI. The “X-inactivation center (Xic)” contains Xist and a handful of other genes that initiate and maintain the heterochromatinization of the inactive X (Xi) to form the Barr body. In humans, XY males and XO females do not display XCI, whereas XX/XXX/XXXX females and XXY males do display XCI [241]. Only recently has the “counting” mechanism by which the cell can sense the number of X-chromosomes begun to be elucidated. XCI occurs in both marsupial and placental mammals, however, the cellular choice of the Xi differs. In marsupials, there is non-random imprinted inactivation of the paternal X-chromosome, whereas in placental mammals there is random inactivation of one X-chromosome on a cell-by-cell basis leading to mosaicism [242]. For the remaining text, we will be referring to placental mammals.
During the early stages of differentiation, the two X-chromosomes pair at their Xic [243], causing activation of the XCI programming. In this way, only cells with more than one X can induce XCI. After pairing of the Xic’s, Xist is expressed only from the Xi, coating the chromosome with Xist and recruiting chromatin modifiers to stably repress transcription from Xi. On the active X-chromosome (Xa), the expression of the antisense transcript of Xist (Tsix) blocks Xist expression [244]. Maintenance of XCI occurs through epigenomic mechanisms, including repressive histone modifications and hypermethylation of cytosine residues in CG contexts [245] (Figure 4). Despite the strong heterochromatic state of the Xi and tethering to the nuclear lamina [246], a subset of X-chromosome genes are consistently expressed from the Xi, leading to higher overall gene expression in females [247, 248]. Recent work has shown that the upregulation of Xist is linked with certain types of cancer in both males and females [249]. Further, escape from XCI in females has been implicated in the female bias in autoimmune diseases [218, 250] and AD [251]. Dichotomous expression of Y-chromosome encoded genes (including Sry) in males and Xist expression in females are examples of how sex dimorphisms can contribute to sex effects beyond sex determination and differentiation. Further, ectopic expression of male- or female- genes in the opposite sex or alterations in the tissue-specific patterning of sexually dimorphic genes can contribute to disease.
Figure 4. Epigenomic mechanisms of XCI.
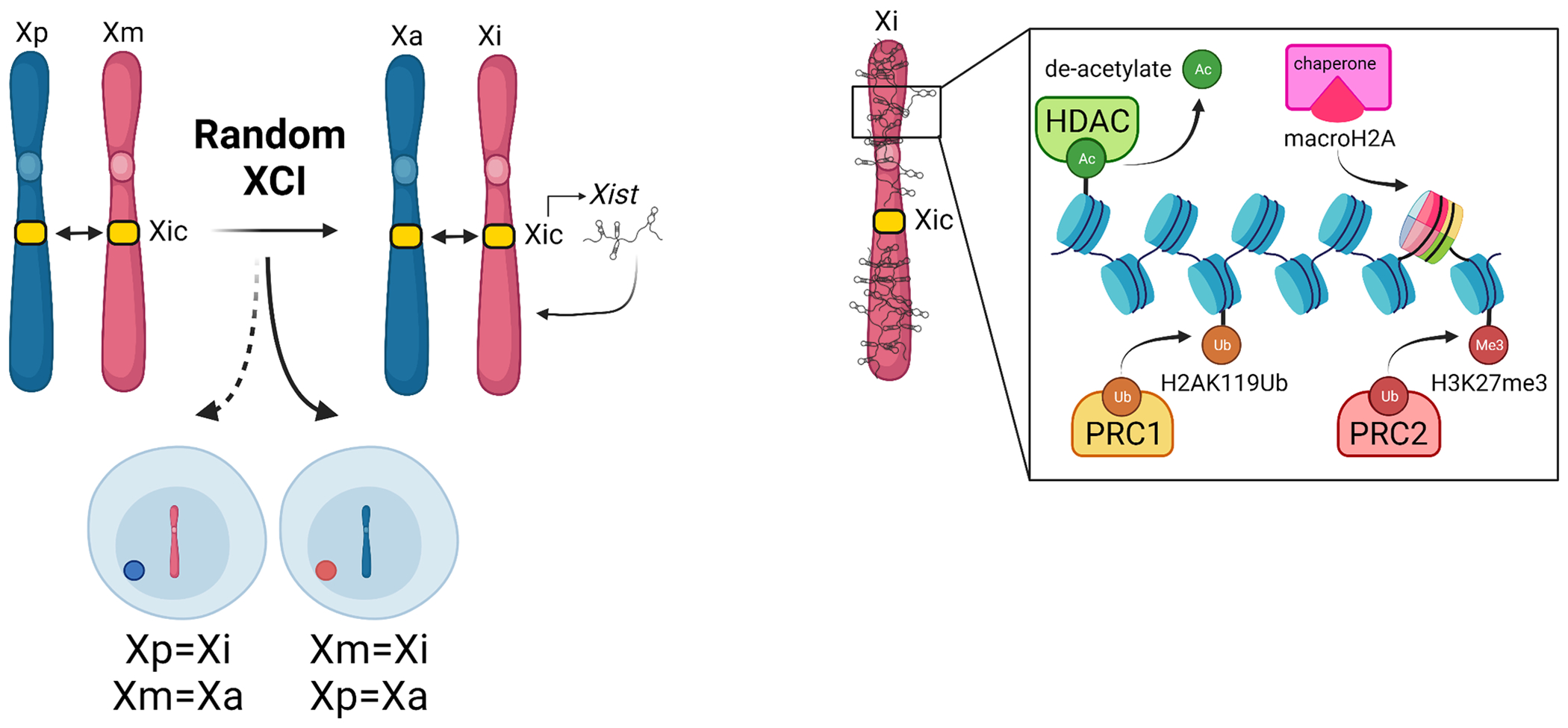
XCI starts with pairing of the X-inactivation centers (Xic), which triggers random selection of the inactive X (Xi) from either the maternal X (Xm) or paternal X (Xp). Following pairing of the Xic’s, the Xi expresses the lncRNA Xist to coat the Xi. Xist recruits chromatin modifiers to induce heterochromatization of the Xi. Created with Biorender.com.
In mammals, the X-chromosome is believed to have a greater number of immune-related genes which may account for female biases observed in autoimmune diseases such as multiple sclerosis and systemic lupus erythematosus [42, 252, 253]. Incomplete X-chromosome inactivation and subsequent escape from inactivation of some genes contribute to sex effects in microglial immune responses [108, 247]. Females are characterized by enhanced immune responses with increased resistance to infection [247, 254]. Further, sex chromosomes have been implicated in sex differences in neuroinflammatory pathways in the mouse hippocampus [223]. More studies are needed to determine the sex chromosomal mechanisms regulating sex effects in microglial-mediated immunity across the lifespan.
3.4. Gender regulation of sex effects
Often, scientists and clinicians interchangeably use the words sex and gender to refer to biological sex [255]. However, gender is a societal construct that is influenced by an individual’s perception of their sex, as well as their social and physical environments [223]. Although gender likely impacts health and disease outcomes [256], it is not possible to discern gender in non-human animal models. Additionally, the complex nature of human behavior and the variation between individuals’ experiences make it difficult to study the diverse role that gender plays in health and disease. Current studies on gender-affirming hormones work to understand the hormonal contributions to gender incongruence [257, 258]. Future human studies will need to further assess the role of gender in modulating sex effects.
4. Models of study
4.1. Models to study hormonal contributions to sex effects
Gonadectomy (GDX) -
Since the gonads are the main producers of sex steroids, surgical removal of the ovaries (OVX) or testes (OCX) can be used to better understand hormonal differences in aging and their effects on microglial reactivity. In males, gonadectomy has been used to investigate the associations of androgens with aging and metabolism [259–261]. OVX mice have been used as models of menopause, either to better understand the effects of the lack of steroid production or to test new drugs (reviewed by [262]). OCX in male mice led to decreased microglial proliferation and increased inflammation [263]. In female mice, OVX increased the number of Iba1+ microglia in the hypothalamus [8]. Thus, GDX is a well-established method for modulating sex steroid hormones to investigate mechanisms of sex effects. However, GDX also depletes non-hormonal factors produced by the gonads (i.e., microRNAs [264]) that may confound the interpretation of results. Additionally, GDX is uncommon in humans which brings into question the potential translatability of results.
Chemical OVX -
It is also possible to inactivate the steroidogenic potential of the ovaries by depleting the follicular reserve, instead of physically removing the ovaries by surgery [265]. Intraperitoneal injection of 4-vinyl cyclohexene diepoxide (VCD) [266] damages the primordial and primary ovarian follicles and causes them to go into atresia [267]. As soon as 50 days after treatment, the ovaries become completely depleted of follicles and are unable to produce E2. Since the ovaries are still present, they are able to secrete other factors which models human menopause more accurately. On the other hand, VCD-induced ovotoxicity induces pro-apoptotic signaling pathways and alters oxidative stress response pathways, which does not accurately model natural menopause [268].
Contributing to the lack of knowledge regarding the effects of menopausal hormonal changes on microglial sex effects is that rodents do not phenocopy this change. Specifically, female rodents do not undergo full estrogen depletion after reproductive senescence [269–271]. Experimental models of menopause (OVX or VCD) come close to modeling humans, but the extent of research on effects of microglia is limited. Almost completely unexplored are any effects of widely used hormonal birth control methods. Clearly a great deal more research is needed in these areas.
Hormone treatment –
Sex steroid hormones (i.e. E2, T) can be replaced after GDX by implantation of hormone-containing Silastic capsules subcutaneously, routine injections, oral gavage, or commercially available pellets [272]. Using this approach, it was shown that the replacement of E2 in aged OVX mice decreased the number of microglia in the hippocampus [273]. Since hormone levels are constantly changing (i.e., during estrous cyclicity [147]), the replacement of hormones fails to recapitulate the natural hormone environment.
Gonadal transplant –
After GDX, gonadal transplantation from one individual to another can also provide insights about the roles played by the gonads on sex divergences and aging. For instance, when ovaries from young mice were transplanted into older female mice, these old mice had increased lifespan and healthspan with reduced inflammation [274–277]. Gonadal transplant surgeries are technically challenging and require advanced training.
Hormone receptor knockouts –
Knockout of sex steroid receptors (i.e., ERs, AR, PR) in combination can be used to determine which receptors hormones are acting to generate sex-specific responses to various stimuli. For example, using ERα and ERβ global knockout (KO) mouse lines, Vegeto et al. [278] showed that ERα deficiency promoted reactive microglial phenotypes in a region-dependent manner and that the anti-inflammatory effects of E2 act through ERα in response to LPS challenge. Microglial-specific deletion of sex steroid receptors can be achieved using the Cre/lox system [279] by crossing a well-established microglial-specific Cre line [280–285] with a floxed mouse line for a sex steroid receptor [286–289]. In combination with GDX, hormone treatment can be used to elucidate the receptors necessary for specific hormone actions.
Parabiosis -
Parabiosis has been used as a model to understand the effects of circulating factors and hormones since its description by Paul Bert in the 19th century [290]. The surgical joining of two organisms such that they develop a shared circulation is useful in studying factors, whether physiological or pathological, that produce system-wide effects. Alternatively, virtual parabiosis or plasmapheresis also presents a more feasible parabiosis approach and poses limited practical challenges as compared to traditional parabiosis. The transfer of plasma from one animal to another has been gaining attention lately and provides similar benefits experimentally to the surgical parabiosis approach [291, 292]. The transfer of plasma from female animals to male animals or vice versa could provide insights into the holistic effects of sex-specific circulating factors and how they influence sex differentiation and specific effects on the neuroimmune system with aging. It is important to note that varying levels of hormonal and non-hormonal circulating factors may confound the interpretation of the results. More targeted approaches will be needed to determine the plasma constituents responsible for any therapeutic effect observed.
4.2. Models to study sex chromosomal contributions to sex effects
Four Core Genotypes mouse model -
The Four Core Genotypes mouse model (FCG) is a tractable model to study the distinct contributions of sex chromosomes and sex hormones to phenotypic sex effects. Generation of the FCG was achieved through two genetic components. First, a spontaneous deletion of the testes-determining Sry gene (Y-Sry) [293] resulted in an XY genetic male with ovaries (XYF). Insertion of the Sry transgene onto an autosome (A) of the Y-Sry mouse (XY-SryASry) resulted in a genetically male (XY) mouse with testes (FCG-XYM) in which the chromosomal and gonadal determination of male sex was uncoupled. Breeding the FCG-XYM with a wild-type XXF (WT-XXF) thus generates the “four core genotypes”: XX and XY-Sry mice with ovaries (XXF/XYF) and XXASry and XY-SryASry mice with testes (XXM/XYM). Thus, the use of the FCG allows for a two-way design to study the separate effects of sex chromosomes and sex hormones on phenotypes, as well as potential interactive effects. Since the generation of the FCG-XYM requires two genetic manipulations, a comparison of findings in the FCG-XYM to WT-XYM would serve as an important control to ensure that phenotypic sex effects are not a result of the transgenics.
Mouse models of sex chromosome aneuploidies –
Arnold reviewed mouse models that can be used to evaluate sex chromosomal effects in non-gonadal tissues [294] and how sex chromosomes and hormones impact autoimmunity, behavior, and the brain [295]. The XO mouse model of Turner Syndrome [296] can be compared to wild-type XX females to determine the effects of X-chromosome dosage on sex effects using standard t-tests. Crossing dams with XXY-Sry with XYASry males produce litters with six male genotypes: XY, XYY-Sry, XXASry, XXY-Sry, XYASry, and XYY-SryASry [297] allowing for the interrogation of sex chromosome number on sex-specific phenotypes using a two-way design (number of X chromosomes, number of Y chromosomes). The XY* mouse model has a functional centromere on the Y-chromosome that allows for recombination of the X and Y*, resulting in XY*X (XO), XX, XY* (XY), and XXY* (XXY) mice to interrogate X-chromosome dosage while controlling for gonadal sex in a manner similar to the FCG [295, 298]. Of note, complete absence of an X-chromosome is not described in naturally occurring sex chromosome aneuploidies or mouse models, signifying that the X-chromosome is essential for organismal survival.
Direct genetic manipulation of individual X or Y genes –
Instead of manipulating the whole sex chromosome, individual X or Y genes can be manipulated using global knockouts, cell type-specific knockouts or overexpression in vitro or in vivo.
Mouse models to study XCI –
Mating C57BL/6 mice (M. musculus) with a non-functional copy of Xist to M. spretus males leads to skewed XCI in which the paternal X-chromosome is inactivated in all cells. In transcriptomic or epigenomic studies, determining if the sequence came from Xi or XA is based on single nucleotide polymorphisms (SNPs) comparison of the musculus and spretus genomes [248, 299]. This allows for concrete determination of genes that escape XCI and epigenomic patterns associated with these trends. One limitation of this model is that it relies on detecting SNPs known to vary between M. musculus and M. spretus, which may not be found in the gene of interest. However, the variation rate between M. spretus and M. musculus is ~1 in 50 base pairs [300], which makes the chance of getting at least one SNP in each gene very likely. Mice with floxed GFP and tdTomato reporters in the X-linked Hrpt locus were generated such that a fluorescent signal can be used to distinguish which parental X-chromosome had undergone XCI [301]. This method can be used to study XCI mosaicism or skewing, as well as for sorting cells to compare phenotypes depending on XCI parent-of-origin.
The models and methods presented in this section may also be combined to generate information that is too complex for a single model to explain. When combining approaches, machine learning models may be necessary to rank the importance of each factor in generating the observed phenotype.
5. Future Directions and Conclusions
While sex effects are primarily thought of in terms of reproduction, both genetic and gonadal sex can cause widespread sex effects across body systems. Sex effects in the brain not only include developmental differentiation of brain regions that involve sex-specific behaviors [46, 187, 302], but also occur in brain regions not canonically associated with sex differentiation (such as the hippocampus) that are also responsive to hormonal changes across the lifespan [4, 143, 250, 303]. Sex effects established during development and those that change with aging can contribute to the sex differences in the prevalence and pathogenesis of neurodevelopmental diseases [303–307] and neurodegenerative diseases [36, 38, 159, 308, 309].
Previous studies from our group have demonstrated that sex effects in the mouse hippocampus are primarily involved in microglial reactivity [4, 5] and that differences in neuroinflammatory signaling are regulated by both gonadal and hormonal sex [223]. Microglia react to aging and brain pathology through the upregulation of pro-inflammatory cytokines and chemokines, changes in phagocytosis and lysosomal processing, and transition to disease-associated phenotypic states [13, 23, 26, 310, 311]. However, the basic mechanisms governing the sexually divergent microglial response (whether hormonal or sex chromosomal) to brain aging and disease have not been fully elucidated, in part due to the difficulty in controlling for the hormonal milieu across the lifespan as well as separating effects of sex chromosomes.
Estrogens have been shown to exert anti-inflammatory effects on microglia [157, 200, 273, 278, 312]. One interesting future line of research includes microglial-specific modulation of estrogen signaling. Microglia express Esr1, the gene coding for ERα, at a higher level than other cell types in the mouse brain [313]. Since gonadal sex contributes to female-biased neuroinflammatory signaling pathways in the hippocampus, it stands to reason that signaling through microglial ERα may contribute to sex effects. E2 is of particular interest since it is the most potent estrogen and the prominent form present in pre-menopausal women [314].
In addition to hormonally-mediated sex effects, escape from XCI with aging is another potential mechanism of sex divergence in microglial reactivity. The brain is unique in that many cells, especially neurons, are post-mitotic and long-lived. Although rodent microglia are able to proliferate and repopulate [315, 316], in the absence of infection, human microglia have a median turnover rate of ~28% per year and can survive for >20 years [317]. Chronic, low-grade, sterile inflammation in the aging brain may cause the re-activation of silenced genes on the Xi skewing inflammatory pathways with aging in long-lived cells. For example, Tlr7 (an X-encoded gene) acts upstream of IRF7, a transcription factor in the type-I interferon response pathway [318]. In human plasma dendritic cells, Tlr7 escape from XCI leading to sex differences in type-I interferon response [319]. It stands to reason that aging or brain disease could lead to sex divergence of Tlr7 and alteration of interferon-response pathways.
In summary, microglia are a promising target for future modulation of sex-specific inflammatory signaling. Identifying the upstream regulatory mechanisms (whether hormonal, chromosomal, or their interaction) will guide the development of sex-specific interventions that may aid in the treatment of age-related brain diseases.
Acknowledgments:
Research reported in this publication was supported by the Office Of The Director, National Institutes Of Health under Award Number DP5OD033443. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by grants from the National Institutes of Health (NIH) P30AG050911, R01AG059430, T32AG052363, F31AG064861, F99AG079813, and BrightFocus Foundation (M2020207). This work was also supported in part by awards I01BX003906 and 1IK6BX006033 from the United States (U.S.) Department of Veterans Affairs, Biomedical Laboratory Research and Development Service. The authors would like to acknowledge Kevin Pham for reviewing and editing the work.
Footnotes
Conflict of Interest: Authors report no conflict of interest.
References
- 1.Kraus A, Buckley KM, and Salinas I, Sensing the world and its dangers: An evolutionary perspective in neuroimmunology. eLife, 2021. 10: p. e66706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein SL and Flanagan KL, Sex differences in immune responses. Nature Reviews Immunology, 2016. 16(10): p. 626–638. [DOI] [PubMed] [Google Scholar]
- 3.Osborne BF, Turano A, and Schwarz JM, Sex Differences in the Neuroimmune System. Curr Opin Behav Sci, 2018. 23: p. 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mangold CA, et al. , CNS-wide Sexually Dimorphic Induction of the Major Histocompatibility Complex 1 Pathway With Aging. J Gerontol A Biol Sci Med Sci, 2017. 72(1): p. 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangold CA, et al. , Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation, 2017. 14(1): p. 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanguas-Casás N, et al. , Aging and sex: Impact on microglia phagocytosis. Aging Cell, 2020. 19(8): p. e13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villa A, et al. , Sex-Specific Features of Microglia from Adult Mice. Cell Rep, 2018. 23(12): p. 3501–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debarba LK, et al. , 17-α-Estradiol Has Sex-Specific Effects on Neuroinflammation That Are Partly Reversed by Gonadectomy. J Gerontol A Biol Sci Med Sci, 2022. 77(1): p. 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alliot F, Godin I, and Pessac B, Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Developmental Brain Research, 1999. 117(2): p. 145–152. [DOI] [PubMed] [Google Scholar]
- 10.Aguzzi A, Barres BA, and Bennett ML, Microglia: Scapegoat, Saboteur, or Something Else? Science, 2013. 339(6116): p. 156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z and Trapp BD, Microglia and neuroprotection. Journal of Neurochemistry, 2016. 136(S1): p. 10–17. [DOI] [PubMed] [Google Scholar]
- 12.Parkhurst CN, et al. , Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell, 2013. 155(7): p. 1596–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casali BT and Reed-Geaghan EG, Microglial Function and Regulation during Development, Homeostasis and Alzheimer’s Disease. Cells, 2021. 10(4): p. 957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filipello F, et al. , The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity, 2018. 48(5): p. 979–991.e8. [DOI] [PubMed] [Google Scholar]
- 15.VanRyzin JW, et al. , Microglia and sexual differentiation of the developing brain: A focus on extrinsic factors. Glia, 2020. 68(6): p. 1100–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niraula A, Sheridan JF, and Godbout JP, Microglia Priming with Aging and Stress. Neuropsychopharmacology, 2017. 42(1): p. 318–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mosher KI and Wyss-Coray T, Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem Pharmacol, 2014. 88(4): p. 594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haruwaka K, et al. , Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun, 2019. 10(1): p. 5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon HS and Koh S-H, Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Translational Neurodegeneration, 2020. 9(1): p. 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen GY and Nuñez G, Sterile inflammation: sensing and reacting to damage. Nature Reviews Immunology, 2010. 10(12): p. 826–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kigerl KA, et al. , Pattern recognition receptors and central nervous system repair. Exp Neurol, 2014. 258: p. 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Streit WJ, et al. , Dystrophic microglia in the aging human brain. Glia, 2004. 45(2): p. 208–212. [DOI] [PubMed] [Google Scholar]
- 23.Von Bernhardi R, Eugenin-von Bernhardi L, and Eugenin J, Microglial cell dysregulation in Brain Aging and Neurodegeneration. Frontiers in Aging Neuroscience, 2015. 7(124). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paolicelli R, et al. , Defining Microglial States and Nomenclature: A Roadmap to 2030. SSRN Electronic Journal, 2022. [Google Scholar]
- 25.Guzman-Martinez L, et al. , Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Frontiers in Pharmacology, 2019. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heneka MT, et al. , Neuroinflammation in Alzheimer’s disease. The Lancet. Neurology, 2015. 14(4): p. 388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivasan K, et al. , Alzheimer’s Patient Microglia Exhibit Enhanced Aging and Unique Transcriptional Activation. Cell Rep, 2020. 31(13): p. 107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efthymiou AG and Goate AM, Late onset Alzheimer’s disease genetics implicates microglial pathways in disease risk. Molecular Neurodegeneration, 2017. 12(1): p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sobue A, et al. , Microglial gene signature reveals loss of homeostatic microglia associated with neurodegeneration of Alzheimer’s disease. Acta Neuropathologica Communications, 2021. 9(1): p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch EC and Standaert DG, Ten Unsolved Questions About Neuroinflammation in Parkinson’s Disease. Mov Disord, 2021. 36(1): p. 16–24. [DOI] [PubMed] [Google Scholar]
- 31.Hamza TH, et al. , Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet, 2010. 42(9): p. 781–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noelker C, et al. , Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep, 2013. 3: p. 1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grigoriadis N, et al. , Neuroinflammation in multiple sclerosis: evidence for autoimmune dysregulation, not simple autoimmune reaction. Clin Neurol Neurosurg, 2006. 108(3): p. 241–4. [DOI] [PubMed] [Google Scholar]
- 34.Irvine K, et al. , Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. J Clin Exp Neuropsychol, 2012. 34(9): p. 989–98. [DOI] [PubMed] [Google Scholar]
- 35.Sala Frigerio C, et al. , The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Aβ Plaques. Cell Rep, 2019. 27(4): p. 1293–1306.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferretti MT, et al. , Sex differences in Alzheimer disease - the gateway to precision medicine. Nat Rev Neurol, 2018. 14(8): p. 457–469. [DOI] [PubMed] [Google Scholar]
- 37.Burke SL, et al. , Sex differences in the development of mild cognitive impairment and probable Alzheimer’s disease as predicted by hippocampal volume or white matter hyperintensities. J Women Aging, 2019. 31(2): p. 140–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dumitrescu L, et al. , Sex differences in the genetic predictors of Alzheimer’s pathology. Brain, 2019. 142(9): p. 2581–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang L, et al. , Sex-specific DNA methylation differences in Alzheimer’s disease pathology. Acta neuropathologica communications, 2021. 9(1): p. 77–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cerri S, Mus L, and Blandini F, Parkinson’s Disease in Women and Men: What’s the Difference? J Parkinsons Dis, 2019. 9(3): p. 501–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaidya B, et al. , Parkinson’s disease in women: Mechanisms underlying sex differences. Eur J Pharmacol, 2021. 895: p. 173862. [DOI] [PubMed] [Google Scholar]
- 42.Kalincik T, et al. , Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain, 2013. 136(12): p. 3609–3617. [DOI] [PubMed] [Google Scholar]
- 43.Voskuhl RR, et al. , Sex differences in brain atrophy in multiple sclerosis. Biology of Sex Differences, 2020. 11(1): p. 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lynch MA, Exploring Sex-Related Differences in Microglia May Be a Game-Changer in Precision Medicine. Front Aging Neurosci, 2022. 14: p. 868448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarthy MM, et al. , Sex differences in the brain: the not so inconvenient truth. J Neurosci, 2012. 32(7): p. 2241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hughes IA, Minireview: Sex Differentiation. Endocrinology, 2001. 142(8): p. 3281–3287. [DOI] [PubMed] [Google Scholar]
- 47.Fechner PY, The role of SRY in mammalian sex determination. Acta Paediatr Jpn, 1996. 38(4): p. 380–9. [DOI] [PubMed] [Google Scholar]
- 48.Kashimada K and Koopman P, Sry: the master switch in mammalian sex determination. Development, 2010. 137(23): p. 3921–3930. [DOI] [PubMed] [Google Scholar]
- 49.Medawar PB, Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol, 1948. 29(1): p. 58–69. [PMC free article] [PubMed] [Google Scholar]
- 50.Neuwelt EA and Clark WK, Unique aspects of central nervous system immunology. Neurosurgery, 1978. 3(3): p. 419–30. [DOI] [PubMed] [Google Scholar]
- 51.Galea I, Bechmann I, and Perry VH, What is immune privilege (not)? Trends Immunol, 2007. 28(1): p. 12–8. [DOI] [PubMed] [Google Scholar]
- 52.Ransohoff RM and Cardona AE, The myeloid cells of the central nervous system parenchyma. Nature, 2010. 468(7321): p. 253–262. [DOI] [PubMed] [Google Scholar]
- 53.Vincenti I and Merkler D, New advances in immune components mediating viral control in the CNS. Current Opinion in Virology, 2021. 47: p. 68–78. [DOI] [PubMed] [Google Scholar]
- 54.Boulanger LM, Huh GS, and Shatz CJ, Neuronal plasticity and cellular immunity: shared molecular mechanisms. Curr Opin Neurobiol, 2001. 11(5): p. 568–78. [DOI] [PubMed] [Google Scholar]
- 55.Veerhuis R, Nielsen HM, and Tenner AJ, Complement in the brain. Mol Immunol, 2011. 48(14): p. 1592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elward K and Gasque P, “Eat me” and “don’t eat me” signals govern the innate immune response and tissue repair in the CNS: emphasis on the critical role of the complement system. Mol Immunol, 2003. 40(2–4): p. 85–94. [DOI] [PubMed] [Google Scholar]
- 57.Hanke ML and Kielian T, Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin Sci (Lond), 2011. 121(9): p. 367–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radisky DC, et al. , Single proteins might have dual but related functions in intracellular and extracellular microenvironments. Nat Rev Mol Cell Biol, 2009. 10(3): p. 228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stevens B, et al. , The classical complement cascade mediates CNS synapse elimination. Cell, 2007. 131(6): p. 1164–78. [DOI] [PubMed] [Google Scholar]
- 60.Schafer DP, et al. , Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron, 2012. 74(4): p. 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glynn MW, et al. , MHCI negatively regulates synapse density during the establishment of cortical connections. Nat Neurosci, 2011. 14(4): p. 442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoshida TM, Wang A, and Hafler DA, Basic principles of neuroimmunology. Semin Immunopathol, 2022. [DOI] [PubMed] [Google Scholar]
- 63.Croese T, Castellani G, and Schwartz M, Immune cell compartmentalization for brain surveillance and protection. Nat Immunol, 2021. 22(9): p. 1083–1092. [DOI] [PubMed] [Google Scholar]
- 64.Høglund RA and Maghazachi AA, Multiple sclerosis and the role of immune cells. World J Exp Med, 2014. 4(3): p. 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abbott NJ, et al. , Structure and function of the blood-brain barrier. Neurobiol Dis, 2010. 37(1): p. 13–25. [DOI] [PubMed] [Google Scholar]
- 66.Alvarez JI, et al. , The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science, 2011. 334(6063): p. 1727–31. [DOI] [PubMed] [Google Scholar]
- 67.Wosik K, et al. , Angiotensin II controls occludin function and is required for blood brain barrier maintenance: relevance to multiple sclerosis. J Neurosci, 2007. 27(34): p. 9032–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Summers L, et al. , Activation of brain endothelial cells by interleukin-1 is regulated by the extracellular matrix after acute brain injury. Mol Cell Neurosci, 2013. 57: p. 93–103. [DOI] [PubMed] [Google Scholar]
- 69.Thurgur H and Pinteaux E, Microglia in the Neurovascular Unit: Blood-Brain Barrier-microglia Interactions After Central Nervous System Disorders. Neuroscience, 2019. 405: p. 55–67. [DOI] [PubMed] [Google Scholar]
- 70.Weber CM and Clyne AM, Sex differences in the blood-brain barrier and neurodegenerative diseases. APL Bioeng, 2021. 5(1): p. 011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morris G, et al. , Leaky brain in neurological and psychiatric disorders: Drivers and consequences. Aust N Z J Psychiatry, 2018. 52(10): p. 924–948. [DOI] [PubMed] [Google Scholar]
- 72.Obermeier B, Daneman R, and Ransohoff RM, Development, maintenance and disruption of the blood-brain barrier. Nat Med, 2013. 19(12): p. 1584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao Z, et al. , Establishment and Dysfunction of the Blood-Brain Barrier. Cell, 2015. 163(5): p. 1064–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morrison HW and Filosa JA, Stroke and the neurovascular unit: glial cells, sex differences, and hypertension. Am J Physiol Cell Physiol, 2019. 316(3): p. C325–C339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keaney J and Campbell M, The dynamic blood-brain barrier. FEBS J, 2015. 282(21): p. 4067–79. [DOI] [PubMed] [Google Scholar]
- 76.Morris G, et al. , A narrative review on the similarities and dissimilarities between myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and sickness behavior. BMC Med, 2013. 11: p. 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morris G and Maes M, Oxidative and Nitrosative Stress and Immune-Inflammatory Pathways in Patients with Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Curr Neuropharmacol, 2014. 12(2): p. 168–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meszaros A, et al. , Neurovascular Inflammaging in Health and Disease. Cells, 2020. 9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Franceschi C and Campisi J, Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. The Journals of Gerontology: Series A, 2014. 69(Suppl_1): p. S4–S9. [DOI] [PubMed] [Google Scholar]
- 80.López-Otín C, et al. , The hallmarks of aging. Cell, 2013. 153(6): p. 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liguori I, et al. , Oxidative stress, aging, and diseases. Clin Interv Aging, 2018. 13: p. 757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Godbout JP, et al. , Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb j, 2005. 19(10): p. 1329–31. [DOI] [PubMed] [Google Scholar]
- 83.Frank MG, et al. , mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging, 2006. 27(5): p. 717–22. [DOI] [PubMed] [Google Scholar]
- 84.Griffin R, et al. , The age-related attenuation in long-term potentiation is associated with microglial activation. Journal of Neurochemistry, 2006. 99(4): p. 1263–1272. [DOI] [PubMed] [Google Scholar]
- 85.Lucin KM and Wyss-Coray T, Immune activation in brain aging and neurodegeneration: too much or too little? Neuron, 2009. 64(1): p. 110–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheng C-H, et al. , Alterations in high-order diffusion imaging in veterans with Gulf War Illness is associated with chemical weapons exposure and mild traumatic brain injury. Brain, behavior, and immunity, 2020. 89: p. 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Davalos D, et al. , ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci, 2005. 8(6): p. 752–8. [DOI] [PubMed] [Google Scholar]
- 88.Manwani B, et al. , Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol, 2013. 249: p. 120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hefendehl JK, et al. , Homeostatic and injury-induced microglia behavior in the aging brain. Aging cell, 2014. 13(1): p. 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schimmel SJ, Acosta S, and Lozano D, Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain circulation, 2017. 3(3): p. 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang Y and Le W, Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol, 2016. 53(2): p. 1181–1194. [DOI] [PubMed] [Google Scholar]
- 92.DePaula-Silva AB, et al. , Differential transcriptional profiles identify microglial- and macrophage-specific gene markers expressed during virus-induced neuroinflammation. Journal of Neuroinflammation, 2019. 16(1): p. 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fabbri E, et al. , Association Between Accelerated Multimorbidity and Age-Related Cognitive Decline in Older Baltimore Longitudinal Study of Aging Participants without Dementia. J Am Geriatr Soc, 2016. 64(5): p. 965–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tegeler C, et al. , The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function--data from the Berlin Aging Study II. Neurobiol Aging, 2016. 38: p. 112–117. [DOI] [PubMed] [Google Scholar]
- 95.Hendel-Chavez H, et al. , Immunological hallmarks of JC virus replication in multiple sclerosis patients on long-term natalizumab therapy. J Virol, 2013. 87(10): p. 6055–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Borst K, Dumas AA, and Prinz M, Microglia: Immune and non-immune functions. Immunity, 2021. 54(10): p. 2194–2208. [DOI] [PubMed] [Google Scholar]
- 97.Boche D, Perry VH, and Nicoll JAR, Review: Activation patterns of microglia and their identification in the human brain. Neuropathology and Applied Neurobiology, 2013. 39(1): p. 3–18. [DOI] [PubMed] [Google Scholar]
- 98.Dubbelaar ML, et al. , The Kaleidoscope of Microglial Phenotypes. Front Immunol, 2018. 9: p. 1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hammond TR, et al. , Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity, 2019. 50(1): p. 253–271.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keren-Shaul H, et al. , A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell, 2017. 169(7): p. 1276–1290.e17. [DOI] [PubMed] [Google Scholar]
- 101.Marschallinger J, et al. , Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nature Neuroscience, 2020. 23(2): p. 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Absinta M, et al. , A lymphocyte-microglia-astrocyte axis in chronic active multiple sclerosis. Nature, 2021. 597(7878): p. 709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Q, et al. , Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron, 2019. 101(2): p. 207–223.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Safaiyan S, et al. , White matter aging drives microglial diversity. Neuron, 2021. 109(7): p. 1100–1117.e10. [DOI] [PubMed] [Google Scholar]
- 105.Gorgoulis V, et al. , Cellular Senescence: Defining a Path Forward. Cell, 2019. 179(4): p. 813–827. [DOI] [PubMed] [Google Scholar]
- 106.Ogrodnik M, et al. , Whole-body senescent cell clearance alleviates age-related brain inflammation and cognitive impairment in mice. Aging Cell, 2021. 20(2): p. e13296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han J, et al. , Uncovering sex differences of rodent microglia. Journal of neuroinflammation, 2021. 18(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kodama L and Gan L, Do microglial sex differences contribute to sex differences in neurodegenerative diseases? Trends in molecular medicine, 2019. 25(9): p. 741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Villa A, Della Torre S, and Maggi A, Sexual differentiation of microglia. Frontiers in Neuroendocrinology, 2019. 52: p. 156–164. [DOI] [PubMed] [Google Scholar]
- 110.Darling J and Sanchez K, The Role of Microglia in Brain Aging: A Focus on Sex Differences. 2020. [Google Scholar]
- 111.Yanguas-Casás N, Physiological sex differences in microglia and their relevance in neurological disorders. Neuroimmunology and Neuroinflammation, 2020. 7(1): p. 13–22. [Google Scholar]
- 112.Schwarz JM, Sholar PW, and Bilbo SD, Sex differences in microglial colonization of the developing rat brain. J Neurochem, 2012. 120(6): p. 948–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McClelland K, Bowles J, and Koopman P, Male sex determination: Insights into molecular mechanisms. Asian journal of andrology, 2011. 14: p. 164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weisz J and Ward IL, Plasma Testosterone and Progesterone Titers of Pregnant Rats, Their Male and Female Fetuses, and Neonatal Offspring*. Endocrinology, 1980. 106(1): p. 306–316. [DOI] [PubMed] [Google Scholar]
- 115.Thion MS, et al. , Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell, 2018. 172(3): p. 500–516.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schwarz JM, Sholar PW, and Bilbo SD, Sex differences in microglial colonization of the developing rat brain. Journal of neurochemistry, 2012. 120(6): p. 948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lenz KM, et al. , Microglia are essential to masculinization of brain and behavior. Journal of Neuroscience, 2013. 33(7): p. 2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Phoenix CH, et al. , Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology, 1959. 65: p. 369–82. [DOI] [PubMed] [Google Scholar]
- 119.Yanguas-Casás N, et al. , Sex differences in the phagocytic and migratory activity of microglia and their impairment by palmitic acid. Glia, 2018. 66(3): p. 522–537. [DOI] [PubMed] [Google Scholar]
- 120.Mangold CA, et al. , Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. Journal of Neuroinflammation, 2017. 14(1): p. 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guneykaya D, et al. , Transcriptional and translational differences of microglia from male and female brains. Cell reports, 2018. 24(10): p. 2773–2783. [DOI] [PubMed] [Google Scholar]
- 122.Patel T, et al. , Transcriptional landscape of human microglia implicates age, sex, and APOE-related immunometabolic pathway perturbations. Aging Cell, 2022. 21(5): p. e13606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Berchtold NC, et al. , Gene expression changes in the course of normal brain aging are sexually dimorphic. Proceedings of the National Academy of Sciences, 2008. 105(40): p. 15605–15610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wierman ME, Sex steroid effects at target tissues: mechanisms of action. Advances in Physiology Education, 2007. 31(1): p. 26–33. [DOI] [PubMed] [Google Scholar]
- 125.Drummond AE, The role of steroids in follicular growth. Reprod Biol Endocrinol, 2006. 4: p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Y, et al. , Steroidogenesis in Leydig cells: effects of aging and environmental factors. Reproduction, 2017. 154(4): p. R111–r122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Phumsatitpong C, Wagenmaker ER, and Moenter SM, Neuroendocrine interactions of the stress and reproductive axes. Frontiers in Neuroendocrinology, 2021. 63: p. 100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clements JA, et al. , Studies on human sexual development. III. Fetal pituitary and serum, and amniotic fluid concentrations of LH, CG, and FSH. J Clin Endocrinol Metab, 1976. 42(1): p. 9–19. [DOI] [PubMed] [Google Scholar]
- 129.Kaplan SL and Grumbach MM, The ontogenesis of human foetal hormones. II. Luteinizing hormone (LH) and follicle stimulating hormone (FSH). Acta Endocrinol (Copenh), 1976. 81(4): p. 808–29. [DOI] [PubMed] [Google Scholar]
- 130.Beck-Peccoz P, et al. , Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J Clin Endocrinol Metab, 1991. 73(3): p. 525–32. [DOI] [PubMed] [Google Scholar]
- 131.Eggers S and Sinclair A, Mammalian sex determination-insights from humans and mice. Chromosome Res, 2012. 20(1): p. 215–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Berenbaum SA and Beltz AM, Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Front Neuroendocrinol, 2011. 32(2): p. 183–200. [DOI] [PubMed] [Google Scholar]
- 133.Turano A, Osborne BF, and Schwarz JM, Sexual Differentiation and Sex Differences in Neural Development. Curr Top Behav Neurosci, 2019. 43: p. 69–110. [DOI] [PubMed] [Google Scholar]
- 134.Renault CH, et al. , Minipuberty of human infancy - A window of opportunity to evaluate hypogonadism and differences of sex development? Ann Pediatr Endocrinol Metab, 2020. 25(2): p. 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Spaziani M, et al. , Hypothalamo-Pituitary axis and puberty. Mol Cell Endocrinol, 2021. 520: p. 111094. [DOI] [PubMed] [Google Scholar]
- 136.Plant TM, 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-pituitary-gonadal axis. J Endocrinol, 2015. 226(2): p. T41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.McLean AC, et al. , Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp, 2012(67): p. e4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Najmabadi S, et al. , Menstrual bleeding, cycle length, and follicular and luteal phase lengths in women without known subfertility: A pooled analysis of three cohorts. Paediatric and Perinatal Epidemiology, 2020. 34(3): p. 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Butcher RL, Collins WE, and Fugo NW, Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology, 1974. 94(6): p. 1704–8. [DOI] [PubMed] [Google Scholar]
- 140.Inoue S, Neural basis for estrous cycle-dependent control of female behaviors. Neurosci Res, 2022. 176: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 141.Viau V and Meaney MJ, Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology, 1991. 129(5): p. 2503–11. [DOI] [PubMed] [Google Scholar]
- 142.DiCarlo LM, Vied C, and Nowakowski RS, The stability of the transcriptome during the estrous cycle in four regions of the mouse brain. J Comp Neurol, 2017. 525(15): p. 3360–3387. [DOI] [PubMed] [Google Scholar]
- 143.Jaric I, et al. , Chromatin organization in the female mouse brain fluctuates across the oestrous cycle. Nature Communications, 2019. 10(1): p. 2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kumar P and Magon N, Hormones in pregnancy. Niger Med J, 2012. 53(4): p. 179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Berry A, et al. , Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-beta levels in female rats. Horm Behav, 2008. 54(1): p. 143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ramos-Martinez E, et al. , The role of prolactin in central nervous system inflammation. 2021. 32(3): p. 323–340. [DOI] [PubMed] [Google Scholar]
- 147.Lovick TA, Estrous cycle and stress: influence of progesterone on the female brain. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas, 2012. 45(4): p. 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pfaff D and Keiner M, Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J Comp Neurol, 1973. 151(2): p. 121–58. [DOI] [PubMed] [Google Scholar]
- 149.Warembourg M, Radioautographic localization of estrogen-concentrating cells in the brain and pituitary of the guinea pig. Brain Res, 1977. 123(2): p. 357–62. [DOI] [PubMed] [Google Scholar]
- 150.Sar M, Estradiol is concentrated in tyrosine hydroxylase-containing neurons of the hypothalamus. Science, 1984. 223(4639): p. 938–40. [DOI] [PubMed] [Google Scholar]
- 151.Paterni I, et al. , Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids, 2014. 90: p. 13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mitra SW, et al. , Immunolocalization of Estrogen Receptor β in the Mouse Brain: Comparison with Estrogen Receptor α. Endocrinology, 2003. 144(5): p. 2055–2067. [DOI] [PubMed] [Google Scholar]
- 153.Fink G, et al. , Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol, 1996. 16(3): p. 325–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McEwen BS and Alves SE, Estrogen actions in the central nervous system. Endocr Rev, 1999. 20(3): p. 279–307. [DOI] [PubMed] [Google Scholar]
- 155.Gillies GE, et al. , Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson’s disease. Pharmacol Biochem Behav, 2004. 78(3): p. 513–22. [DOI] [PubMed] [Google Scholar]
- 156.Song YJ, et al. , The Effect of Estrogen Replacement Therapy on Alzheimer’s Disease and Parkinson’s Disease in Postmenopausal Women: A Meta-Analysis. Front Neurosci, 2020. 14: p. 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vegeto E, Benedusi V, and Maggi A, Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Front Neuroendocrinol, 2008. 29(4): p. 507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mulnard RA, et al. , Estrogen Replacement Therapy for Treatment of Mild to Moderate Alzheimer DiseaseA Randomized Controlled Trial. JAMA, 2000. 283(8): p. 1007–1015. [DOI] [PubMed] [Google Scholar]
- 159.Rahman A, et al. , Sex and Gender Driven Modifiers of Alzheimer’s: The Role for Estrogenic Control Across Age, Race, Medical, and Lifestyle Risks. Frontiers in Aging Neuroscience, 2019. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Snyder AM and Hull EM, Perinatal progesterone affects learning in rats. Psychoneuroendocrinology, 1980. 5(2): p. 113–9. [DOI] [PubMed] [Google Scholar]
- 161.Schumacher M, et al. , Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm IGF Res, 2004. 14 Suppl A: p. S18–33. [DOI] [PubMed] [Google Scholar]
- 162.Azcoitia I, Barreto GE, and Garcia-Segura LM, Molecular mechanisms and cellular events involved in the neuroprotective actions of estradiol. Analysis of sex differences. Front Neuroendocrinol, 2019. 55: p. 100787. [DOI] [PubMed] [Google Scholar]
- 163.Giatti S, et al. , Neuroactive steroids, neurosteroidogenesis and sex. Prog Neurobiol, 2019. 176: p. 1–17. [DOI] [PubMed] [Google Scholar]
- 164.Picazo O, Azcoitia I, and Garcia-Segura LM, Neuroprotective and neurotoxic effects of estrogens. Brain Res, 2003. 990(1–2): p. 20–7. [DOI] [PubMed] [Google Scholar]
- 165.Green JS, et al. , The effects of exercise training on abdominal visceral fat, body composition, and indicators of the metabolic syndrome in postmenopausal women with and without estrogen replacement therapy: the HERITAGE family study. Metabolism, 2004. 53(9): p. 1192–6. [DOI] [PubMed] [Google Scholar]
- 166.Musatov S, et al. , Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci U S A, 2007. 104(7): p. 2501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Broekmans FJ, Soules MR, and Fauser BC, Ovarian aging: mechanisms and clinical consequences. Endocr Rev, 2009. 30(5): p. 465–93. [DOI] [PubMed] [Google Scholar]
- 168.Shifren JL, Gass ML, and N.R.f.C.C.o.M.W.W. Group, The North American Menopause Society recommendations for clinical care of midlife women. Menopause, 2014. 21(10): p. 1038–62. [DOI] [PubMed] [Google Scholar]
- 169.Allshouse A, Pavlovic J, and Santoro N, Menstrual Cycle Hormone Changes Associated with Reproductive Aging and How They May Relate to Symptoms. Obstet Gynecol Clin North Am, 2018. 45(4): p. 613–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sowers M, et al. , Bone mineral density and its change in pre-and perimenopausal white women: the Michigan Bone Health Study. J Bone Miner Res, 1998. 13(7): p. 1134–40. [DOI] [PubMed] [Google Scholar]
- 171.Henderson VW, et al. , Cognitive effects of estradiol after menopause: A randomized trial of the timing hypothesis. Neurology, 2016. 87(7): p. 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wellons M, et al. , Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause, 2012. 19(10): p. 1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shen L, et al. , Effects of early age at natural menopause on coronary heart disease and stroke in Chinese women. Int J Cardiol, 2017. 241: p. 6–11. [DOI] [PubMed] [Google Scholar]
- 174.Imtiaz B, et al. , Postmenopausal hormone therapy and Alzheimer disease: A prospective cohort study. Neurology, 2017. 88(11): p. 1062–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shumaker SA, et al. , Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA, 2004. 291(24): p. 2947–58. [DOI] [PubMed] [Google Scholar]
- 176.Shumaker SA, et al. , Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA, 2003. 289(20): p. 2651–62. [DOI] [PubMed] [Google Scholar]
- 177.Yaffe K, et al. , Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA, 1998. 279(9): p. 688–95. [DOI] [PubMed] [Google Scholar]
- 178.Singh M, et al. , Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res, 1994. 644(2): p. 305–12. [DOI] [PubMed] [Google Scholar]
- 179.Miller VM, et al. , The Kronos Early Estrogen Prevention Study (KEEPS): what have we learned? Menopause, 2019. 26(9): p. 1071–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Carroll JC and Pike CJ, Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology, 2008. 149(5): p. 2607–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Saleh RNM, et al. , Hormone replacement therapy is associated with improved cognition and larger brain volumes in at-risk APOE4 women: results from the European Prevention of Alzheimer’s Disease (EPAD) cohort. Alzheimer’s Research & Therapy, 2023. 15(1): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gorski RA, et al. , Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res, 1978. 148(2): p. 333–46. [DOI] [PubMed] [Google Scholar]
- 183.Simerly RB, Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci, 2002. 25: p. 507–36. [DOI] [PubMed] [Google Scholar]
- 184.Morris JA, Jordan CL, and Breedlove SM, Sexual differentiation of the vertebrate nervous system. Nature Neuroscience, 2004. 7(10): p. 1034–1039. [DOI] [PubMed] [Google Scholar]
- 185.Arnold AP and Gorski RA, Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci, 1984. 7: p. 413–42. [DOI] [PubMed] [Google Scholar]
- 186.Lenz KM, et al. , Microglia are essential to masculinization of brain and behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2013. 33(7): p. 2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nugent BM, et al. , Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci, 2015. 18(5): p. 690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bain J, Testosterone and the aging male: to treat or not to treat? Maturitas, 2010. 66(1): p. 16–22. [DOI] [PubMed] [Google Scholar]
- 189.Harman SM, et al. , Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab, 2001. 86(2): p. 724–31. [DOI] [PubMed] [Google Scholar]
- 190.Vance ML, Andropause. Growth Horm IGF Res, 2003. 13 Suppl A: p. S90–2. [DOI] [PubMed] [Google Scholar]
- 191.Singh P, Andropause: Current concepts. Indian J Endocrinol Metab, 2013. 17(Suppl 3): p. S621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Thilers PP, Macdonald SW, and Herlitz A, The association between endogenous free testosterone and cognitive performance: a population-based study in 35 to 90 year-old men and women. Psychoneuroendocrinology, 2006. 31(5): p. 565–76. [DOI] [PubMed] [Google Scholar]
- 193.Janowsky JS, The role of androgens in cognition and brain aging in men. Neuroscience, 2006. 138(3): p. 1015–20. [DOI] [PubMed] [Google Scholar]
- 194.Sierra A, et al. , Steroid hormone receptor expression and function in microglia. Glia, 2008. 56(6): p. 659–74. [DOI] [PubMed] [Google Scholar]
- 195.Saijo K, et al. , An ADIOL-ERβ-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell, 2011. 145(4): p. 584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lei B, et al. , Anti-Inflammatory Effects of Progesterone in Lipopolysaccharide-Stimulated BV-2 Microglia. PLOS ONE, 2014. 9(7): p. e103969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Bali N, et al. , Progesterone Antagonism of Neurite Outgrowth Depends on Microglial Activation via Pgrmc1/S2R. Endocrinology, 2013. 154(7): p. 2468–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Garcia-Segura LM, et al. , Aromatase: a neuroprotective enzyme. Prog Neurobiol, 2003. 71(1): p. 31–41. [DOI] [PubMed] [Google Scholar]
- 199.Kokras N, et al. , Sex differences in behavioral and neurochemical effects of gonadectomy and aromatase inhibition in rats. Psychoneuroendocrinology, 2018. 87: p. 93–107. [DOI] [PubMed] [Google Scholar]
- 200.Acosta-Martínez M, Shaping Microglial Phenotypes Through Estrogen Receptors: Relevance to Sex-Specific Neuroinflammatory Responses to Brain Injury and Disease. Journal of Pharmacology and Experimental Therapeutics, 2020. 375(1): p. 223. [DOI] [PubMed] [Google Scholar]
- 201.Slowik A, et al. , Impact of steroid hormones E2 and P on the NLRP3/ASC/Casp1 axis in primary mouse astroglia and BV-2 cells after in vitro hypoxia. The Journal of Steroid Biochemistry and Molecular Biology, 2018. 183: p. 18–26. [DOI] [PubMed] [Google Scholar]
- 202.Yun J, et al. , Estrogen deficiency exacerbates Aβ-induced memory impairment through enhancement of neuroinflammation, amyloidogenesis and NF-κB activation in ovariectomized mice. Brain, Behavior, and Immunity, 2018. 73: p. 282–293. [DOI] [PubMed] [Google Scholar]
- 203.Thakkar R, et al. , NLRP3 inflammasome activation in the brain after global cerebral ischemia and regulation by 17β-estradiol. Oxidative medicine and cellular longevity, 2016. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Barreto GE, Santos-Galindo M, and Garcia-Segura LM, Selective estrogen receptor modulators regulate reactive microglia after penetrating brain injury. Frontiers in Aging Neuroscience, 2014. 6: p. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Vegeto E, et al. , The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology, 2006. 147(5): p. 2263–72. [DOI] [PubMed] [Google Scholar]
- 206.Aryanpour R, et al. , Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model. International Immunopharmacology, 2017. 51: p. 131–139. [DOI] [PubMed] [Google Scholar]
- 207.Liu C, et al. , Progesterone attenuates neurological deficits and exerts a protective effect on damaged axons via the PI3K/AKT/mTOR-dependent pathway in a mouse model of intracerebral hemorrhage. Aging, 2022. 14(6): p. 2574–2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Barreto G, et al. , Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. European Journal of Neuroscience, 2007. 25(10): p. 3039–3046. [DOI] [PubMed] [Google Scholar]
- 209.Arnold AP, Conceptual frameworks and mouse models for studying sex differences in physiology and disease: why compensation changes the game. Exp Neurol, 2014. 259: p. 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wise AL, Gyi L, and Manolio TA, eXclusion: toward integrating the X chromosome in genome-wide association analyses. American journal of human genetics, 2013. 92(5): p. 643–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Martin J, et al. , Examining Sex-Differentiated Genetic Effects Across Neuropsychiatric and Behavioral Traits. Biological Psychiatry, 2021. 89(12): p. 1127–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Knickmeyer RC and Davenport M, Turner syndrome and sexual differentiation of the brain: implications for understanding male-biased neurodevelopmental disorders. J Neurodev Disord, 2011. 3(4): p. 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Otter M, Schrander-Stumpel CTRM, and Curfs LMG, Triple X syndrome: a review of the literature. European journal of human genetics : EJHG, 2010. 18(3): p. 265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Harris VM, et al. , Klinefelter’s syndrome (47,XXY) is in excess among men with Sjogren’s syndrome. Clin Immunol, 2016. 168: p. 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.van Elst PC, et al. , Evaluating the Scope of Language Impairments in a Patient with Triple X Syndrome: A Brief Report. Developmental Neurorehabilitation, 2020. 23(6): p. 402–406. [DOI] [PubMed] [Google Scholar]
- 216.Kanakis GA and Nieschlag E, Klinefelter syndrome: more than hypogonadism. Metabolism - Clinical and Experimental, 2018. 86: p. 135–144. [DOI] [PubMed] [Google Scholar]
- 217.Wisniewski AB, Gender Development in 46,XY DSD: Influences of Chromosomes, Hormones, and Interactions with Parents and Healthcare Professionals. Scientifica, 2012. 2012: p. 834967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mousavi MJ, Mahmoudi M, and Ghotloo S, Escape from X chromosome inactivation and female bias of autoimmune diseases. Molecular Medicine, 2020. 26(1): p. 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shvetsova E, et al. , Skewed X-inactivation is common in the general female population. European Journal of Human Genetics, 2019. 27(3): p. 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Yurov YB, et al. , X chromosome aneuploidy in the Alzheimer’s disease brain. Molecular Cytogenetics, 2014. 7(1): p. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Maan AA, et al. , The Y chromosome: a blueprint for men’s health? European Journal of Human Genetics, 2017. 25(11): p. 1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vermeulen MC, et al. , Mosaic loss of Chromosome Y in aged human microglia. Genome Res, 2022. 32(10): p. 1795–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ocanas SR, et al. , Differential Regulation of Mouse Hippocampal Gene Expression Sex Differences by Chromosomal Content and Gonadal Sex. Mol Neurobiol, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhang M, et al. , Profiling of Sexually Dimorphic Genes in Neural Cells to Identify Eif2s3y, Whose Overexpression Causes Autism-Like Behaviors in Male Mice. Front Cell Dev Biol, 2021. 9: p. 669798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Snijders Blok L, et al. , Mutations in DDX3X Are a Common Cause of Unexplained Intellectual Disability with Gender-Specific Effects on Wnt Signaling. American journal of human genetics, 2015. 97(2): p. 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hoye ML, et al. , Aberrant cortical development is driven by impaired cell cycle and translational control in a DDX3X syndrome model. eLife, 2022. 11: p. e78203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Davis EJ, et al. , A second X chromosome contributes to resilience in a mouse model of Alzheimer’s disease. Sci Transl Med, 2020. 12(558). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Czech DP, et al. , The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. Journal of neurochemistry, 2012. 122(2): p. 260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lee J, et al. , Sex-specific neuroprotection by inhibition of the Y-chromosome gene, SRY, in experimental Parkinson’s disease. Proceedings of the National Academy of Sciences, 2019. 116(33): p. 16577–16582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lahn Bruce T and Page David C, Functional Coherence of the Human Y Chromosome. Science, 1997. 278(5338): p. 675–680. [DOI] [PubMed] [Google Scholar]
- 231.Kauppi L, Jasin M, and Keeney S, The tricky path to recombining X and Y chromosomes in meiosis. Annals of the New York Academy of Sciences, 2012. 1267: p. 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Baliakas P and Forsberg LA, Chromosome Y loss and drivers of clonal hematopoiesis in myelodysplastic syndrome. Haematologica, 2021. 106(2): p. 329–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhou W, et al. , Mosaic loss of chromosome Y is associated with common variation near TCL1A. Nature Genetics, 2016. 48(5): p. 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Forsberg LA, et al. , Mosaic loss of chromosome Y in peripheral blood is associated with shorter survival and higher risk of cancer. Nature Genetics, 2014. 46(6): p. 624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ouseph MM, et al. , Genomic alterations in patients with somatic loss of the Y chromosome as the sole cytogenetic finding in bone marrow cells. Haematologica, 2021. 106(2): p. 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Dumanski JP, et al. , Mosaic Loss of Chromosome Y in Blood Is Associated with Alzheimer Disease. The American Journal of Human Genetics, 2016. 98(6): p. 1208–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Persani L, et al. , Increased loss of the Y chromosome in peripheral blood cells in male patients with autoimmune thyroiditis. Journal of Autoimmunity, 2012. 38(2): p. J193–J196. [DOI] [PubMed] [Google Scholar]
- 238.Hirata T, et al. , Investigation of chromosome Y loss in men with schizophrenia. Neuropsychiatric disease and treatment, 2018. 14: p. 2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Grassmann F, et al. , Y chromosome mosaicism is associated with age-related macular degeneration. European Journal of Human Genetics, 2019. 27(1): p. 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Fang H, Disteche CM, and Berletch JB, X Inactivation and Escape: Epigenetic and Structural Features. Front Cell Dev Biol, 2019. 7: p. 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lyon MF, Sex chromatin and gene action in the mammalian X-chromosome. American journal of human genetics, 1962. 14(2): p. 135–148. [PMC free article] [PubMed] [Google Scholar]
- 242.Shevchenko AI, Zakharova IS, and Zakian SM, The evolutionary pathway of x chromosome inactivation in mammals. Acta naturae, 2013. 5(2): p. 40–53. [PMC free article] [PubMed] [Google Scholar]
- 243.Xu N, Tsai CL, and Lee JT, Transient homologous chromosome pairing marks the onset of X inactivation. Science, 2006. 311(5764): p. 1149–52. [DOI] [PubMed] [Google Scholar]
- 244.Stavropoulos N, Lu N, and Lee Jeannie T, A functional role for Tsix transcription in blocking Xist RNA accumulation but not in X-chromosome choice. Proceedings of the National Academy of Sciences, 2001. 98(18): p. 10232–10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Fang H, Disteche CM, and Berletch JB, X Inactivation and Escape: Epigenetic and Structural Features. Frontiers in cell and developmental biology, 2019. 7: p. 219–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bonora G and Disteche CM, Structural aspects of the inactive X chromosome. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 2017. 372(1733): p. 20160357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Carrel L and Willard HF, X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature, 2005. 434(7031): p. 400–404. [DOI] [PubMed] [Google Scholar]
- 248.Berletch JB, et al. , Genes that escape from X inactivation. Human Genetics, 2011. 130(2): p. 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chen D.-l., et al. , Long noncoding RNA XIST expedites metastasis and modulates epithelial–mesenchymal transition in colorectal cancer. Cell Death & Disease, 2017. 8(8): p. e3011–e3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Youness A, Miquel C-H, and Guéry J-C, Escape from X Chromosome Inactivation and the Female Predominance in Autoimmune Diseases. International journal of molecular sciences, 2021. 22(3): p. 1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Bajic VP, et al. , The X Files: “The Mystery of X Chromosome Instability in Alzheimer’s Disease”. Frontiers in genetics, 2020. 10: p. 1368–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cooper GS and Stroehla BC, The epidemiology of autoimmune diseases. Autoimmunity reviews, 2003. 2(3): p. 119–125. [DOI] [PubMed] [Google Scholar]
- 253.Hanamsagar R, et al. , Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia, 2017. 65(9): p. 1504–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pinheiro I, Dejager L, and Libert C, X‐chromosome‐located microRNAs in immunity: might they explain male/female differences? The X chromosome‐genomic context may affect X‐located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. Bioessays, 2011. 33(11): p. 791–802. [DOI] [PubMed] [Google Scholar]
- 255.Richie C, Sex, not gender. A plea for accuracy. Experimental & Molecular Medicine, 2019. 51(11): p. 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mauvais-Jarvis F, et al. , Sex and gender: modifiers of health, disease, and medicine. Lancet, 2020. 396(10250): p. 565–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Ramirez K, et al. , Epigenetics Is Implicated in the Basis of Gender Incongruence: An Epigenome-Wide Association Analysis. Frontiers in Neuroscience, 2021. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Unger CA, Hormone therapy for transgender patients. Translational andrology and urology, 2016. 5(6): p. 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Iemura S, et al. , Role of Dkk2 in the Muscle/bone Interaction of Androgen-Deficient Mice. Exp Clin Endocrinol Diabetes, 2021. 129(10): p. 770–775. [DOI] [PubMed] [Google Scholar]
- 260.Gasparini SJ, et al. , Androgens sensitise mice to glucocorticoid-induced insulin resistance and fat accumulation. Diabetologia, 2019. 62(8): p. 1463–1477. [DOI] [PubMed] [Google Scholar]
- 261.Gaignard P, et al. , Sex differences in brain mitochondrial metabolism: influence of endogenous steroids and stroke. J Neuroendocrinol, 2018. 30(2). [DOI] [PubMed] [Google Scholar]
- 262.Medina-Contreras J, et al. , Ovariectomized rodents as a menopausal metabolic syndrome model. A minireview. Mol Cell Biochem, 2020. 475(1–2): p. 261–276. [DOI] [PubMed] [Google Scholar]
- 263.Peng R, et al. , Gonadal hormone trigger the dynamic microglial alterations through Traf6/TAK1 axis that correlate with depressive behaviors. J Psychiatr Res, 2022. 152: p. 128–138. [DOI] [PubMed] [Google Scholar]
- 264.Toms D, Pan B, and Li J, Endocrine Regulation in the Ovary by MicroRNA during the Estrous Cycle. Front Endocrinol (Lausanne), 2017. 8: p. 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cao LB, et al. , Systemic changes in a mouse model of VCD-induced premature ovarian failure. Life Sci, 2020. 262: p. 118543. [DOI] [PubMed] [Google Scholar]
- 266.Brooks HL, Pollow DP, and Hoyer PB, The VCD Mouse Model of Menopause and Perimenopause for the Study of Sex Differences in Cardiovascular Disease and the Metabolic Syndrome. Physiology (Bethesda), 2016. 31(4): p. 250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kappeler CJ and Hoyer PB, 4-vinylcyclohexene diepoxide: a model chemical for ovotoxicity. Syst Biol Reprod Med, 2012. 58(1): p. 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Cao LB, et al. , Hormone-Like Effects of 4-Vinylcyclohexene Diepoxide on Follicular Development. Frontiers in Cell and Developmental Biology, 2020. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nelson JF, et al. , A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod, 1982. 27(2): p. 327–39. [DOI] [PubMed] [Google Scholar]
- 270.Felicio LS, Nelson JF, and Finch CE, Longitudinal studies of estrous cyclicity in aging C57BL/6J mice: II. Cessation of cyclicity and the duration of persistent vaginal cornification. Biol Reprod, 1984. 31(3): p. 446–53. [DOI] [PubMed] [Google Scholar]
- 271.Yan Y, et al. , Estrogen deficiency is associated with hippocampal morphological remodeling of early postmenopausal mice. Oncotarget, 2017. 8(13): p. 21892–21902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Majewski AR, et al. , Sterilization of Silastic Capsules Containing 17β-Estradiol for Effective Hormone Delivery in Mus musculus. J Am Assoc Lab Anim Sci, 2018. 57(6): p. 679–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lei DL, et al. , Effects of estrogen and raloxifene on neuroglia number and morphology in the hippocampus of aged female mice. Neuroscience, 2003. 121(3): p. 659–66. [DOI] [PubMed] [Google Scholar]
- 274.Habermehl TL, et al. , Aging-associated changes in motor function are ovarian somatic tissue-dependent, but germ cell and estradiol independent in post-reproductive female mice exposed to young ovarian tissue. Geroscience, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Mason JB, et al. , Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A Biol Sci Med Sci, 2009. 64(12): p. 1207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Mason JB, et al. , Transplantation of young ovaries restored cardioprotective influence in postreproductive-aged mice. Aging Cell, 2011. 10(3): p. 448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Mason JB, et al. , Manipulation of Ovarian Function Significantly Influenced Trabecular and Cortical Bone Volume, Architecture and Density in Mice at Death. PLoS One, 2015. 10(12): p. e0145821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Vegeto E, et al. , Estrogen receptor-α mediates the brain antiinflammatory activity of estradiol. Proceedings of the National Academy of Sciences, 2003. 100(16): p. 9614–9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Sauer B, Inducible gene targeting in mice using the Cre/lox system. Methods, 1998. 14(4): p. 381–92. [DOI] [PubMed] [Google Scholar]
- 280.Masuda T, et al. , Novel Hexb-based tools for studying microglia in the CNS. Nature Immunology, 2020. 21(7): p. 802–815. [DOI] [PubMed] [Google Scholar]
- 281.McKinsey GL, et al. , A new genetic strategy for targeting microglia in development and disease. Elife, 2020. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Jung S, et al. , Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol, 2000. 20(11): p. 4106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Yona S, et al. , Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity, 2013. 38(1): p. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sahasrabuddhe V and Ghosh HS, Cx3Cr1-Cre induction leads to microglial activation and IFN-1 signaling caused by DNA damage in early postnatal brain. Cell Rep, 2022. 38(3): p. 110252. [DOI] [PubMed] [Google Scholar]
- 285.Kaiser T and Feng G, Tmem119-EGFP and Tmem119-CreERT2 Transgenic Mice for Labeling and Manipulating Microglia. eNeuro, 2019. 6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Wong WP, et al. , Extranuclear estrogen receptor-alpha stimulates NeuroD1 binding to the insulin promoter and favors insulin synthesis. Proc Natl Acad Sci U S A, 2010. 107(29): p. 13057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Maneix L, et al. , Estrogen receptor β exon 3-deleted mouse: The importance of non-ERE pathways in ERβ signaling. Proceedings of the National Academy of Sciences, 2015. 112(16): p. 5135–5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.MacLean HE, et al. , A floxed allele of the androgen receptor gene causes hyperandrogenization in male mice. Physiol Genomics, 2008. 33(1): p. 133–7. [DOI] [PubMed] [Google Scholar]
- 289.Fernandez-Valdivia R, et al. , A mouse model to dissect progesterone signaling in the female reproductive tract and mammary gland. Genesis, 2010. 48(2): p. 106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Conboy MJ, Conboy IM, and Rando TA, Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging cell, 2013. 12(3): p. 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Villeda SA, et al. , The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature, 2011. 477(7362): p. 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.De Miguel Z, et al. , Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature, 2021. 600(7889): p. 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Lovell-Badge R and Robertson E, XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development, 1990. 109(3): p. 635–46. [DOI] [PubMed] [Google Scholar]
- 294.Arnold AP, Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. J Neuroendocrinol, 2009. 21(4): p. 377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Arnold AP, Four Core Genotypes and XY* mouse models: Update on impact on SABV research. Neuroscience & Biobehavioral Reviews, 2020. 119: p. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Probst FJ, et al. , Genotype, phenotype, and karyotype correlation in the XO mouse model of Turner Syndrome. J Hered, 2008. 99(5): p. 512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Park JH, et al. , Effects of sex chromosome aneuploidy on male sexual behavior. Genes Brain Behav, 2008. 7(6): p. 609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Eicher EM, et al. , The mouse Y* chromosome involves a complex rearrangement, including interstitial positioning of the pseudoautosomal region. Cytogenetic and Genome Research, 1991. 57(4): p. 221–230. [DOI] [PubMed] [Google Scholar]
- 299.Yang F, et al. , Global survey of escape from X inactivation by RNA-sequencing in mouse. Genome Res, 2010. 20(5): p. 614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zhang J, et al. , SNPdetector: a software tool for sensitive and accurate SNP detection. PLoS Comput Biol, 2005. 1(5): p. e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Wu H, et al. , Cellular Resolution Maps of X Chromosome Inactivation: Implications for Neural Development, Function, and Disease. Neuron, 2014. 81(1): p. 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Arnold AP, A general theory of sexual differentiation. Journal of Neuroscience Research, 2017. 95(1–2): p. 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Lai MC, et al. , Biological sex affects the neurobiology of autism. Brain, 2013. 136(Pt 9): p. 2799–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Werling DM and Geschwind DH, Sex differences in autism spectrum disorders. Current opinion in neurology, 2013. 26(2): p. 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Martel MM, et al. , Potential hormonal mechanisms of attention-deficit/hyperactivity disorder and major depressive disorder: a new perspective. Hormones and behavior, 2009. 55(4): p. 465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wang L-J, et al. , Potential role of pre- and postnatal testosterone levels in attention-deficit/hyperactivity disorder: is there a sex difference? Neuropsychiatric disease and treatment, 2017. 13: p. 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Arnett AB, et al. , Sex differences in ADHD symptom severity. Journal of child psychology and psychiatry, and allied disciplines, 2015. 56(6): p. 632–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Filon JR, et al. , Gender Differences in Alzheimer Disease: Brain Atrophy, Histopathology Burden, and Cognition. Journal of Neuropathology & Experimental Neurology, 2016. 75(8): p. 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Dubal DB, Broestl L, and Worden K, Sex and gonadal hormones in mouse models of Alzheimer’s disease: what is relevant to the human condition? Biology of sex differences, 2012. 3(1): p. 24–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Avignone E, et al. , Altered morphological dynamics of activated microglia after induction of status epilepticus. J Neuroinflammation, 2015. 12: p. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Schetters STT, et al. , Neuroinflammation: Microglia and T Cells Get Ready to Tango. Front Immunol, 2017. 8: p. 1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Sierra A, et al. , Steroid hormone receptor expression and function in microglia. Glia, 2008. 56(6): p. 659–674. [DOI] [PubMed] [Google Scholar]
- 313.Zhang Y, et al. , An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. The Journal of Neuroscience, 2014. 34(36): p. 11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Barha CK, Dalton GL, and Galea LAM, Low Doses of 17α-Estradiol and 17β-Estradiol Facilitate, Whereas Higher Doses of Estrone and 17α- and 17β-Estradiol Impair, Contextual Fear Conditioning in Adult Female Rats. Neuropsychopharmacology, 2010. 35(2): p. 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Ajami B, et al. , Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci, 2007. 10(12): p. 1538–43. [DOI] [PubMed] [Google Scholar]
- 316.Füger P, et al. , Microglia turnover with aging and in an Alzheimer’s model via long-term in vivo single-cell imaging. Nat Neurosci, 2017. 20(10): p. 1371–1376. [DOI] [PubMed] [Google Scholar]
- 317.Reu P, et al. , The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep, 2017. 20(4): p. 779–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Coleman LG, Zou J, and Crews FT, Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. Journal of Neuroinflammation, 2017. 14(1): p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Hagen SH, et al. , Heterogeneous Escape from X Chromosome Inactivation Results in Sex Differences in Type I IFN Responses at the Single Human pDC Level. Cell Reports, 2020. 33(10): p. 108485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Krasemann S, et al. , The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity, 2017. 47(3): p. 566–581.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]


