Movement-evoked pain is associated with synovitis and pain sensitization in patients with painful knee osteoarthritis.
Keywords: Knee osteoarthritis, Movement-evoked pain, Pain sensitization, Synovitis, Bone marrow lesions
Abstract
Introduction:
Movement-evoked pain (MEP) is the primary symptom in patients with knee osteoarthritis (KOA).
Objectives:
This study aimed to investigate the contribution of joint structural changes and pain sensitization to the mechanisms of MEP in patients with KOA.
Methods:
A total of 86 patients were assessed for demographic characteristics, osteoarthritis severity, Whole-Organ Magnetic Resonance Imaging Score–Hoffa synovitis and bone marrow lesions, pressure pain threshold and temporal summation of pain at the knee and forearm, Central Sensitization Inventory-9, and MEP. In measure of MEP, knee pain was scored using a numerical rating scale (NRS, 0–10) before and every minute during a 6-minute walking test (6MWT), and the MEP index was defined as the change in NRS pain score from baseline to the sixth minute of walking.
Result:
On average, pain during 6MWT increased by 1.4 ± 1.5 points on the NRS relative to baseline, with 30.2% of patients showing an increase of 2 points or more. The hierarchical linear regression analysis revealed that Hoffa synovitis, pressure pain threshold at the forearm, and temporal summation of pain at the knee were associated with the MEP index.
Conclusion:
The findings of this study suggest that both synovitis and neural mechanisms, such as pain sensitization, play a role in the development of MEP in KOA.
1. Introduction
Knee osteoarthritis (KOA) is the most common chronic joint disease in adults ≥50 years old worldwide. Currently, KOA is considered a whole joint disease with multiple pathological factors, including the destruction of articular cartilage, synovitis, and subchondral bone changes.46 Pain is the primary symptom of KOA, and it is referred to as movement-evoked pain (MEP), which occurs during loading movements rather than at rest. Movement-evoked pain is a major cause of disability and reduced quality of life in patients with KOA, and KOA with severe pain may have a sensitized response to exercise.14,47 Various factors, such as joint inflammation, increased pain sensitivity, and overweight, may contribute to MEP in patients with KOA37,47; however, the etiology of MEP in KOA, whether because of joint structural changes or pain sensitization, remains poorly understood.
Most elderly patients with knee pain display joint structural changes on radiographs, and the prevalence of pain tends to increase with the progression of osteoarthritis severity.6,38 However, many individuals with Kellgren–Lawrence (KL) grade 3 or higher have no pain, and pain does not often correlate with the severity of KOA.29 Recently, joint inflammation measured by magnetic resonance imaging (MRI), such as synovitis and bone marrow lesions (BMLs), has also been considered a trigger for pain; however, the relationship between self-reported pain and MRI findings is only weakly correlated.8,9,18,48 Therefore, the pathology of MEP in KOA is not solely determined by local mechanisms.
Previous studies using quantitative sensory testing (QST), including pressure pain threshold (PPT) and temporal summation of pain (TSP), have reported that pain in KOA results from the sensitization of nociceptors (peripheral sensitization) and pathological neural signaling in the central nervous system (central sensitization).3,11,43 Pain sensitization is a factor that decreases treatment response to exercise therapy and pharmacotherapy.10,15,16 Moreover, alterations in the central nervous system may cause various symptoms, such as fatigue and poor sleep, which are called central sensitization-related symptoms and affect KOA pain symptoms.2,15 Thus, factors contributing to pain symptoms are varied, but which ones are strongly associated with MEP in KOA remains unclear.
This study aimed to examine factors associated with MEP among KOA severity, synovitis, BMLs, pain sensitization, and central sensitization-related symptoms in patients with KOA. We hypothesized that both pain sensitization and structural abnormalities would be associated with MEP.
2. Methods
2.1. Participants
Patients with symptomatic KOA, newly referred for physiotherapy by an orthopedic surgeon, were recruited from the Maehara Orthopedic Rehabilitation Clinic in Japan between January 2022 and December 2022 for this cross-sectional study. The inclusion criteria were as follows: a diagnosis of KOA confirmed by radiographic findings (KL grade ≥1); men and women aged between 50 and 90 years; experiencing pain intensity of ≥2 on an 11-point numerical rating scale (NRS); and the presence of chronic joint pain for ≥6 months. The exclusion criteria included systemic inflammatory diseases (eg, rheumatoid arthritis); cognitive impairment; severe medical comorbidities (eg, neurological, psychological, or cardiovascular diseases and cancer); serious hip or ankle pathology (eg, osteoarthritis and unhealed fractures); leg pain referred from the lumbar spine; previous joint replacement; and use of centrally acting medications (eg, antidepressants and anxiolytics). In cases of bilateral KOA, the affected side was defined as the knee with more severe pain.
This study was approved by the Institutional Ethics Committees of Kobe Gakuin University in Kobe, Japan (No.: 21-17) and Maehara Orthopedic Rehabilitation Clinic in Aichi, Japan (No.: 21-02). This study was conducted in compliance with the Declaration of Helsinki and its later amendments. All participants gave informed consent.
2.2. Procedure
All patients were assessed for demographic data (age, sex, body mass index [BMI], pain intensity using NRS, and pain duration), X-ray and MRI findings, PPT, TSP, and Central Sensitization Inventory-9 (CSI-9). Pain on the NRS was evaluated in reference to the following question, “what is the peak knee pain you felt in the past week?” In the case of bilateral symptomatic KOA, knee pain on the most painful side was assessed. Subsequently, MEP was assessed during a 6-minute walking test (6MWT). Pressure pain threshold, TSP, and MEP were performed in a quiet, temperature-controlled room.
2.3. Data collection
2.3.1. Movement-evoked pain
Movement-evoked pain is a measure of the sensitized responses to movements.45,47 The patients were asked to rate the pain intensity before and at each minute during the 6MWT.45,47 Pain immediately before walking was assessed by the current pain in the static standing position. Pain intensity was assessed using NRS, wherein “0” indicated “no pain” and “10” indicated “worst possible pain.” The MEP index was defined as the change in NRS reported at the sixth minute of walking minus the NRS immediately before the walking test. In the 6MWT, a 20-m course between 2 cones was used. Patients were encouraged to walk as far as possible within 6 minutes and were informed that they were not allowed to run.
2.3.2. Osteoarthritis severity
The KL grade was used as a measure of the radiological osteoarthritis severity.22 The characteristics of each KL grade are as follows: grade 1, doubtful osteoarthritis with the presence of minor osteophytes of doubtful importance; grade 2, minimal osteoarthritis with definite osteophytes but an unimpaired joint space; grade 3, moderate osteoarthritis with osteophytes and moderate diminution of the joint space; and grade 4, severe osteoarthritis with a greatly impaired joint space and sclerosis of the subchondral bone.
2.3.3. Magnetic resonance imaging findings
All patients underwent MRI (Echelon RX 1.5T [Nikko Medical, Chiba, Japan]). Imaging sequences included T2-weighted fast spin echo with fat suppression in coronal (repetition time: 3272 ms; echo time: 36 ms; field of view: 29.7 × 20 mm; matrix: 288 × 256; slice thickness: 3 mm; interslice gap: 3.3 mm) and sagittal (repetition time: 3272 ms; echo time: 36 ms; field of view: 29.7 × 20 mm; matrix: 288 × 256; slice thickness: 3 mm; interslice gap: 3.3 mm) planes. All images were evaluated by a single orthopedic surgeon with extensive experience in the care of knee disease. Synovitis and BMLs were assessed using the Whole-Organ Magnetic Resonance Imaging Score (WORMS).33 The synovitis score was graded on a scale of 0 to 3 (0 = normal, 1 = <33% of the maximum potential distension, 2 = 33%–66% of the maximum potential distension, and 3 = >66% of the maximum potential distension) in the intercondylar region of fat pad (Hoffa's fat pad). This site is called Hoffa synovitis,17 reflecting true synovitis.36 The BMLs were graded on a scale of 0 to 3 at all 15 areas around the knee joint (0 = none, 1 = <25%, 2 = 25%–50%, and 3 = >50%). The sum of BMLs grade at 15 areas was calculated as the BMLs score. The examples of MRI findings are shown in Figure 1.
Figure 1.
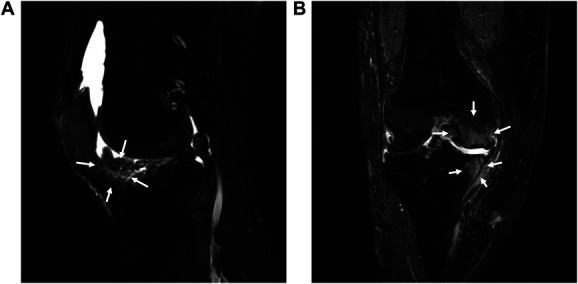
Examples of magnetic resonance imaging findings. (A) Hoffa synovitis and (B) bone marrow lesions.
2.3.4. Pain sensitization
Pressure pain threshold, a measure of pain sensitivity by pressure stimulus, was assessed by a physiotherapist at the knee joint and extensor carpi radialis longus (forearm, 5 cm distal to the lateral epicondyle of the humerus) using a hand-held pressure algometer (Algometer Type II, Somedic AB, Sweden) with a 1-cm2 probe at a pressing rate of 30 kPa/s.4 The PPT was measured on the affected side. The assessment sites of the knee joint were as follows: 2 cm distal to the inferior medial edge of the patella, 2 cm distal to the inferior lateral edge of the patella, 3 cm lateral to the midpoint on the lateral edge of the patella, and 3 cm medial to the mid-point on the medial edge of the patella (Fig. 2).4 The PPT at the knee was defined as the lowest PPT at the 4 sites.16 The mean of the 2 measurements was used for the analysis.
Figure 2.
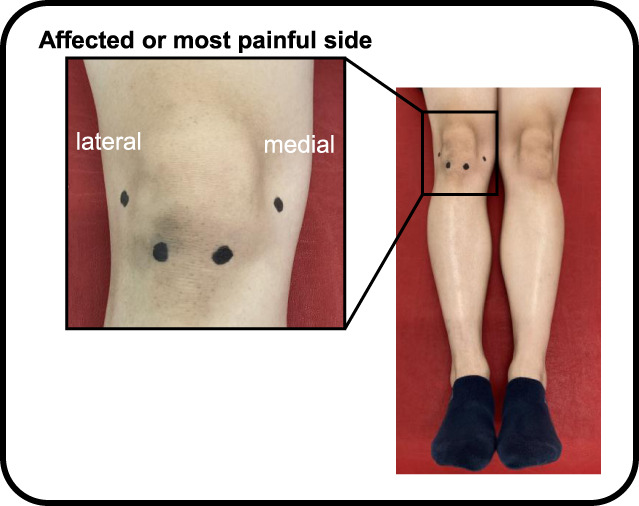
Measurement sites of pressure pain threshold around the patella. The measurement sites of the knee joint were as follows: 2 cm distal to the inferior medial edge of the patella, 2 cm distal to the inferior lateral edge of the patella, 3 cm lateral to the midpoint on the lateral edge of the patella, and 3 cm medial to the mid-point on the medial edge of the patella.
Temporal summation of pain, a measure of segmental sensitization of spinal cord, was assessed in the knee joint and forearm.4 The TSP at the knee joint was assessed at the lowest PPT site. After the first stimulation, patients were asked to rate the pain intensity on a visual analog scale (VAS), in which “0” indicated “no pain” and “100” indicated the “worst possible pain.” Subsequently, 10 sequential stimuli were applied to the same site in 1-second intervals, and the patients reported the pain intensity for the last stimulation on the VAS. The pressure intensity of the sequential stimuli was equal to the PPT level. The TSP value was calculated as the difference between the 10th VAS score and the first VAS score.16
2.3.5. Central sensitization-related symptoms
Central sensitization-related symptoms were assessed using the CSI-9.31 The CSI-9, consisting of 9 items, is a short version of the 25-item Central Sensitization Inventory (CSI), and its reliability and validity have been previously established.31 The total score is classified as “subclinical” for scores ranging from 0 to 9, “mild” for scores ranging from 10 to 19, and “moderate/severe” for scores ranging from 20 to 36. Central sensitization-related symptoms were classified as follows: emotional distress; urological and general symptoms; muscle symptoms; headache and jaw symptoms; and sleep disturbance, and CSI-9 contains all 5 factors.
2.4. Statistical approach
The data analysis in this study was conducted in 2 steps. First, changes in pain during 6MWT were identified. Because of the nonnormality in the distribution of MEP index, Friedman analysis of variance (ANOVA) with time (pre, 1, 2, 3, 4, 5, and 6) was used to describe the increase in the NRS of pain during the 6MWT. The percentage of participants with an increase in the NRS of 2 or more was calculated as a clinically meaningful change in patients with KOA.44
Second, hierarchical linear regression analysis was used to examine the association between the MEP index and the independent variables. Standardized residual scatter plots, P–P plots, and histograms were used to check the underlying assumptions for multiple regression analysis, including the assumption of linear relationships, homoscedasticity, independency, and normality of residuals.39 To avoid multicollinearity, the correlation coefficients among all variables were calculated by correlation analysis. If the correlation coefficient (r) was > 0.80 for 2 variables, the variable with the stronger association with the MEP index was included in the hierarchical linear regression analysis. Additionally, correlation analysis of the MEP index was performed for the variables, and variables with P < 0.05 were included in the hierarchical linear regression analysis. To differentiate the effects of demographic data and other variables, the regression was entered in 2 steps. The control variables (age, sex, and BMI), if significantly correlated with the MEP index, were entered into the hierarchical linear regression models in step 1, followed by KL grade, WORMS, PPT, TSP, and CSI-9 in step 2.
Sample size was estimated using G*Power and Green method.12 Previous studies have demonstrated that demographic data, inflammatory findings, PPT, and TSP may be associated with pain intensity during movement.16,48 Therefore, the final regression model was assumed to include a maximum of 4 explanatory variables, including the control variables, and the sample size for this study was calculated to be at least 86 participants (power = 0.80, α = 0.05, effect size [f2] = 0.15). A significance level of P < 0.05 was used for each analysis. Statistical analyses were performed using SPSS version 29.0 (IBM Corporation, Armonk, NY).
3. Results
3.1. Demographic data
Demographic data are shown in Table 1. The total number of patients was 86. The mean age of the patients was 66.2 ± 10.9 years, and females accounted for 83.7% of all patients. The NRS ranged from 2 to 10 (mean, 4.6 ± 2.0). The pain duration ranged from 6 to 240 months (mean, 62.5 ± 56.2 months).
Table 1.
Demographic data of patients with knee osteoarthritis.
| Characteristics | Value | Range |
|---|---|---|
| N | 86 | |
| Age (y) | 66.2 ± 10.9 | 50–86 |
| Sex, n (%) | ||
| Male | 14 (16.3) | |
| Female | 72 (83.7) | |
| BMI (kg/m2) | 24.1 ± 3.6 | 19.0–39.4 |
| Pain intensity (NRS) | 4.6 ± 2.0 | 2–10 |
| Pain duration (mo) | 62.5 ± 56.2 | 6–240 |
Data are presented as the mean ± SD.
BMI, body mass index; NRS, numerical rating scale.
3.2. Movement-evoked pain
The change in MEP is shown in Figure 3. The MEP index ranged from 0 to 6 points. On average, pain during the 6MWT increased by 1.4 ± 1.5 points on the NRS relative to baseline, with 30.2% of patients demonstrating an increase of 2 points or more. Analysis using Friedman ANOVA revealed statistically significant increases in NRS with each minute (P < 0.001).
Figure 3.
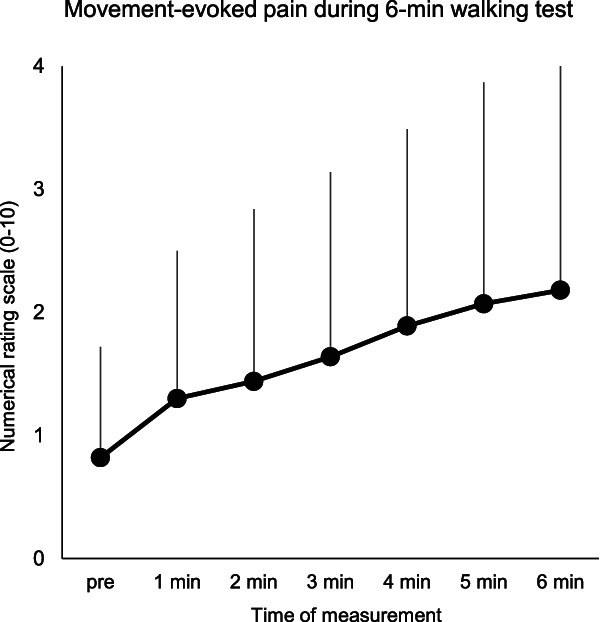
Change in movement-evoked pain during a 6-minute walking test.
3.3. Factors associated with movement-evoked pain
Table 2 shows the mean KL grade, WORMS, PPT, TSP, and CS-9. As the correlation of independent variables was r < 0.80, all variables were used in the analysis. Age, sex, and BMI did not demonstrate a statistically significant correlation with the MEP index and thus were not incorporated into the hierarchical linear regression analysis.
Table 2.
Mean values and standard deviation of primary assessments.
| Variables | Value | Range |
|---|---|---|
| MEP index | 1.4 ± 1.5 | 0 to 6 |
| KL grade (%) | ||
| 1 | 32 (37.2) | |
| 2 | 27 (31.4) | |
| 3 | 22 (25.6) | |
| 4 | 5 (5.8) | |
| WORMS-synovitis | 0.6 ± 0.9 | 0 to 3 |
| WORMS-BMLs | 2.5 ± 3.4 | 0 to 14 |
| PPT-knee | 314.6 ± 180.8 | 14 to 1158 |
| PPT-forearm | 334.5 ± 140.1 | 14 to 954 |
| TSP-knee | 13.0 ± 14.4 | −13 to 63 |
| TSP-forearm | 8.2 ± 6.5 | 0 to 34 |
| CSI-9 | 8.8 ± 5.6 | 0 to 25 |
Data are presented as the mean ± SD.
BMLs, bone marrow lesions; CSI-9, Central Sensitization Inventory-9; KL, Kellgren–Lawrence; MEP, movement-evoked pain; PPT, pressure pain threshold; TSP, temporal summation of pain; WORMS, Whole-Organ Magnetic Resonance Imaging Score.
All underlying assumptions for linear regression, including the assumption of linear relationships, homoscedasticity, independency and normality of residuals, were appropriately met. The MEP index significantly correlated with WORMS-synovitis (rSpearman = 0.404, P < 0.001), PPT at the knee (rSpearman = −0.529, P < 0.001) and forearm (rSpearman = −0.450, P < 0.001), and TSP at the knee (rSpearman = 0.529, P < 0.001), but not with WORMS-BMLs (rSpearman = 0.187, P = 0.086), TSP at the forearm (rSpearman = 0.148, P = 0.174), and CSI-9 (rSpearman = 0.191, P = 0.078).
The variables associated with the MEP index are shown in Table 3. Whole-Organ Magnetic Resonance Imaging Score-synovitis, PPT at the forearm, and TSP at the knee were significantly associated with the MEP index (R2 = 0.543, R2adj = 0.520, P < 0.001). All tolerances were > 0.1, and all variance inflation factors were < 10; thus, multicollinearity did not occur.
Table 3.
Hierarchical linear regression analysis.
| Variables | β | t | P | Collinearity | |
|---|---|---|---|---|---|
| Tolerance | VIF | ||||
| WORMS-synovitis | 0.287 | 3.339 | <0.001 | 0.764 | 1.309 |
| PPT-knee | −0.030 | −0.266 | 0.791 | 0.432 | 2.322 |
| PPT-forearm | −0.226 | −2.142 | 0.035 | 0.509 | 1.966 |
| TSP-knee | 0.437 | 5.035 | <0.001 | 0.751 | 1.332 |
PPT, pressure pain threshold; TSP, temporal summation of pain; VIF, variance inflation factor; WORMS, Whole-Organ Magnetic Resonance Imaging Score.
4. Discussion
This study aimed to examine the contribution of structural abnormalities and pain sensitization to MEP in KOA. In this study, the pain intensity gradually increased during 6MWT, and 30.2% of patients with KOA showed an increase of 2 or more on NRS. The hierarchical linear regression analysis showed that Hoffa synovitis on MRI findings, lower PPT at the forearm, and facilitated TSP at the knee, an index of central sensitization, were associated with the MEP index independently of the control variables. The findings of this study suggest that both synovitis and neural mechanisms, such as pain sensitization, play a role in the development of MEP in KOA.
4.1. Factors associated with movement-evoked pain
In this study, on average, the pain increased by 1.4 ± 1.5 points on the NRS relative to baseline during the walking task and that 30.2% of patients exhibited an increase in NRS of 2 or more. Previous studies have shown that pain increases in patients with KOA with loading movements, such as walking and stair climbing.14,47 Similarly, approximately 30% of patients with low back pain experience an increase in pain during a 6-minute walking test.45 Exercise in the affected area could have led to increased clinical pain that persisted for up to 2 days in patients with chronic neck and shoulder pain.13 These findings, in conjunction with the results of our study, suggest that MEP is a primary manifestation of musculoskeletal pain and that some patients with KOA may exhibit sensitized responses to movements. Generally, the female sex, old age, and high BMI are factors in the development of pain symptoms; however, the present study demonstrated that demographic data were not associated with MEP. Several other studies that examined MEP also identified that age and sex were not predictors of MEP.45,47 There results may suggest that factors other than demographic data (eg, inflammation and sensitization) have a large influence.
The pathology of KOA pain is multifactorial, with several proposed mechanisms, including structural changes, biomechanical factors, inflammatory processes, and pain sensitization (Fig. 4). Our findings demonstrate that Hoffa synovitis is a contributing factor to MEP. Synovitis releases pro-inflammatory and pain neurotransmitters, such as substance P and nerve growth factor.26 Studies using sensitive imaging modalities have confirmed a high prevalence of synovitis in all KL grades and correlated it with pain symptoms in KOA.8,48 Moreover, chronic postoperative pain after total knee arthroplasty is associated with the degree of Hoffa synovitis23; therefore, these findings suggest that synovitis may be involved in chronic knee joint pain. Conversely, BMLs and KL grades were not found to be contributing factors to MEP in this study. To date, clinical data have shown that BMLs are associated with pain during movement more than synovitis.24,37 However, these reports were based on patients with high BMI and high KL grade. Our study included patients with low BMI, and early KOA patients accounted for a significant proportion of the sample. Thus, the discrepancy in the results between the present study and previous studies may be attributed to subject characteristics.
Figure 4.
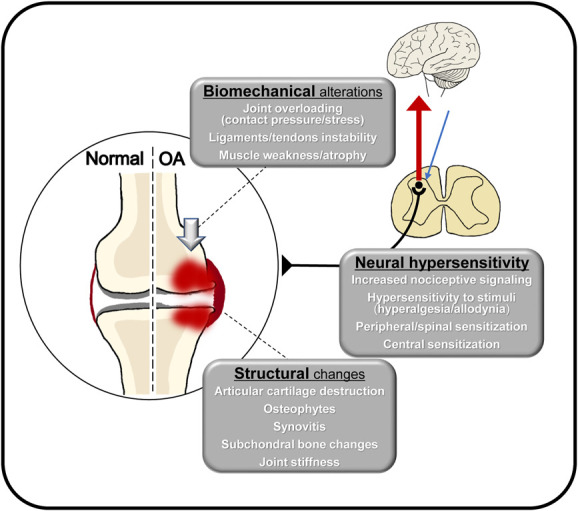
Pathological characteristics of knee osteoarthritis. OA, osteoarthritis.
Interestingly, our findings indicate that patients with severe MEP exhibit an increase in the TSP, which is an indicator of segmental sensitization of spinal cord.3 It has been reported that almost all patients with chronic peripheral sensitization in osteoarthritis exhibit central sensitization.46 The walking task imposes repetitive weight loads on the knee joint; thus, it is plausible that central sensitization may be associated with MEP, particularly that produced by loading movements. Additionally, we found that the MEP index was associated with PPT of the forearm, an indicator of widespread hyperalgesia. Vaegter et al. have reported that MEP in patients with low back pain was associated with PPT at a remote site, which is congruent with the findings of the present study.45 Conversely, our findings indicate that increased pain during walking is not linked to PPT at the knee. Lower PPT in the local area reflects peripheral sensitization caused by structural abnormalities20; therefore, our results may indicate that MEP in patients with KOA is influenced by central sensitization rather than peripheral sensitization. However, identifying the peripheral mechanisms is difficult with only PPT at the knee. In the present study, synovitis was associated with MEP, suggesting that at least the peripheral mechanisms based on structural abnormalities affects pain symptoms. Another intriguing finding is the lack of association between the CSI-9 and MEP index. In recent clinical studies, CSI did not correlate with pain symptoms or treatment response in patients with KOA.28 The CSI encompasses the criteria for nociplastic pain as proposed by the International Association for the Study of Pain.30 Therefore, MEP was not considered to be directly associated with factors related to nociplastic pain in KOA.
4.2. Implications for the management of movement-evoked pain
Exercise and physical activity are internationally recognized treatments for osteoarthritis, and their role in improving pain and functional disability has been demonstrated.5,40 In particular, walking is a versatile exercise modality that has been shown to alleviate pain and sensitivity.19 Recent evidence indicates that 8 to 12 weeks of exercise are required to improve pain symptoms1,5,34,40,41; however, MEP inhibits the introduction or continuation of exercise.21 Our findings suggest that Hoffa synovitis and pain sensitization lead to increased MEP in KOA. Therefore, it is necessary to introduce exercise combined with pharmacotherapy in patients with severe MEP. Additionally, Meeus et al.27 have advocated an approach that incorporates graded exercise in conjunction with pain neuroscience education for patients at risk for exercise-induced hyperalgesia. Low-intensity exercise decreases pain sensitization in both exercise and nonexercise sites.32 Moreover, a weight bearing exercise may have a greater analgesic effect than a non-weight bearing exercise in KOA.7 Therefore, it may be beneficial to introduce low-intensity or nonloading exercises, in combination with patient education, for patients at risk of increased MEP. However, effective treatments to improve synovitis and pain sensitization have not yet been established,35,46 and further studies are required.
4.3. Limitation
This study had several limitations. First, the proportion of patients with severe KL grades was limited in this study; thus, the results of this study may not be generalizable to other populations with severe structural abnormalities. Second, this study used a cross-sectional design. Future studies should longitudinally examine the relationship between MEP, inflammation, and sensitization. To date, pain sensitization assessed by QST parameters has been demonstrated to predict response to total knee arthroplasty, pharmacotherapy, and exercise therapy10,16,23; however, their predictive ability and sensitivity are insufficient. Considering the results of the present study, MEP may be an indicator of pain sensitization and may be a useful predictive tool for treatment outcome. Movement-evoked pain can be measured without the use of specific instruments, which makes it a useful assessment in clinical practice. Future studies comparing the clinical usefulness of QST and MEP are needed. Third, psychological factors were not assessed in this study. There are evidences that psychological factors, such as pain catastrophizing and fear of movement, are associated with MEP in chronic musculoskeletal pain, including low back pain42 and osteoarthritis.47 These factors have been linked to lower top-down pain inhibition25; thus, our findings should be interpreted with caution.
5. Conclusion
This study provides evidence that the pathogenesis of MEP in KOA is not solely determined by local mechanisms, such as joint structural changes, but also by neural mechanisms, including central sensitization. These findings may aid in identifying potential targets for treatment and in predicting the risk of exercise-induced pain in pretreatment. Future research could further optimize treatment algorithms for KOA by examining the changes in MEP over time with interventions that target these factors.
Disclosures
The authors have no conflict of interest to declare.
Acknowledgements
The authors extend their gratitude to all the participants examined in this study and the Department of Rehabilitation, Maehara Orthopedics Rehabilitation Clinic staff for their invaluable assistance with data collection.
Author contributions: T.H., S.O., K.S., and T.M. designed the research; T.H. undertook data collection; T.H. performed the statistical analyses; T.H., S.O., K.S., and T.M. interpreted the results; T.H. and T.M. wrote the paper; all authors discussed the results and commented on the manuscript.
This research received no specific grant from any funding agency.
Data availability statement: The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Satoshi Ohga, Email: ohga@reha.kobegakuin.ac.jp.
Kazuhiro Shimo, Email: shimo@reha.kobegakuin.ac.jp.
Takako Matsubara, Email: matsubar@reha.kobegakuin.ac.jp.
References
- [1].Ageberg E, Nilsdotter A, Kosek E, Roos EM. Effects of neuromuscular training (NEMEX-TJR) on patient-reported outcomes and physical function in severe primary hip or knee osteoarthritis: a controlled before-and-after study. BMC Musculoskelet Disord 2013;14:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aguila MER, Rebbeck T, Leaver AM, Lagopoulos J, Brennan PC, Hübscher M, Refshauge KM. The association between clinical characteristics of migraine and brain GABA levels: an exploratory study. J Pain 2016;17:1058–67. [DOI] [PubMed] [Google Scholar]
- [3].Arendt-Nielsen L. Pain sensitisation in osteoarthritis. Clin Exp Rheumatol 2017;3(suppl 107):68–74. [PubMed] [Google Scholar]
- [4].Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. PAIN 2010;149:573–81. [DOI] [PubMed] [Google Scholar]
- [5].Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, Espinosa R, Haugen IK, Lin J, Mandl LA, Moilanen E, Nakamura N, Snyder-Mackler L, Trojian T, Underwood M, McAlindon TE. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27:1578–89. [DOI] [PubMed] [Google Scholar]
- [6].Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord 2008;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bennell KL, Dobson F, Roos EM, Skou ST, Hodges P, Wrigley TV, Kyriakides M, Metcalf B, Hunt MA, Hinman RS. Influence of biomechanical characteristics on pain and function outcomes from exercise in medial knee osteoarthritis and varus malalignment: exploratory analyses from a randomized controlled trial. Arthritis Care Res 2015;67:1281–8. [DOI] [PubMed] [Google Scholar]
- [8].Dainese P, Wyngaert KV, De Mits S, Wittoek R, Van Ginckel A, Calders P. Association between knee inflammation and knee pain in patients with knee osteoarthritis: a systematic review. Osteoarthritis Cartilage 2022;30:516–34. [DOI] [PubMed] [Google Scholar]
- [9].Daugaard CL, Riis RG, Bandak E, Gudbergsen H, Henriksen M, Bliddal H, Boesen M. Perfusion in bone marrow lesions assessed on DCE-MRI and its association with pain in knee osteoarthritis: a cross-sectional study. Skeletal Radiol 2020;49:757–64. [DOI] [PubMed] [Google Scholar]
- [10].Edwards RR, Dolman AJ, Martel MO, Finan PH, Lazaridou A, Cornelius M, Wasan AD. Variability in conditioned pain modulation predicts response to NSAID treatment in patients with knee osteoarthritis. BMC Musculoskelet Disord 2016;17:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fingleton C, Smart K, Moloney N, Fullen BM, Doody C. Pain sensitization in people with knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage 2015;23:1043–56. [DOI] [PubMed] [Google Scholar]
- [12].Green SB. How many subjects does it take to do a regression analysis. Multivariate Behav Res 1991;26:499–510. [DOI] [PubMed] [Google Scholar]
- [13].Grimby-Ekman A, Ahlstrand C, Gerdle B, Larsson B, Sandén H. Pain intensity and pressure pain thresholds after a light dynamic physical load in patients with chronic neck-shoulder pain. BMC Musculoskelet Disord 2020;21:266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harden RN, Wallach G, Gagnon CM, Zereshki A, Mukai A, Saracoglu M, Kuroda MM, Graciosa JR, Bruehl S. The osteoarthritis knee model: psychophysical characteristics and putative outcomes. J Pain 2013;14:281–9. [DOI] [PubMed] [Google Scholar]
- [15].Hattori T, Shimo K, Niwa Y, Katsura Y, Tokiwa Y, Ohga S, Matsubara T. Pain sensitization and neuropathic pain-like symptoms associated with effectiveness of exercise therapy in patients with hip and knee osteoarthritis. Pain Res Manag 2022;2022:4323045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hattori T, Ohga S, Shimo K, Niwa Y, Tokiwa Y, Matsubara T. Predictive value of pain sensitization associated with response to exercise therapy in patients with knee osteoarthritis: a prospective cohort study. J Pain Res 2022;15:3537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hayashi D, Roemer FW, Katur A, Felson DT, Yang SO, Alomran F, Guermazi A. Imaging of synovitis in osteoarthritis: current status and outlook. Semin Arthritis Rheum 2011;41:116–30. [DOI] [PubMed] [Google Scholar]
- [18].Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, Gale D, Grainger A, Conaghan P, Felson DT. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis 2007;66:1599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hviid JCT, Thorlund JB, Vaegter HB. Walking increases pain tolerance in humans: an experimental cross-over study. Scand J Pain 2019;19:813–22. [DOI] [PubMed] [Google Scholar]
- [20].Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, de Souza LP, Cutait MM, Fregni F, Camanho GL. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Rheum 2008;59:1424–31. [DOI] [PubMed] [Google Scholar]
- [21].Jack K, McLean SM, Moffett JK, Gardiner E. Barriers to treatment adherence in physiotherapy outpatient clinics: a systematic review. Man Ther 2010;15:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kurien T, Kerslake RW, Graven-Nielsen T, Arendt-Nielsen L, Auer DP, Edwards K, Scammell BE, Petersen KK. Chronic postoperative pain after total knee arthroplasty: the potential contributions of synovitis, pain sensitization and pain catastrophizing—an explorative study. Eur J Pain 2022;26:1979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lo GH, McAlindon TE, Niu J, Zhang Y, Beals C, Dabrowski C, Le Graverand MP, Hunter DJ; OAI Investigators Group. Bone marrow lesions and joint effusion are strongly and independently associated with weight-bearing pain in knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2009;17:1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Malfliet A, Coppieters I, Van Wilgen P, Kregel J, De Pauw R, Dolphens M, Ickmans K. Brain changes associated with cognitive and emotional factors in chronic pain: a systematic review. Eur J Pain 2017;21:769–86. [DOI] [PubMed] [Google Scholar]
- [26].Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther 2017;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Meeus M, Nijs J, Van Wilgen P, Noten S, Goubert D, Huijnen I. Moving on to movement in patients with chronic joint pain. PAIN 2016;1:1–8. [Google Scholar]
- [28].Mibu A, Nishigami T, Tanaka K, Manfuku M, Yono S. Difference in the impact of central sensitization on pain-related symptoms between patients with chronic low back pain and knee osteoarthritis. J Pain Res 2019;12:1757–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Muraki S, Oka H, Akune T, Mabuchi A, En-yo Y, Yoshida M, Saika A, Suzuki T, Yoshida H, Ishibashi H, Yamamoto S, Nakamura K, Kawaguchi H, Yoshimura N. Prevalence of radiographic knee osteoarthritis and its association with knee pain in the elderly of Japanese population-based cohorts: the ROAD study. Osteoarthritis Cartilage 2009;17:1137–43. [DOI] [PubMed] [Google Scholar]
- [30].Nijs J, Lahousse A, Kapreli E, Bilika P, Saraçoğlu İ, Malfliet A, Coppieters I, De Baets L, Leysen L, Roose E, Clark J, Voogt L, Huysmans E. Nociplastic pain criteria or recognition of central sensitization? pain phenotyping in the past, present and future. J Clin Med 2021;10:3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Nishigami T, Tanaka K, Mibu A, Manfuku M, Yono S, Tanabe A. Development and psychometric properties of short form of central sensitization inventory in participants with musculoskeletal pain: a cross-sectional study. PLoS One 2018;13:e0200152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Niwa Y, Shimo K, Ohga S, Tokiwa Y, Hattori T, Matsubara T. Effects of exercise-induced hypoalgesia at different aerobic exercise intensities in healthy young adults. J Pain Res 2022;15:3615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, Kothari M, Lu Y, Fye K, Zhao S, Genant HK. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage 2004;12:177–90. [DOI] [PubMed] [Google Scholar]
- [34].Raposo F, Ramos M, Lúcia Cruz A. Effects of exercise on knee osteoarthritis: a systematic review. Musculoskeletal Care 2021;19:399–435. [DOI] [PubMed] [Google Scholar]
- [35].Riis RGC, Henriksen M, Klokker L, Bartholdy C, Ellegaard K, Bandak E, Hansen BB, Bliddal H, Boesen M. The effects of intra-articular glucocorticoids and exercise on pain and synovitis assessed on static and dynamic magnetic resonance imaging in knee osteoarthritis: exploratory outcomes from a randomized controlled trial. Osteoarthritis Cartilage 2017;25:481–91. [DOI] [PubMed] [Google Scholar]
- [36].Roemer FW, Guermazi A, Zhang Y, Yang M, Hunter DJ, Crema MD, Bohndorf K. Hoffa's fat pad: evaluation on unenhanced MR images as a measure of patellofemoral synovitis in osteoarthritis. AJR Am J Roentgenol 2009;192:1696–700. [DOI] [PubMed] [Google Scholar]
- [37].Satake Y, Izumi M, Aso K, Igarashi Y, Sasaki N, Ikeuchi M. Comparison of predisposing factors between pain on walking and pain at rest in patients with knee osteoarthritis. J Pain Res 2021;14:1113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schaible HG. Mechanisms of chronic pain in osteoarthritis. Curr Rheumatol Rep 2012;14:549–56. [DOI] [PubMed] [Google Scholar]
- [39].Schneider A, Hommel G, Blettner M. Linear regression analysis: part 14 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2010;107:776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Skou ST, Rasmussen S, Laursen MB, Rathleff MS, Arendt-Nielsen L, Simonsen O, Roos EM. The efficacy of 12 weeks non-surgical treatment for patients not eligible for total knee replacement: a randomized controlled trial with 1-year follow-up. Osteoarthritis Cartilage 2015;23:1465–75. [DOI] [PubMed] [Google Scholar]
- [41].Skou ST, Roos EM. Good Life with osteoArthritis in Denmark (GLA:D™): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Musculoskelet Disord 2017;18:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sullivan MJL, Larivière C, Simmonds M. Activity-related summation of pain and functional disability in patients with whiplash injuries. PAIN 2010;151:440–6. [DOI] [PubMed] [Google Scholar]
- [43].Thakur M, Dickenson AH, Baron R. Osteoarthritis pain: nociceptive or neuropathic? Nat Rev Rheumatol 2014;10:374–80. [DOI] [PubMed] [Google Scholar]
- [44].Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, Bombardier C, Felson D, Hochberg M, van der Heijde D, Dougados M. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis 2005;64:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Vaegter HB, Petersen KK, Sjodsholm LV, Schou P, Andersen MB, Graven-Nielsen T. Impaired exercise-induced hypoalgesia in individuals reporting an increase in low back pain during acute exercise. Eur J Pain 2021;25:1053–63. [DOI] [PubMed] [Google Scholar]
- [46].Vincent TL. Peripheral pain mechanisms in osteoarthritis. PAIN 2020;161(suppl 1):138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wideman TH, Finan PH, Edwards RR, Quartana PJ, Buenaver LF, Haythornthwaite JA, Smith MT. Increased sensitivity to physical activity among individuals with knee osteoarthritis: relation to pain outcomes, psychological factors, and responses to quantitative sensory testing. PAIN 2014;155:703–11. [DOI] [PubMed] [Google Scholar]
- [48].Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis 2011;70:60–7. [DOI] [PubMed] [Google Scholar]


