In Brief
Researchers studied whether the Rotterdam model (RM) score to predict postoperative cerebellar mutism syndrome (CMS) will predict the incidence of postoperative mutism in a high-expertise pediatric neurosurgical center. They found poor applicability and generalizability of the RM, as the incidence of postoperative CMS was significantly lower in the cohort of patients who were at high risk for postoperative mutism compared with what was predicted by RM. These findings point to an inadequacy of radiology-based models predicting postoperative CMS, likely because these models do not take into account preoperative symptoms and deficits and surgical experience and expertise.
Keywords: cerebellar mutism, posterior fossa syndrome, medulloblastoma, Rotterdam model, surgical injury, tumor
ABBREVIATIONS : CMS = cerebellar mutism syndrome, CMS1 = type 1, CMS2 = CMS type 2, GTR = gross-total resection, d(sagittal) = depth of invasion and/or compression of the brainstem by tumor, D(sagittal) = line connecting the upper and lower points of the brainstem invaded by tumor, MCP = middle cerebellar peduncle, NTR = near-total resection, NWNS = non-WNT/non-SHH, RM = Rotterdam model, SCP = superior cerebellar peduncle, SHH = sonic hedgehog, STR = subtotal resection
Abstract
OBJECTIVE
Postoperative cerebellar mutism syndrome (CMS) develops in up to 40% of children with medulloblastoma. The Rotterdam model (RM) has been reported to predict a 66% risk of CMS in patients with a score of ≥ 100. The aim of this study was to retrospectively apply the RM to an independent cohort of patients with newly diagnosed medulloblastoma and study the applicability of the RM in predicting postoperative CMS.
METHODS
Participants had to have their first tumor resection at the authors’ institution and be enrolled in the SJMB12 protocol (NCT01878617). All participants underwent structured serial neurological evaluations before and then periodically after completing radiation therapy. Imaging was reviewed by the study neurologist who was blinded to CMS status when reviewing the scans and retrospectively applied RM score to each participant.
RESULTS
Forty participants were included (14 females and 26 males). Four (10%) patients had CMS. The median age at tumor resection was 11.7 years (range 3.5–17.8 years). Tumor location was midline in 30 (75%), right lateral in 6 (15%), and left lateral in 4 (10%). The median Evans index was 0.3 (range 0.2–0.4), and 34 (85%) patients had an Evans index ≥ 0.3. Five participants required a ventricular shunt. The median tumor volume was 51.97 cm3 (range 20.13–180.58 cm3). Gross-total resection was achieved in 35 (87.5%) patients, near-total resection in 4 (10%), and subtotal in 1. The median RM score was 90 (range 25–145). Eighteen participants had an RM score of ≥ 100, and of these 16.7% (n = 3) had CMS. Of the 22 patients with an RM score < 100, 1 child developed CMS (4.5%, CI 0.1%–22.8%); 3 of the 18 patients with an RM score ≥ 100 developed CMS (16.7%, CI 3.6%–41.4%). The observed rate of CMS in the cohort of children with an RM score ≥ 100 was significantly lower than the observed rate in the original RM cohort (66.7%, CI 51%–80.0%, p < 0.001). A greater risk of CMS in patients with an RM score ≥ 100 could not be confirmed (p = 0.31).
CONCLUSIONS
At the authors’ institution, the incidence of CMS in patients who had an RM ≥ 100 was significantly lower than the RM cohort. These findings raise questions regarding generalizability of RM; however, fewer cases of CMS and a relatively small cohort limit this conclusion.
An estimated 500 children are diagnosed with medulloblastoma each year in the United States.1 Of those children who undergo resection, up to 40% may develop postoperative cerebellar mutism syndrome (CMS), also known as posterior fossa syndrome.2,3 CMS is characterized by a dramatic reduction in speech, ranging from severe limitation to total mutism. The syndrome may include a variety of other symptoms, including ataxia, cranial nerve palsies, involuntary movements, and behavioral changes.4,5 Mutism usually resolves spontaneously, and other deficits improve with time, but many survivors will have long-term neurological and cognitive impairment, and many will be wheelchair bound at 1 year after surgery.5,6 The exact pathophysiology of CMS has not been elucidated. However, evidence suggests that perioperative injury to the deep cerebellar nuclei (fastigial and dentate), right paramedian cerebellum, and cerebellar outflow tract to the cortex via superior cerebellar peduncle are major contributors to the development of CMS.7–9
Midline tumor location, diagnosis of medulloblastoma, and younger age at the time of surgery are recognized risk factors for CMS.5,10 The Rotterdam model (Table 1)11 uses multiple features of preoperative imaging to predict the risk of CMS. According to this model, patients with a score of ≥ 100 have a 66% absolute risk of developing CMS. We previously reported that surgical experience, as defined by annual institutional volume and/or pediatric neurosurgery fellowship training, influences the risk of CMS.5 Since our institution qualifies, according to this definition,5 as a high-volume pediatric neurosurgical center, we wanted to assess applicability and generalizability of the RM. We hypothesized that at our institution, being a high-volume center for pediatric neurosurgical oncology, the rate of CMS would be lower than that predicted by the RM, and that the RM model will not be applicable. We assessed this by retrospectively applying the RM to a cohort of children who had their first surgery for medulloblastoma at our institution.
TABLE 1.
Predicting factors used in the RM and their associated risk scores
| Predictor | Risk Score |
|---|---|
| Radiological diagnosis |
|
| Medulloblastoma |
15 |
| Other |
0 |
| Tumor location on MRI |
|
| Midline |
10 |
| Cerebellar hemisphere |
0 |
| Brainstem invasion |
35 |
| MCP |
|
| Invasion lt |
20 |
| Invasion rt |
40 |
| Bilat invasion &/or compression |
40 |
| SCP invasion |
|
| Lt |
10 |
| Rt |
20 |
| Bilat |
25 |
| d(sagittal) ≥ 0.58 cm | 20 |
Modified from Dhaenens et al. Childs Nerv Syst. 2020;36(7):1471–1480.12
© The authors, published with permission. CC BY 4.0 (https://creativecommons.org/licenses/by/4.0/).
Methods
Subject Data Collection
The institutional review board exempted this study from requiring patient/parent consent. All children enrolled in our multi-institutional medulloblastoma trial (SJMB12 protocol; ClinicalTrials.gov identifier no. NCT 01878617) who underwent their first tumor surgery at our institution were included in this study. To be included in the SJMB12 study, patients had to be older than 3 years at the time of enrollment and have a histological diagnosis of medulloblastoma. The SJMB12 protocol was approved by the St. Jude Institutional Review Board. Patients’ legal guardians signed written informed consent prior to the study. Patients with prior chemo- or radiotherapy, or those with a delayed start of treatment by more than 36 days were excluded from the study. All participants in SJMB12 study received a detailed and structured neurological assessment and examination on arrival to our institution, after completion of radiation treatment, and then every 3–6 months. All neurological assessments were performed by the study neurologists.5 Neurological examination at each visit included quantified assessment of speech, gait, stance, and dysmetria using the modified Scale for Assessment and Rating of Ataxia score.12
Diagnosis of CMS required language impairment, and participants were divided into two groups: type 1, with complete mutism (CMS1); and type 2, characterized by paucity of speech and an inability to string 3-word sentences (CMS2).
Definitions and Comparison
The RM provides a scoring system to predict overall probability of developing the syndrome after surgery (Table 1). These features include the diagnosis of medulloblastoma, midline tumor location, brainstem invasion, middle cerebellar peduncle (MCP) invasion and/or compression, superior cerebellar peduncle (SCP) invasion, and d(sagittal) (i.e., depth of invasion and/or compression of the brainstem by tumor) ≥ 0.58 cm (Fig. 1).11 Invasion of the cerebellar peduncles is defined as visible signal change on MRI indicating loss of distinction between tumor border and MCP or SCP at their interface. The d(sagittal) value can be calculated by drawing D(sagittal), which is the line connecting the upper and lower points of the brainstem invaded by tumor and measuring the distance from the line created by D(sagittal) to the back of the brainstem (Fig. 1).
FIG. 1.
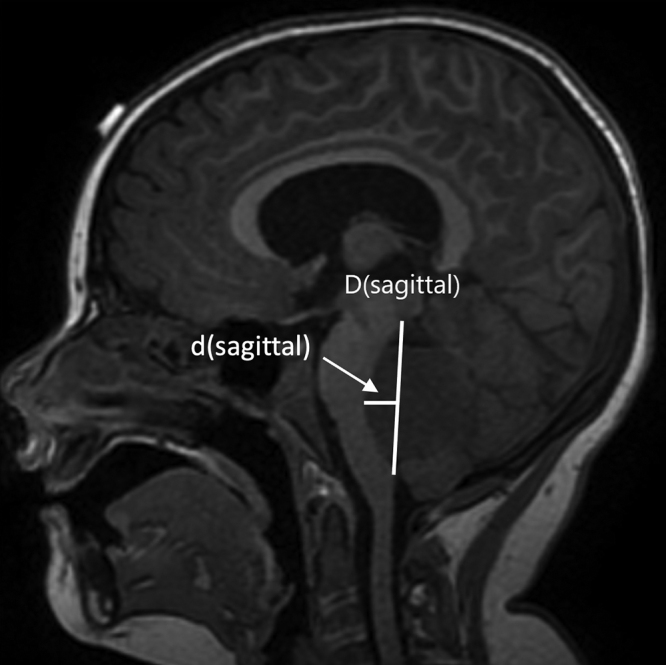
Visual representation of D(sagittal) and d(sagittal) measurements.
In addition to the features described by the RM,11 patient demographics, tumor volume, tumor type, the presence of ventriculomegaly as defined by the Evans index, and the extent of tumor resection were recorded. Tumor size was recorded in three planes (height × width × anteroposterior diameter) to calculate the tumor volume. The degree of ventriculomegaly was calculated using the Evans index (maximum cranium width/frontal horn width on axial imaging). Gross-total resection (GTR) was defined as no radiologically visible tumor, near-total resection (NTR) was defined as a residual tumor < 1.5 cm in size, and subtotal resection (STR) was defined as a residual tumor > 1.5 cm in size. The study neurologist (R.B.K.), with more than 20 years of experience in neuro-oncology, reviewed all preoperative MRI scans while blinded to CMS status. The RM was then retrospectively applied to each patient to determine their predicted risk of developing CMS.
Surgical Approach
The study pediatric neurosurgeons (F.A.B. and P.K.) have an oncological surgery experience of more than 30 and 15 years. For tumors located within the fourth ventricle, surgeons at our institution use telovelar dissection. This approach facilitates exposure of the entire fourth ventricle, from the obex up to the aqueduct and both lateral recesses. The vermis is not resected except minimally in selected cases. Several common strategies are used to rapidly resect the tumor from within the fourth ventricle while minimizing retraction on the walls of the fourth ventricle. Fixed retractors are never used; handheld brain ribbons are used, if needed, but there is no prolonged retraction of normal tissue. Once the tumor is entered, speed of resection is critical. Depending on tumor consistency, a handheld sucker or an ultrasonic aspirator are used. Tumor bleeding is most effectively controlled by rapid extirpation, allowing the expanded walls of the fourth ventricle to come down in a controlled fashion, rather than with the use of retraction. If tumor is found to be adherent to the ependymal boundaries of the fourth ventricle (i.e., floor, lateral recess, and cerebellar peduncles), slow microdissection to minimize manipulation and judicious use of low-setting bipolar diathermy are employed. The tumor is never chased below the level of the ependyma. Minimal retraction of the cerebellum, including the vermis/roof, reduces the risk of injury to deep cerebellar nuclei (including the fastigial nucleus) and superior cerebellar peduncles.
Statistical Analysis
The incidence of CMS was summarized using exact binomial confidence intervals. Associations between categorical variables were tested using the Fisher exact test. Logistic regression approaches were used to study associations with continuous CMS scores; p < 0.05 was considered statistically significant.
Results
Forty participants (14 males and 26 females) met inclusion criteria for this study; 30 were included in an earlier publication of 178 trial participants.5 The median age at the time of tumor resection was 11.7 years (range 3.5–17.8 years). GTR was achieved in 35 (87.5%) patients, NTR in 4 (10%), and STR in 1 (2.5%). The median tumor volume was 51.97 cm3 (20.13–180.58 cm3). Tumors were midline in 30 (75%), right lateral in 6 (15%), and left lateral in 4 (10%). Tumor types included 21 non-WNT/non–sonic hedgehog (NWNS), 12 sonic hedgehog (SHH), and 7 WNT. Eighteen (45%) participants had a d(sagittal) ≥ 0.58 cm, indicating clinically significant invasion/compression of brainstem by the tumor. The median Evans index was 0.3 (range 0.2–0.4); 34 (85%) participants had an Evans index ≥ 0.3, indicating the presence of ventriculomegaly. Five participants required a ventricular shunt.
The median RM score for our cohort was 90 (range 25–146); 18 (45%) participants had a score ≥ 100 (Table 2). The distribution of participants with scores < 100 versus ≥ 100 was similar between the current cohort and the cohort on which the RM was based11 (p = 0.45). Of the 22 patients with an RM score < 100 in our cohort, 1 child developed CMS (4.5%, CI 0.1%–22.8%) and 3 of the 18 patients with an RM score ≥ 100 developed CMS (16.7%, CI 3.6%–41.4%). The anticipated rate number per RM was 12 participants (95% CI 9–14). The observed rate of CMS in our cohort of children with an RM score ≥ 100 was significantly lower compared with the observed rate in the original RM cohort11 (66.7%, CI 51%–80.0%; p < 0.001) (Fig. 2). There was no difference in the observed rate of CMS with RM < 100 in the two cohorts (1/22 vs 1/75, p = 0.40).
TABLE 2.
Demographics, tumor characteristics, and RM scores of patients with CMS and without CMS
| Variable | No. of Pts in the Cohort w/ CMS (n = 4) | Total Cohort (n = 40) |
|---|---|---|
| Sex |
|
|
| Male |
3 |
26 |
| Female |
1 |
14 |
| Median age at tumor resection, yrs (range) |
9.5 (3.5–17.8) |
11.7 (3.5–17.8) |
| IHC group |
|
|
| SHH, lt side |
0 |
4 |
| SHH, rt side |
0 |
6 |
| SHH, midline |
0 |
2 |
| WNT |
1 |
7 |
| NWNS |
3 |
21 |
| Extent of resection |
|
|
| GTR |
3 |
35 |
| NTR |
1 |
4 |
| STR |
0 |
1 |
| Median tumor vol, cm3 |
70.2 |
51.97 |
| Evans index ≥0.3 |
2 (50%) |
34 (85%) |
| Location |
|
|
| Midline |
4 |
30 |
| Rt cerebellar |
0 |
6 |
| Lt cerebellar |
0 |
4 |
| d(sagittal) |
|
|
| <0.58 |
0 |
22 |
| ≥0.58 |
4 |
18 |
| Ventricular shunting |
|
|
| Yes |
1 |
5 |
| No |
3 |
35 |
| RM score |
|
|
| 0–30 |
0 |
5 |
| 31–70 |
0 |
9 |
| 71–99 |
1 |
8 |
| ≥100 | 3 | 18 |
IHC = immunohistochemistry; pt = patient.
Values are presented as the number of patients unless stated otherwise.
FIG. 2.
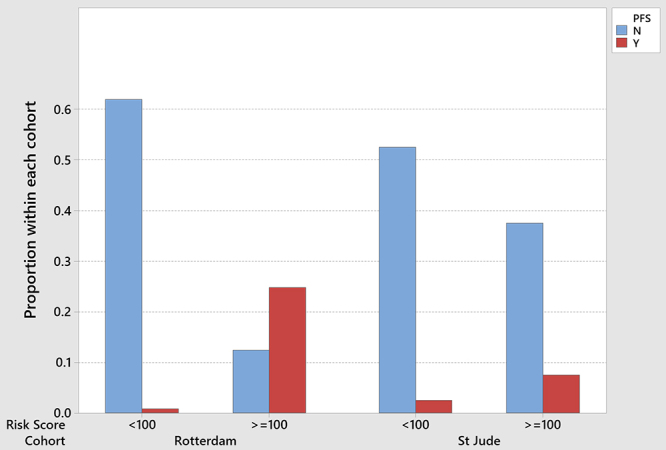
Comparison between the risk of CMS (posterior fossa syndrome [PFS]) in our and RM cohorts. The observed rate of CMS in our cohort of children with RM score ≥ 100 was significantly lower compared with the observed rate in the RM cohort, p < 0.001. There was no difference in the observed rate of CMS with RM < 100 in the two cohorts, p = 0.40. Figure is available in color online only.
All 4 (10%) participants who were diagnosed with CMS were classified as having CMS1, 3 had GTR, and 1 an NTR. Among these 4 patients, the median age at tumor resection was 9.5 years (range 3.5–17.8 years), the median tumor volume was 70.2 cm3 (46.1–111.75 cm3), and the tumor location was uniformly midline. Tumor types included 3 NWNS and 1 WNT. All 4 patients (100%) had a d(sagittal) ≥ 0.58 cm, 2 (50%) had an Evans index ≥ 0.3 (indicating the presence of significant ventriculomegaly), and 1 (25%) required a ventricular shunt. Speech returned in all 4 patients at a median of 28 days (range 20–67 days) after tumor resection and independent gait at 66 days (range 20–122 days). The RM scores of these 4 patients were 85, 125, 142, and 145. The child who experienced CMS with an RM of < 100 was an 11-year, 11-month-old male who underwent GTR of a midline fourth ventricular NWNS tumor. Tumor dimensions were 4.31 cm × 3.36 cm × 3.18 cm.
There was no difference in the rate of CMS in our cohort between those with an RM score < 100 versus those with an RM score ≥ 100 (p = 0.31). Given that the dichotomized RM score was not associated with the incidence of CMS in our cohort, we also investigated via single-parameter logistic regression whether continuous RM scores were associated with incidence of CMS, but no association was detected (p = 0.12).
Discussion
CMS can be a devastating complication of posterior fossa tumor surgery and continues to have a very high incidence in children undergoing surgery for medulloblastoma. Indeed, a recent large prospective study reported a 45% prevalence in children who underwent surgery for medulloblastoma.13 Yet, despite this high prevalence, there is little to no consensus on what causes the syndrome. Results evaluating different surgical approaches have been inconsistent, and the influence of surgical experience on risk for CMS has been debated. Most recently, the RM proposed an objective scoring system that assigns a risk score based on intrinsic tumor properties that does not take approach or surgical experience into account. However, our recent report suggested that patients seen in high-volume pediatric neurosurgical centers were less likely to develop CMS after medulloblastoma surgery and those who developed CMS had less severe symptoms.5 Moreover, an earlier report by Cobourn et al. also suggested that improving surgical techniques lowers the incidence of CMS.10 While it could be argued that the lower prevalence of CMS in these reports may be due to preponderance of low-risk patients according to the RM, we sought to test the generalizability of the RM and validity on our institution experience as a high-volume surgical center.
Among the 40 participants in our cohort, only 4 (10%) developed CMS, while 18 met criteria for high risk according to the RM, with RM scores ≥ 100. According to the RM, the estimated probability of developing CMS in the high-risk cohort is 66% and, from a cohort of 18, 12 (95% CI 9–14) would be expected to develop CMS. Therefore, the observed rate of CMS in our cohort was significantly lower than what would be expected per the RM. Furthermore, we did not observe a difference in the rate of CMS between the RM high-risk versus low-risk groups in our cohort (16.7% vs 4.5%, p = 0.31). These results suggest that the RM model overestimated the risk of CMS in our cohort, thus raising questions about its generalizability. Moreover, these results support our hypothesis that surgical experience may be a contributor to the risk of developing CMS.
The questions that should be asked are what surgical techniques, or maneuvers, are critical at reducing the risk of CMS, and can these be taught? There are many retrospective studies with divergent conclusions, but a large recent prospective study did not find that the telovelar approach was conclusively better at preventing CMS.13 Recent studies have suggested the importance of injury to the fastigial nucleus in the development of CMS.8,14 The fastigial nucleus is a paramedian nucleus in the anterior superior vermis just above the roof of the fourth ventricle and perhaps will be at higher risk of injury if a purely transvermian approach is used for tumor resection. Recent studies and our data do suggest that surgical technique, approach, and experience are important factors contributing to CMS development and its severity.5,10 In our earlier prospective study of CMS,5 almost half of those with CMS1 had not regained independent gait 1 year after the surgery and that most of these nonambulatory children underwent surgery at low-volume centers. Consistent with this, all 4 CMS1 children in this study regained speech and independent gait within 4 months of tumor resection.
Limitations of this study include a single-institution source, retrospective nature, relatively small numbers, and, especially, a small number of events (CMS). The small number of events is important, as more events need to happen to validate a model.15 However, brain tumors are rare in children, and this was a retrospective analysis of prospectively collected data in a clinical trial. Additionally, imaging reviewer was not blinded to tumor histology as all children had medulloblastoma. However, in modern imaging, the presence of diffusion restriction in medulloblastoma, along with other features, makes it easy to be strongly suspected in preoperative imaging.
Conclusions
We report a much lower rate of CMS at our institution in children that were classified as high risk for postoperative CMS by the RM, thus raising questions about generalizability of the RM. We also could not confirm the utility of the cutoff score of 100 in separating low- versus high-risk patients. This may be due to low number of events in our cohort and limited data set on which the RM development was based, especially as RM fails to account for surgeon’s experience and preoperative symptoms. Further studies are needed to identify variables that contribute to CMS and improve risk prediction.
Acknowledgments
This work was supported in part by the National Cancer Institute (St. Jude Cancer Center Support [CORE] grant no. P30-CA21765) and the American Lebanese Syrian Associated Charities.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Khan, Boop, Gajjar. Acquisition of data: Khan, Gajjar, Robinson. Analysis and interpretation of data: Khan, Klimo, Onar-Thomas, Boop, Gajjar, Robinson. Drafting the article: Bush, Klimo, Robinson. Critically revising the article: Khan, Bush, Klimo, Onar-Thomas, Gajjar, Robinson. Reviewed submitted version of manuscript: Khan, Klimo, Onar-Thomas, Huang, Boop, Gajjar, Robinson. Approved the final version of the manuscript on behalf of all authors: Khan. Statistical analysis: Onar-Thomas, Huang. Administrative/technical/material support: Gajjar. Study supervision: Khan, Boop.
Supplemental Information
Previous Presentations
These data were presented at the Posterior Fossa Society–First Global Meeting, Liverpool, United Kingdom, September 2022.
References
- 1. Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci. 2012;19(11):1541–1544. doi: 10.1016/j.jocn.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 2. Küper M, Timmann D. Cerebellar mutism. Brain Lang. 2013;127(3):327–333. doi: 10.1016/j.bandl.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 3. Renne B, Radic J, Agrawal D, et al. Cerebellar mutism after posterior fossa tumor resection in children: a multicenter international retrospective study to determine possible modifiable factors. Childs Nerv Syst. 2020;36(6):1159–1169. doi: 10.1007/s00381-019-04058-7. [DOI] [PubMed] [Google Scholar]
- 4. Gudrunardottir T, Morgan AT, Lux AL, et al. Consensus paper on post-operative pediatric cerebellar mutism syndrome: the Iceland Delphi results. Childs Nerv Syst. 2016;32(7):1195–1203. doi: 10.1007/s00381-016-3093-3. [DOI] [PubMed] [Google Scholar]
- 5. Khan RB, Patay Z, Klimo P, et al. Clinical features, neurologic recovery, and risk factors of postoperative posterior fossa syndrome and delayed recovery: a prospective study. Neuro Oncol. 2021;23(9):1586–1596. doi: 10.1093/neuonc/noab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schreiber JE, Palmer SL, Conklin HM, et al. Posterior fossa syndrome and long-term neuropsychological outcomes among children treated for medulloblastoma on a multi-institutional, prospective study. Neuro Oncol. 2017;19(12):1673–1682. doi: 10.1093/neuonc/nox135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morris EB, Phillips NS, Laningham FH, et al. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain. 2009;132(Pt 11):3087–3095. doi: 10.1093/brain/awp241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McAfee SS, Zhang S, Zou P, et al. Fastigial nuclei surgical damage and focal midbrain disruption implicate PAG survival circuits in cerebellar mutism syndrome. Neuro Oncol. 2023;25(2):375–385. doi: 10.1093/neuonc/noac168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAfee SS, Robinson G, Gajjar A, et al. Cerebellar mutism is linked to midbrain volatility and desynchronization from speech cortices Brain. Published online June 21, 2023 10.1093/brain/awad209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cobourn K, Marayati F, Tsering D, et al. Cerebellar mutism syndrome: current approaches to minimize risk for CMS. Childs Nerv Syst. 2020;36(6):1171–1179. doi: 10.1007/s00381-019-04240-x. [DOI] [PubMed] [Google Scholar]
- 11. Bae D, Mlc VV, Catsman-Berrevoets CE. Preoperative prediction of postoperative cerebellar mutism syndrome. Validation of existing MRI models and proposal of the new Rotterdam pCMS prediction model. Childs Nerv Syst. 2020;36(7):1471–1480. doi: 10.1007/s00381-020-04535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmitz-Hübsch T, du Montcel ST, Baliko L, et al. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006;66(11):1717–1720. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 13. Grønbæk JK, Wibroe M, Toescu S, et al. Postoperative speech impairment and surgical approach to posterior fossa tumours in children: a prospective European multicentre cohort study. Lancet Child Adolesc Health. 2021;5(11):814–824. doi: 10.1016/S2352-4642(21)00274-1. [DOI] [PubMed] [Google Scholar]
- 14. Jabarkheel R, Amayiri N, Yecies D, et al. Molecular correlates of cerebellar mutism syndrome in medulloblastoma. Neuro Oncol. 2020;22(2):290–297. doi: 10.1093/neuonc/noz158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vergouwe Y, Steyerberg EW, Eijkemans MJ, Habbema JD. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J Clin Epidemiol. 2005;58(5):475–483. doi: 10.1016/j.jclinepi.2004.06.017. [DOI] [PubMed] [Google Scholar]


