Abstract
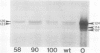
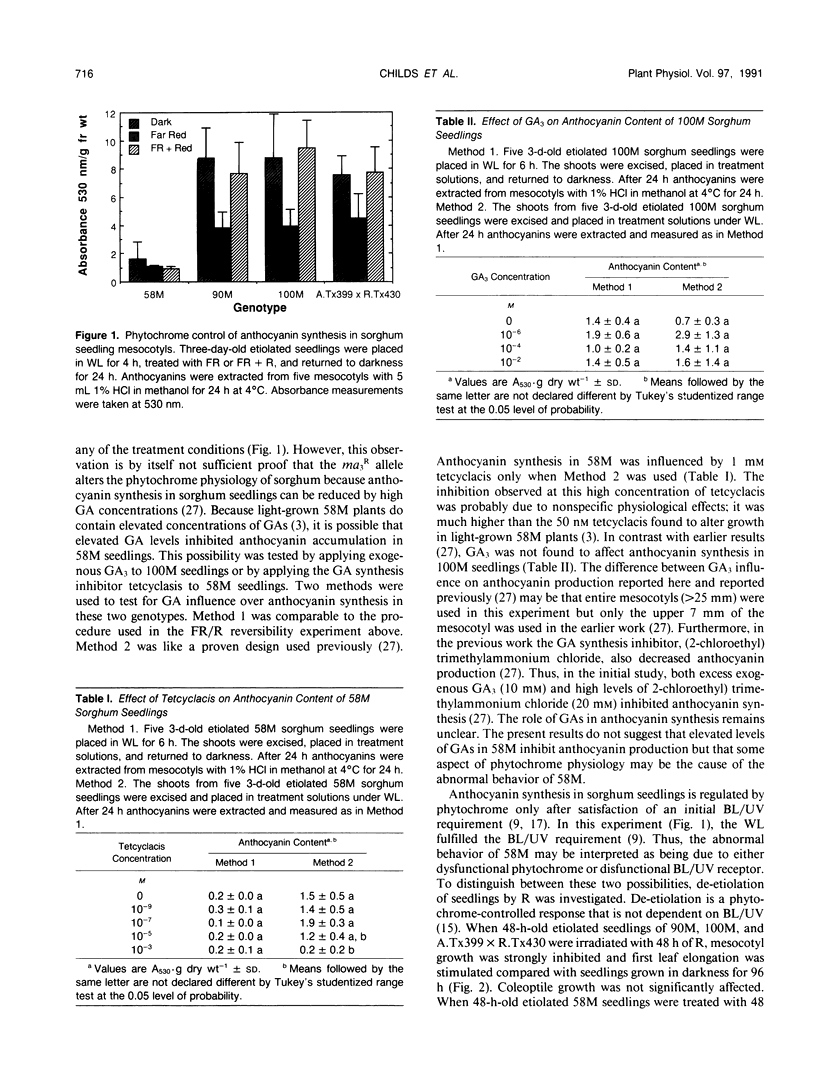
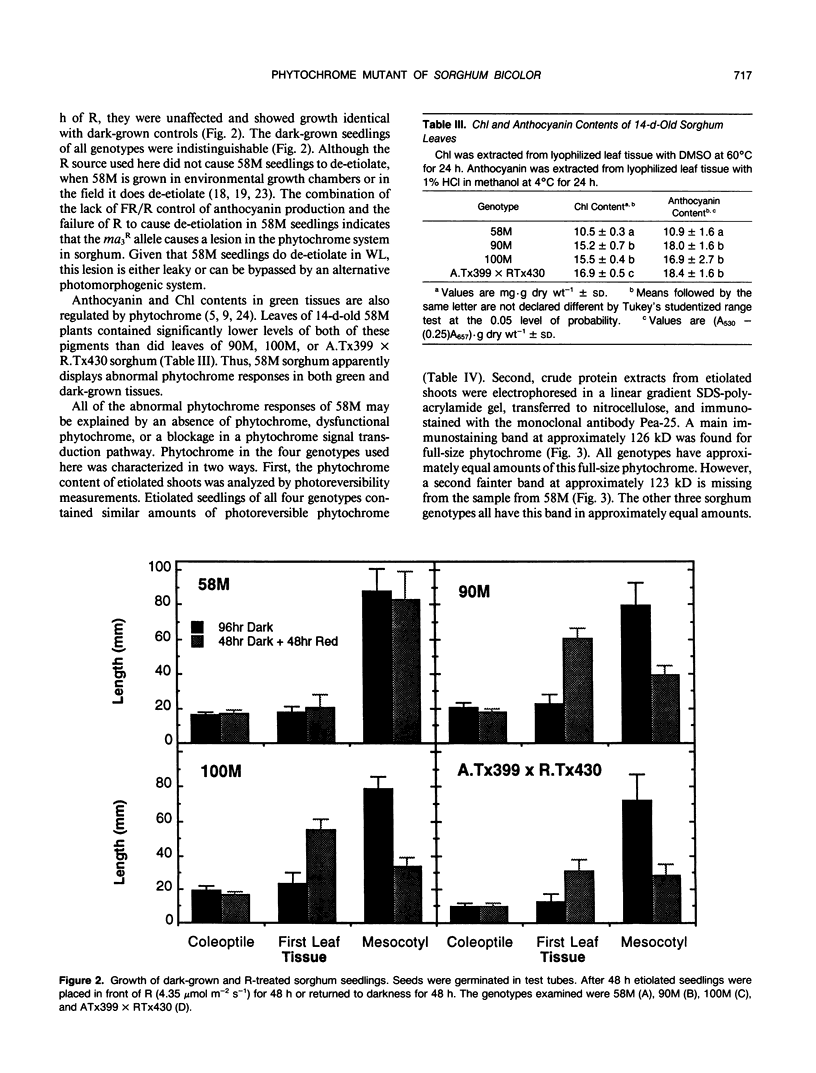
Physiological processes controlled by phytochrome were examined in three near-isogenic genotypes of Sorghum bicolor, differing at the allele of the third maturity gene locus. Seedlings of 58M (ma3R ma3R) did not show phytochrome control of anthocyanin synthesis. In contrast, seedlings of 90M (ma3ma3) and 100M (Ma3Ma3) demonstrated reduced anthocyanin synthesis after treatment with far red and reversal of the far red effect by red. De-etiolation of 48-hour-old 90M and 100M dark-grown seedlings occurred with 48 hours of continuous red. Dark-grown 58M seedlings did not de-etiolate with continuous red treatment. Treatment of seedlings with gibberellic acid or tetcyclacis, a gibberellin synthesis inhibitor, did not alter anthocyanin synthesis. Levels of chlorophyll and anthocyanin were lower in light-grown 58M seedlings than in 90M and 100M. Etiolated seedlings of all three genotypes have similar amounts of photoreversible phytochrome. Crude protein extracts from etiolated seedlings were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Phytochrome was visualized with Pea-25, a monoclonal antibody directed to phytochrome from etiolated peas. The samples from all three genotypes contained approximately equivalent amounts of a prominent, immunostaining band at 126 kD. However, the sample from 58M did not show a fainter, secondary band at 123 kD that was present in 90M and 100M. The identity and importance of this secondary band at 123 kD is unknown. We propose that 58M is a phytochrome-related mutant that contains normal amounts of photoreversible phytochrome and normal phytochrome protein when grown in the dark.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamse P., Jaspers P. A., Bakker J. A., Kendrick R. E., Koornneef M. Photophysiology and phytochrome content of long-hypocotyl mutant and wild-type cucumber seedlings. Plant Physiol. 1988 May;87(1):264–268. doi: 10.1104/pp.87.1.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall F. D., Morgan P. W., Mander L. N., Miller F. R., Babb K. H. Genetic Regulation of Development in Sorghum bicolor: V. The ma(3) Allele Results in Gibberellin Enrichment. Plant Physiol. 1991 Jan;95(1):116–125. doi: 10.1104/pp.95.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campell B. R., Bonner B. A. Evidence for Phytochrome Regulation of Gibberellin A(20) 3beta-Hydroxylation in Shoots of Dwarf (lele) Pisum sativum L. Plant Physiol. 1986 Dec;82(4):909–915. doi: 10.1104/pp.82.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J., Peto C. A., Ashbaugh M., Saganich R., Pratt L., Ausubel F. Different Roles for Phytochrome in Etiolated and Green Plants Deduced from Characterization of Arabidopsis thaliana Mutants. Plant Cell. 1989 Sep;1(9):867–880. doi: 10.1105/tpc.1.9.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier M. M., Greppin H., Pratt L. H. Identification of a highly conserved domain on phytochrome from angiosperms to algae. Plant Physiol. 1986 Apr;80(4):982–987. doi: 10.1104/pp.80.4.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs R. J., Siegelman H. W. Photocontrol of Anthocyanin Synthesis in Milo Seedlings. Plant Physiol. 1963 Jan;38(1):25–30. doi: 10.1104/pp.38.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandoli D. F., Briggs W. R. Phytochrome control of two low-irradiance responses in etiolated oat seedlings. Plant Physiol. 1981 Apr;67(4):733–739. doi: 10.1104/pp.67.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelmüller R., Mohr H. Mode of coaction between blue/UV light and light absorbed by phytochrome in light-mediated anthocyanin formation in the milo (Sorghum vulgare Pers.) seedling. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6124–6128. doi: 10.1073/pnas.82.18.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. I., Morgan P. W. Genetic Regulation of Development in Sorghum bicolar: I. Role of the Maturity Genes. Plant Physiol. 1986 Oct;82(2):575–580. doi: 10.1104/pp.82.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. I., Morgan P. W. Genetic Regulation of Development in Sorghum bicolor: II. Effect of the ma(3) Allele Mimicked by GA(3). Plant Physiol. 1986 Oct;82(2):581–584. doi: 10.1104/pp.82.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabino I., Mancinelli A. L. Light, temperature, and anthocyanin production. Plant Physiol. 1986 Jul;81(3):922–924. doi: 10.1104/pp.81.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]