Combined heart-liver transplantation (CHLT) is a complex procedure that treats multiorgan disease and organ failure.1-6 CHLT has become increasingly common in recent years because of advancements in surgical techniques and immunosuppression.2 The most common indications for CHLT include familial amyloidosis, cardiac cirrhosis caused by congenital heart disease and dilated cardiomyopathy, and homozygous familial hypercholesterolemia.3 Additionally, with the aging population, the need for cardiac interventions such as coronary artery bypass surgery in combination with liver transplantation is becoming increasingly common.7 There are several major challenges to the CHLT procedure, including hemodynamic instability following implantation and prolonged cold ischemia during organ preservation when managing the logistics of dual organ implantation or surgery. The recent availability of advanced perfusion in the United States has opened the possibility of a paradigm shift in safe duration of ex situ organ preservation.8,9 In CHLT procedures, machine perfusion allows more flexibility in preservation time to allow the opportunity for hemodynamic stabilization and weaning of cardiopulmonary bypass following the cardiac portion of the procedure.8 Additionally, it lessens metabolic disturbances with hepatic reperfusion including hyperkalemia, fluid overload, and acidosis, thereby lessening the stress on the newly implanted cardiac graft or revascularized heart.10 Our objective was to describe the first 3 cases of heart-liver procedures by means of ex situ NMP at Mayo Clinic Florida.
CASE DESCRIPTION
Case 1
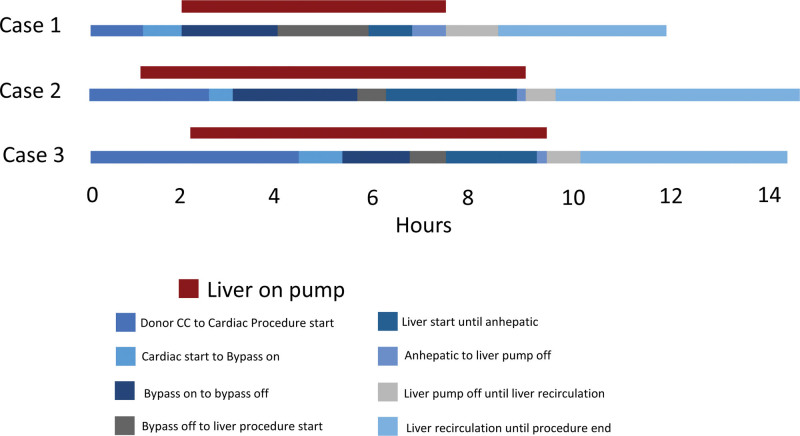
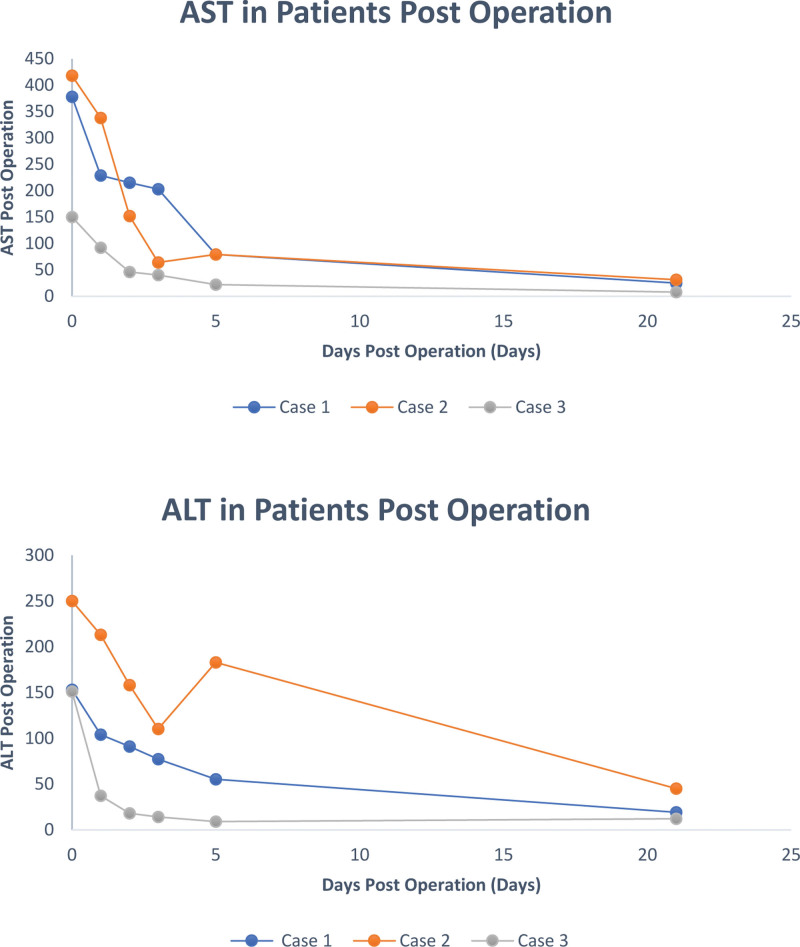
A 58-y-old male presented with a history of cirrhotic stage liver disease secondary to steatotic liver disease with a component of both alcohol and metabolic syndrome, complicated by esophageal varices, complete portal vein thrombosis (Yerdel grade III), hepatocellular carcinoma within Milan (treated with transarterial chemoembolization and stereotactic body radiation therapy), and Yttrium 90 radioembolization. MRI of the abdomen showed 3 OPTN 5 lesions in segment 5 measuring 2.2, 2.0, and 1.6 cm). He had a medical history of coronary artery disease status post percutaneous coronary intervention to left anterior descending and right coronary artery, a history of aortic stenosis status post transcatheter aortic valve replacement procedure in 2019, and heart failure with an ejection fraction of 30% without evidence of ischemic cardiac disease. He had a history of a cardiac arrest and atrial fibrillation. He developed prosthetic aortic valve endocarditis complicated by aortic root abscess/pseudoaneurysm/mycotic superior mesenteric artery aneurysm, and status post percutaneous blade septostomy with progressive structural decay related to endocarditis resulting in severe perivalvular insufficiency. The patient was reviewed by structural cardiology and was without any option for percutaneous intervention and deemed high risk for surgical repair given his cirrhosis. For these reasons, he was listed for a CHLT. At the time of transplant, he was in the intensive care unit for management. He developed ascites requiring large volume paracentesis and acute kidney injury requiring continuous renal replacement therapy (CRRT). He was listed as a United Network of Organ Sharing status 1 for heart transplant. model for end-stage liver disease (MELD)-Na score at listing was 32. Share distance for the donor organ was 198 nautical miles (NM) so air travel was used. CHLT was performed on January 2023; the donor liver was pumped on TransMedics Organ Care System normothermic machine perfusion (NMP). For all 3 cases, liver NMP perfusion was initiated at the donor hospital. Lactate and bile production was monitored for all the pumped livers. Terminal lactate while on pump for all 3 cases was <1 mmol/L. Donor details can be seen in Table 1. The recipient was weaned from cardiopulmonary bypass and stabilized before commencement of the liver transplant portion of the procedure. The total liver preservation time was 9.2 h, total preservation time of the donor heart was 205 min, cardiopulmonary bypass time was 160 min, and total operating time was 14 h (840 min). A timeline of the procedure can be seen in Figure 1. A piggyback liver transplantation without bypass was performed. The complete portal vein thrombosis was managed with thromboembolectomy. The recipient did not experience post reperfusion syndrome (PRS) and they did not develop early allograft dysfunction (EAD). A Graph demonstrating the recipient aspartate transaminase and alanine transaminase for the first 21 d post CHLT procedure can be seen in Figure 2. Following the transplant, the patient’s renal function improved and CRRT was transitioned to hemodialysis. Hemodialysis was stopped 19 d posttransplant. There were no significant complications posttransplants, and the patient was discharged 25 d postoperation.
TABLE 1.
Characteristics of CHLT procedure patients.
| Patients | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Sex | Male | Male | Male |
| Calculated MELD | 32 | 11 | 42 |
| Procedure | Heart transplant and liver transplant | Heart transplant and liver transplant | CABG and liver transplant |
| Transplant date | January 29, 2023 | October 19, 2022 | May 8, 2023 |
| Age at transplant, y | 58 | 54 | 63 |
| Donor organ type | DBD | DBD | DBD |
| Machine perfusion start | January 28, 2023 20:25 | October 19, 2022 8:25 | May 8, 2023 5:37 |
| Machine perfusion end | January 29, 2023 1:56 | October 19, 2022 16:27 | May 8, 2023 13:02 |
| Total pump time (h) | 5.52 | 8.03 | 7.42 |
| Share distance (NM) | 198 | 458 | 257 |
| UNOS “CIT” (h) | 8.77 | 8.72 | 9.25 |
| Recipient WIT (h) | 0.43 | 0.37 | 0.5 |
| Total liver preservation time (h) | 9.2 | 9.08 | 9.75 |
| Actual CIT (h) | 3.25 | 0.69 | 1.83 |
| Final hepatic artery flow on pump (L/min) | 0.58 | 0.79 | 0.53 |
| Total OR time (h) | 11.6 | 10.6 | 8.7 |
| Intraoperative RBC (units) | 9 | 5 | 15 |
| Intraoperative FFP (units) | 5 | 3 | 13 |
| Intraoperative PLTs (units) | 8 | 1 | 4 |
| Intraoperative cryo (units) | 0 | 2 | 2 |
| INR post 7 d | 1.3 | 1.1 | 0.9 |
| Bilirubin post 7 d (mg/dL) | 0.7 | 0.6 | 1.2 |
| Cr at 48 h post (mg/dL) | 1.26 | 0.84 | 2.03 |
| Discharged | February 24, 2023 | November 9, 2022 | May 24, 2023 |
CABG, coronary artery bypass graft; CHLT, combined heart-liver transplantation; Cr, creatinine; cryo, cryoprecipitate; DBD, donation after brain death; FFP, fresh–frozen plasma; INR, international normalization ratio; MELD, model for end-stage liver disease; NM, nautical miles; OR, operative time; PLT, platelets; RBC, red blood cells; UNOS “CIT”, United Network of Organ Sharing cold ischemia time (defined as donor cross clamp until recipient recirculation); WIT, warm ischemia time.
FIGURE 1.
Timeline of CHLT procedure cases (hours). CC, cross clamp.
FIGURE 2.
Graphs demonstrating the AST and ALT in patients over the first 21 d post CHLT procedure patients. ALT, alanine transaminase; AST, aspartate transaminase.
Case 2
A 54-y-old male had end-stage liver disease secondary to cardiac cirrhosis complicated by ascites. A liver biopsy demonstrated focal sinusoidal dilatation associated with perisinusoidal fibrosis consistent with chronic venous outflow impairment with stage IV fibrosis consistent with cirrhosis secondary to cardiac etiology. He had a history of atrial fibrillation/flutter and underwent radiofrequency ablation in 2014. He subsequently underwent atrioventricular nodal ablation and cardiac resynchronization therapy placement on December 2015, with closure of a patent foramen ovale. He developed New York Heart Association 3B functional heart failure with chronic volume overload which progressed for the next 6 y. He developed atypical hypertrophic cardiomyopathy and resulting acute cardiogenic shock. He was listed as United Network of Organ Sharing status 2 for heart transplant with a MELD-Na score of 11 at that time. Share distance for the donor organs was 458 NM so a flight was used. Dual heart-liver transplant was performed on October 2022. The recipient was weaned from cardiopulmonary bypass and stabilized before commencement of the liver transplant portion of the procedure. The liver was pumped on Organ Care System NMP. The total liver preservation time was 9.08 h; total preservation time for heart was 4 h 11 min, cardiopulmonary bypass time was 156 min, and total operating time was 11 h 57 min (717 min). A timeline of the procedure can be seen in Figure 1. Given the history of chronic venous outflow impairment, the hepatic veins and short caval veins were very distended. A piggyback liver transplant without bypass was performed. The recipient did not experience PRS or EAD. There were no complications posttransplant, and the patient was discharged 18 d posttransplant.
Case 3
A 63-y-old male presented with end-stage liver disease secondary to alcohol-related cirrhosis with risk factors for metabolic associated liver disease, including diabetes, complicated by large volume ascites, hepatic encephalopathy, hepatorenal syndrome requiring CRRT, and hyponatremia. CRRT was initiated on April 22, 2023, then prolonged intermittent renal replacement therapy was initiated on May 1–4, and finally transitioned to intermittent hemodialysis on May 6. With regard to risk factors for coronary artery disease, he had a history of hypertension, hyperlipidemia, type 2 diabetes, and significant family history of coronary artery disease. On echocardiogram his ejection fraction was 69% with normal diastolic and normal right ventricular systolic function. Transesophageal echocardiogram confirmed a 4 × 4 mm nodule at the sinotubular junction which appeared to be consistent with atherosclerosis. He had evidence of a moderate immobile atheroma without evidence of ulceration. Nuclear perfusion study was negative. He underwent coronary angiography which demonstrated coronary artery disease (left main coronary artery 60% obstructed by a discrete lesion, proximal left anterior descending artery 40% obstructed by diffuse disease, distal circumflex artery 30% lesion, and first obtuse marginal 30% lesion, ramus 20% lesion, and proximal right coronary artery 20% obstructed by diffuse disease). Given his cirrhosis, he was felt to be too high risk for coronary artery bypass graft (CABG) surgery but also too high risk to undergo liver transplant without coronary revascularization. He was listed for liver transplant with a calculated MELD-Na score of 42. Share distance for the donor organ was 257 NM so a plane was used. He underwent CABG surgery at the time of liver transplant. Following CABG surgery, the recipient was weaned from cardiopulmonary bypass and stabilized before commencement of the liver transplant portion of the procedure. The total liver preservation time was 9.75 h, and total operating time was 10 h 32 min (632 min). A timeline of the procedure can be seen in Figure 1. A piggyback liver transplant without bypass was performed. The recipient did not experience PRS or EAD. There were no significant complications during the operation. He continued to require hemodialysis postoperatively. The patient was discharge to an inpatient rehabilitation facility 16 d postoperation. He continued on an MWF (Monday, Wednesday, Friday) dialysis schedule with a plan to list for kidney transplant “safety net.”
DISCUSSION
The present case series describes 3 cases in which liver NMP was utilized to safely perform CHLT surgeries. In these cases, machine perfusion afforded the ability to wait for the patient to be weaned from cardiopulmonary bypass and to be hemodynamically stable following the cardiac portion of the procedure, without the worry of prolonged cold ischemia time. Because 2 of the cases had more challenging hepatectomies (Yerdel grade III portal vein thrombus in case 1 and severely distended hepatic and short caval veins in case 2), having the liver on NMP allowed for a less rushed hepatectomy. In addition, having the liver graft on NMP before implantation also lessened metabolic disturbances with hepatic reperfusion including hyperkalemia, fluid overload, and acidosis, thereby lessening the stress on the newly implanted cardiac graft or revascularized heart. None of the cases experienced PRS or EAD. Finally, having less concerns for cold ischemia time, allowed our team to accept donor organs from a broader distance, despite the complicated nature of these cases. Because of the success of NMP with these cases, we have modified our CHLT protocol so that NMP will be routinely used for all these cases.
Footnotes
I.D. and K.P.C. participated in research design, data analysis, and the writing of the paper. I.D., S.M.P., D.K.P., and K.P.C. participated in the performance of the research.
The authors declare no funding or conflicts of interest.
Contributor Information
Si M. Pham, Email: pham.si@mayo.edu.
Dana K. Perry, Email: perry.dana@mayo.edu.
REFERENCES
- 1.Beal EW, Mumtaz K, Hayes D, Jr, et al. Combined heart-liver transplantation: Indications, outcomes and current experience. Transplant Rev (Orlando). 2016;30:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gong T, Hall S. Considerations and experience driving expansion of combined heart-liver transplantation. Curr Opin Organ Transplant. 2020;25:496–500. [DOI] [PubMed] [Google Scholar]
- 3.Frountzas M, Karampetsou N, Nikolaou C, et al. Combined heart and liver transplantation: an updated systematic review. Ann R Coll Surg Engl. 2022;104:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebray P, Varnous S. Combined heart and liver transplantation: state of knowledge and outlooks. Clin Res Hepatol Gastroenterol. 2019;43:123–130. [DOI] [PubMed] [Google Scholar]
- 5.Axelrod D, Koffron A, Dewolf A, et al. Safety and efficacy of combined orthotopic liver transplantation and coronary artery bypass grafting. Liver Transpl. 2004;10:1386–1390. [DOI] [PubMed] [Google Scholar]
- 6.Rizvi SSA, Challapalli J, Maynes EJ, et al. Indications and outcomes of combined heart-liver transplant: a systematic review and met-analysis. Transplant Rev (Orlando). 2020;34:100517. [DOI] [PubMed] [Google Scholar]
- 7.Jacob S, Nguyen JH, El-Sayed Ahmed MM, et al. Combined cardiac surgery procedures and liver transplant: a single-center experience. Gen Thorac Cardiovasc Surg. 2022;70:714–720. [DOI] [PubMed] [Google Scholar]
- 8.Croome KP. Introducing machine perfusion into routine clinical practice for liver transplantation in the United States: the moment has finally come. J Clin Med. 2023;12:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croome KP, Brown TE, Mabrey RL, et al. Development of a portable abdominal normothermic regional perfusion (A-NRP) program in the United States. Liver Transpl. 2023;29:1282–1291. [DOI] [PubMed] [Google Scholar]
- 10.Markmann JF, Abouljoud MS, Ghobrial RM, et al. Impact of portable normothermic blood-based machine perfusion on outcomes of liver transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022;157:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]