Abstract
Mass occurrences of Periphylla periphylla in Norwegian fjords cause major concerns related to potential regime shifts that could affect ecosystem stability. 15 years of trawl data (2006–2015), complemented with comprehensive sampling in different areas and seasons (2018–2021) allowed new insights on the dynamics, structure and connectivity of P. periphylla populations within and beyond Trondheimsfjorden. Despite assumed population bursts, no clear trend on P. periphylla population size in Trondheimsfjorden were identified. Sampling frequency and population size suggest a local reproduction of P. periphylla, especially in the inner part of the fjord where young-of-the-year (YOY) individuals occur. Size variations occurred in relation to sampling month, thus pointing at seasonal patterns in growth and reproduction. No distinct population structure of P. periphylla populations within Trondheimsfjorden and over larger spatial scales (> 100 km) along the Norwegian coast was observed. Such poor geographic population structure provides evidence for a strong dispersal of P. periphylla, potentially triggered by frequent deep-water renewals of the fjords’ basins that enable a high gene flow. Data on P. periphylla long-term dynamics, population structure and connectivity provide valuable information for ecosystem state assessments and enable the advancement of ecosystem management approaches, thus accounting for both stakeholder and ecosystem demands.
Keywords: connectivity, jellyfish bloom, population genetics, Scyphozoa, zooplankton, Coronatae
INTRODUCTION
Mass occurrences of jellyfish, both natural and anthropogenic driven, are considered a major challenge that can lead to a restructuring of pelagic ecosystems, clogging of power and desalination plant passages, and negatively impact fisheries, aquaculture and tourism (Baumann and Schernewski, 2012; Doney et al., 2012; Graham et al., 2014; Dong, 2018; Halsband 2018). Jellyfish blooms can especially affect enclosed or semi-enclosed ecosystems such as fjords due to bathymetric or topographic barriers that restrict water exchange, dispersal and distribution patterns with severe impacts on local scales (Mills, 2001). The origin of blooms is often unknown since specimens can be advected from adjacent waters into a given (fjord) ecosystem or originate from local populations, or a combination of these. Reliable estimates on jellyfish population dynamics and stock assessments are scarce thus hindering ecosystem-based management.
The helmet jellyfish Periphylla periphylla (Scyphozoa, Coronatae) shows a cosmopolitan distribution with major occurrence in the meso- and bathypelagic zones of the oceans (Arai, 1997; Morita et al., 2017; GBIF.org, 2022). It is considered a strong competitor for fish due to its high growth rates, long life spans and high reproductive success (Jarms et al., 1999; Bamstedt et al., 2020). In contrast to most other metagenetic scyphozoans, P. periphylla has a holoplanktonic life cycle with direct development where the planula, ephyra and polyp stages are missing (Jarms et al., 1999, 2002). The development of this species is divided into 14 stages (oldest stages 14 A-D) reaching maturity in development stage 14D with a coronal diameter (CD) > 7.5 cm and an age of ca. 3–4 years (Jarms et al., 2002). Despite its principal distribution in the deep oceans, diel vertical migration in P. periphylla is common and occurrences even close to the surface have been reported (Youngbluth and Bamstedt, 2001; Dupont et al., 2009; Geoffroy et al., 2018). From the early 1990 onwards, P. periphylla has been frequently reported in deep Norwegian fjords such as Lurefjorden, Sognefjorden, Halsafjorden, and Trondheimsfjorden with established, long-lasting aggregations. In these locations, mass occurrences of several orders of magnitudes higher than in the open ocean were documented (Fossa, 1992; Dalpadado et al., 1998; Jarms et al., 2002; Sornes et al., 2007; Tiller et al., 2017). Despite jellyfish blooms can vary both on a spatial and temporal scale, records of such year-around aggregation of jellyfish are not often reported. As these mass occurrences have been reported with northward shifts in distribution, P. periphylla has sometimes been reported as invasive species in the fjords (Tiller et al., 2015; Tiller et al., 2017). In Trondheimsfjorden, P. periphylla occurrence can be dated almost 100 years back, as specimens are documented in the collection of the NTNU University Museum, Trondheim, Norway (Bakken et al., 2023). Similar to other jellyfish bloom phenomena, considered to be a consequence of anthropogenic stressors i.e. climate change, overfishing (Attrill et al., 2007; Condon et al., 2012; Duarte et al., 2013), the observed northward shift of P. periphylla occurrence has been discussed in the light of global change (Tiller et al., 2017; Geoffroy et al., 2018). Whether observations of P. periphylla in areas further north reflect in fact a northward shift and, therefore, invasion of new areas or whether this is rather related to increased ecosystem surveillance and sampling frequency remains unknown.
Due to its potential threat to fjord ecosystems and fisheries, several previous studies have analysed life-history dynamics, reproductive success, behavior and impacts of P. periphylla populations on artisanal fisheries in Norwegian fjords (Jarms et al., 2002; Kaartvedt et al., 2015; Bozman et al., 2017; Tiller et al., 2017; Bamstedt et al., 2020). However, large uncertainties remain regarding its population dynamics, intra- and interannual variation, population structure and connectivity. To date, the main driving forces shaping P. periphylla populations (e.g. abiotic conditions, anthropogenic drivers) remain elusive.
Usually, low advection is considered as the main factor that causes intense blooms of P. periphylla (Sornes et al., 2007), leading to local reproduction and populations in different fjords. Thus, genetically different populations of the same species, even with small geographical distance, could exhibit differences in bloom timing and magnitude (Dawson et al., 2015). So far, local reproduction has been reported from Lurefjorden at the southern Norwegian coast from 1993 onwards. Here, small individuals (<1 cm) have been classified as the newest cohort and large specimens as the old cohorts (Jarms et al., 1999). In addition, eggs, and developmental stages of P. periphylla are reported from Lurefjorden from mid-1990 with presence of larvae throughout the year and eggs from summer to fall (Jarms et al., 1999). In Trondheimsfjorden, different size classes of P. periphylla medusae have been reported from all three fjord basins (Solheim, 2012; Jøssang, 2015). However, these studies point at a high share of both very small and very large individuals specifically in the inner fjord (Beistadfjorden, Verrasundet and Verrabotten), thus suggesting that the “mother” population of P. periphylla has established in inner Trondheimsfjorden and that major recruitment events happen there.
LOCAL REPRODUCTION AND CONNECTIVITY OF THE BLOOMS
By studying long-term dynamics and intra- and interannual variation of P. periphylla population size in Trondheimsfjorden over the past 15 years, insights on the population dynamics and potential trends in bloom frequency and intensity could be revealed. In addition, results on population genetics and size–weight relationships of P. periphylla can provide information on population structure and connectivity as well as life-history dynamics in Trondheimsfjorden and adjacent Norwegian waters.
The following hypotheses were addressed:
H1: P. periphylla population size has increased over the last 15 years.
H2: Local reproduction of P. periphylla is promoted in the inner part of Trondheimsfjorden where populations accumulate.H3:
H3: No clear population structure between P. periphylla from the inner part of Trondheimsfjorden compared with mid−/outer fjord areas and adjacent waters can be detected.
MATERIALS AND METHODS
Study area
Trondheimsfjorden is Norway’s third-longest fjord (total length: 126 km), situated at 63° north on the Norwegian west coast with a maximum depth of 617 m. It is characterised by three main sills that define the different areas of Trondheimsfjorden (Fig. 1): Agdenes sill (separating the fjord from the outer sea), Tautra sill and Skarnsund sill (dividing the fjord into three basins). The basins are Ytterfjorden (Outer fjord), Midtfjorden (Middle fjord) and Beistadfjorden (Inner fjord) (Bakken, 2000). Mixing and transport of water masses in Trondheimsfjorden is affected by wind, river run-off, tidal energy and inflow from the North Atlantic current and the Norwegian Coastal Current (Jacobson, 1983). As the sills in Trondheimsfjorden are quite deep (ranging from 90 to 195 m water depths), an exchange of bottom water masses usually happens twice a year from the North Atlantic via the Norwegian coastal current (Jacobson, 1983). First, from February to May to June, there is an inflow of high salinity Atlantic deep water that produces a new layer of bottom water in the fjord, driving the old water out of the fjord. Secondly, in the late summer period, there is an inflow of 32–34 saline Norwegian intermediate coastal water that mixes into the bottom water and slowly exchanges the old water (Jacobson, 1983). Rivers have a large effect on surface water mixing in Trondheimsfjorden when considering the multiple river sources.
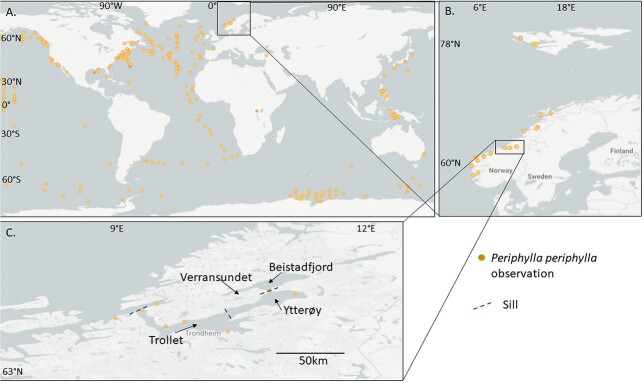
Fig. 1.
Location with P. periphylla observations. (A) Global observation based on GBIF database (accessed 1 December 2021) and (B) observations in Norway and Svalbard (accessed 1 December 2021) as well as (C) in Trondheimsfjorden. Sampling locations in the Outer, Middle and Inner fjord regions are depicted in (C) together with the locations of the three sills in Trondheimsfjorden (dashed lines).
Long-term P. periphylla sampling campaign
A series of research cruises with RV Gunnerus were conducted within the framework of several national projects between 2006 and 2015 (LTS, iKyst, Janus) as well as the EU-project GoJelly (2018–2021) where the helmet jellyfish P. periphylla was sampled for 15 years. From 2006–2021, bottom trawling using a shrimp trawl (inner lining of fine mesh net, stretched 36 mm mesh size) was conducted sporadically in the inner, mid, and outer part of Trondheimsfjorden at several fixed stations (see Table I). In Midfjorden and Ytterfjorden, trawling took place at the stations Stjørnfjorden, Trollet/Røberg, Tautra and Ytterøya, while the stations in the inner fjord were Beistadfjorden, Verrasundet and Verrabotten. The depth of bottom trawling varied depending on stations between a minimum depth of 50 m at Verrabotten and a maximum depth of 507 m in Trollet. Trawling activities took place throughout the year with major activities in the period from spring to autumn. Specimens of P. periphylla were quantified using volumetric measures (L) as an estimate for jellyfish biomass caught during each trawling event. For estimates on the changes in jellyfish biomass over time, the “catch-per-unit effort” (CPUE) for each trawling event was calculated. This was based on the volume of P. periphylla (L) caught during a specific bottom trawling per unit of time (min).
Table I.
Regions, specific locations, geographical coordinates, trawl dates and durations (min.) from bottom trawl deployments in Trondheimsfjorden in the period 2006–2021
| Region | Specific location | Trawl date | Geographic coordinates | Trawl duration (min.) | Total P. periphylla volume (L) | Total P. periphylla abundance (n) | |
|---|---|---|---|---|---|---|---|
| Outer fjord | Stjørnfjorden | 29 August 2018 | N63°43.850’ | E09°56.150’ | 15 | 7.5 | 17 |
| 21 September 2020 | N63°43.850’ | E09°56.150’ | 10 | 17.7 | 40 | ||
| Trollet | 29 August 2019 | N63°29.939’ | E10°13.288’ | 10 | 4 | 9 | |
| 23 September 2019 | N63°29.939’ | E10°13.288’ | 10 | 18.1 | 41 | ||
| 27 August 2020 | N63°29.939’ | E10°13.288’ | 5 | 215.5 | 487 | ||
| Røberg | 12 April 2007 | N63°28.88’ | E09°59.50’ | 10 | 0.4 | 1 | |
| Middle fjord | Tautra | 19 September 2018 | N63°41.312’ | E10°48.001’ | 20 | 14.2 | 32 |
| Tautra | 19 September 2018 | N63°41.312’ | E10°48.001’ | 20 | 14.2 | 32 | |
| 29 August 2019 | N63°41.376’ | E10°47.934’ | 10 | 6.2 | 14 | ||
| 23 September 2019 | N63°41.376’ | E10°47.934’ | 10 | 23.5 | 53 | ||
| Ytterøya | 16 April 2007 | N63°44.98’ | E11°06.55’ | 10 | 40 | ||
| 23 November 2010 | N63°44.98’ | E11°06.55’ | 45 | 1.8 | 4 | ||
| 11 November 2014 | N63°44.98’ | E11°06.55’ | 50 | 38.1 | 86 | ||
| 30 April 2018 | N63°44.98’ | E11°06.55’ | 30 | 69.90 | 158 | ||
| 04 July 2018 | N63°44.98’ | E11°06.55’ | 45 | 44.7 | 101 | ||
| 19 June 2019 | N63°44.98’ | E11°06.55’ | 20 | 6.6 | 15 | ||
| 15 June 2020 | N63°44.98’ | E11°06.55’ | 30 | 93.8 | 212 | ||
| 26 August 2020 | N63°44.98’ | E11°06.55’ | 14 | 15 | 34 | ||
| Inner fjord | Beistadfjorden | 19 April 2007 | N63°56.0198’ | E11°05.5610’ | 10 | 450 | |
| 25 June 2007 | N63°56.0198’ | E11°05.5610’ | 15 | 600 | |||
| 25 October 2007 | N63°56.0198’ | E11°05.5610’ | 10 | 2000 | |||
| 02 April 2008 | N63°56.0198’ | E11°05.5610’ | 30 | 9.3 | 21 | ||
| 02 April 2008 | N63°56.0198’ | E11°05.5610’ | 30 | 17.7 | 40 | ||
| 02 April 2008 | N63°56.0198’ | E11°05.5610’ | 20 | 100 | |||
| 03 April 2008 | N63°56.0198’ | E11°05.5610’ | 10 | 1000 | |||
| 25 March 2009 | N63°56.0198’ | E11°05.5610’ | 15 | 500 | |||
| 23 November 2010 | N63°56.0198’ | E11°05.5610’ | 15 | 1000 | |||
| 13 November 2012 | N63°56.0198’ | E11°05.5610’ | 10 | 600 | |||
| 05 February 2013 | N63°56.0198’ | E11°05.5610’ | 50 | 9.3 | 21 | ||
| 05 February 2013 | N63°56.0198’ | E11°05.5610’ | 10 | 600 | |||
| 09 April 2013 | N63°56.0198’ | E11°05.5610’ | 10 | 300 | |||
| 12 November 2013 | N63°56.0198’ | E11°05.5610’ | 10 | 1200 | |||
| 24 March 2014 | N63°56.0198’ | E11°05.5610’ | 10 | 1000 | |||
| 18 June 2014 | N63°56.0198’ | E11°05.5610’ | 10 | 600 | |||
| 10 November 2015 | N63°56.0198’ | E11°05.5610’ | 20 | 440 | |||
| 29 August 2019 | N63°56.0198’ | E11°05.5610’ | 10 | 62 | 140 | ||
| 27 August 2020 | N63°56.0198’ | E11°05.5610’ | 5 | 310.6 | 702 | ||
| Verrasundet | 24 October 2006 | N63°51.163’ | E10°44.006’ | 40 | 225.70 | 510 | |
| 25 October 2006 | N63°51.163’ | E10°44.006’ | 40 | 230.1 | 520 | ||
| 18 April 2007 | N63°51.163’ | E10°44.006’ | 10 | 750 | |||
| 24 October 2007 | N63°51.163’ | E10°44.006’ | 25 | 2000 | |||
| 01 April 2008 | N63°51.163’ | E10°44.006’ | 40 | 6000 | |||
| 25 March 2009 | N63°51.163’ | E10°44.006’ | 10 | 20 | |||
| 28 March 2011 | N63°51.163’ | E10°44.006’ | 10 | 800 | |||
| 10 April 2013 | N63°51.163’ | E10°44.006’ | 10 | 100 | |||
| 25 March 2014 | N63°51.163’ | E10°44.006’ | 20 | 1500 | |||
| 18 June 2014 | N63°51.163’ | E10°44.006’ | 10 | 700 | |||
| 11 November 2015 | N63°51.163’ | E10°44.006’ | 20 | 550 | |||
| 28 August 2019 | N63°51.163’ | E10°44.006’ | 10 | 110.6 | 250 | ||
| 26 August 2020 | N63°51.163’ | E10°44.006’ | 10 | 865.1 | 1955 | ||
| Verrabotten | 25 October 2006 | N63°49.106’ | E10°38.281’ | 46 | 79.70 | 180 | |
| 17 April 2007 | N63°49.106’ | E10°38.281’ | 51 | 3000 | |||
| 23 October 2007 | N63°49.106’ | E10°38.281’ | 20 | 1240 | |||
| 01 April 2008 | N63°49.106’ | E10°38.281’ | 41 | 520 | |||
| 28 March 2011 | N63°49.106’ | E10°38.281’ | 10 | 300 | |||
| 10 April 2013 | N63°49.106’ | E10°38.281’ | 10 | 200 | |||
| 15 November 2013 | N63°49.106’ | E10°38.281’ | 10 | 800 | |||
| 26 March 2014 | N63°49.106’ | E10°38.281’ | 10 | 2500 | |||
| 18 June 2014 | N63°49.106’ | E10°38.281’ | 10 | 3500 | |||
| 12 November 2015 | N63°49.106’ | E10°38.281’ | 20 | 850 | |||
| 26 August 2020 | N63°49.106’ | E10°38.281’ | 5 | 471.7 | 1066 | ||
| 29 September 2021 | N63°49.106’ | E10°38.281’ | 7 | 497.8 | 1125 | ||
Total Periphylla periphylla volume (L−1) and abundance (n) are provided.
Size and weight estimates of P. periphylla
During the sampling campaigns from 2018 to 2021, a subset of 30 large (> 10 cm CD) and 20 small (< 10 cm CD) individuals of P. periphylla were randomly picked from each catch for size and weight estimations. Sizes of P. periphylla were measured using the CD rounded to the nearest centimeter without decimal point and biomass was achieved by weighing individuals on a Marel M1100 scale (> 1 g) right after trawling. Individual developmental stages were assigned based on size according to the classification of Jarms et al. (2002). Stages from 1 to 8 (< 6 mm CD) were considered as embryonic developmental stages, stages 9-14C as “immature” (6–75 mm CD) and stage 14D (> 75 mm CD) as “mature” (Jarms et al., 2002). Individuals < 1 cm (size range between embryonal developmental stage and immature) correspond to an age class of < 1 year (YOY: young-of-the-year). Tissue pieces from the outer umbrella of P. periphylla were placed in Ethanol (96%) in microcentrifuge tubes and stored at room temperature until further DNA extraction and population genetic analysis. A power function was fitted to the size–weight relationship. A linear mixed effect model by the lme4 package (Bates et al., 2015) was used to partition size variances between individuals based on sampling location and time (year, month).
DNA extraction and sequencing
DNA was extracted from in total 190 randomly selected medusae presenting 15–30 specimens per location per sampling day. Small pieces of jelly tissue, preferably some red tissue, were put into 1.5 (2) ml microcentrifuge tubes and left in the fume hood at room temperature overnight to allow the ethanol to evaporate. Cytochrome c oxidase subunit 1 (mtCOI) was selected as a suitable marker. As a basic DNA barcoding region, this mitochondrial marker allows a great deal of information to be gathered and comparisons to be made with many other scyphozoan species for which population genetics data sets exist (Holland et al., 2004; Dawson, 2005; Prieto et al., 2013). DNA extraction was performed by use of (i) modified Chelex rapid-boiling procedure as explained in Granhag et al. (2012), (ii) EZNA mollusk DNA kit and (iii) Qiagen DNeasy kit, following the manufacturer’s instructions. mtCOI amplifications were performed with a variety of primers: HCO2198 and LCO1490 (Folmer et al., 1994), ChryAtlanF1 and ChryAtlanR1 (Abboud et al., 2018), AnthoF1 and AnthoR1 together with QuantaBio and Phire ® Hot Start DNA polymerases and adjusted PCR programs. Due to the generally low success rate, the best working protocol was by the use of Qiagen DNeasy kit, scyphozoan-specific AnthoF1 and AnthoR1 primers with PCR reaction mix of 20 μL and cycling regime of 5 min at 95°C followed by 40 cycles of 94°C (30 s), 54°C (30 s), 72°C (60 s), with a final extension for 7 min at 72°C. PCR products were verified by electrophoresis on an agarose gel (1.5%) in 1xTAE buffer.
PCR products were purified using Illustra GFX PCR DNA and gel band purification kit following the cleaning procedure recommended by the manufacturer. All PCR products were sequenced by commercial service (Eurofins Sequencing Service, Germany, and Macrogen Europe, Netherlands) using the same primer pairs as described above. The resulting nucleotide sequence electropherograms were checked by eye for poor base calls and sequence quality using Chromas Lite 2.1 (Technelysium Pty Ltd). The good-quality sequences were assembled using BioEdit software (Hall, 1999). All publicly available P. periphylla sequences from GenBank and Bold (mined 03032021) were combined with our sequences and aligned with the MAFFT online service (Katoh et al., 2019). The sequences were aligned using Q-INS-i strategy, which takes RNA secondary structure into account and gap-opening penalty of 1.53 and gap extension penalty of 0.123. The alignments were visually checked, identical sequences were removed, and poorly aligned regions were excluded prior to the analyses. The alignments are available on request. The sequences reported in this paper have been deposited in the European Molecular Biology Laboratory Nucleotide Sequence Database (GenBank Accession numbers: OY764966-OY764990). Bayesian phylogenetic analyses were performed with MrBayes 3.2.7a (Ronquist et al., 2012). Two independent runs with four Markov chains and 1 600 000 generations were carried out (average standard deviation of split frequencies 0.0094). The model was not chosen prior to the analysis but sampled across the GTR model space with gamma-distributed rate variation across sites and a proportion of invariable sites. The resulting estimates (e.g. tree topology) were posterior probability weighted averages of the models. Maximum likelihood bootstrap support values were calculated from 1000 replicates, using GARLI 2.0.1019 (Zwickl, 2006) with jModelTest 0.1.1 (Posada, 2008) AICc criterion selected model (TIM2 + I + G).
Population genetic analysis
Estimates of genetic variation were obtained for all the samples as well as for samples grouped by different seasons and geographical areas. The main genetic structure and differentiation analysis were calculated, for example, the nucleotide diversity (p) and haplotype diversity (h) were estimated using the program DnaSP v5 (Librado & Rozas, 2009) and genetic differentiation was calculated by means of pairwise FST values using 10 000 permutations in ARLEQUIN 3.1 (Excoffier et al., 1992) within the analysis of molecular variance (AMOVA) framework (Excoffier et al., 1992). A median-joining network showing the relationships between the mtDNA haplotypes was constructed using the PopART (http://popart.otago.ac.nz/howtocite.shtml;Bandelt et al., 2000). Tests for population expansion based on Tajima’s D and Fu’s FS (Ramos-Onsins and Rozas, 2002) were carried out using DnaSP v5. Significance levels were corrected using Bonferroni correction.
RESULTS
Periphylla periphylla population size
Overall, P. periphylla was caught at all stations within Trondheimsfjorden. Abundance of P. periphylla in the inner part of the fjord was higher compared with the middle and outer part of the fjord. The highest CPUE of 350 P. periphylla (L min−1) was estimated in June 2014 in the inner fjord (Verrabotten, Fig. 2A). In general, no temporal trend of biomass increases or decreases of P. periphylla was observed in the inner part of Trondheimsfjorden (Fig. 2A). The amount of P. periphylla caught in the middle and outer fjord (Fig. 2B) was 100 times lower compared with the inner fjord. No differences in biomass were observed between stations in the outer and middle fjord and no clear temporal trend was observed over the last 15 years.
Fig. 2.
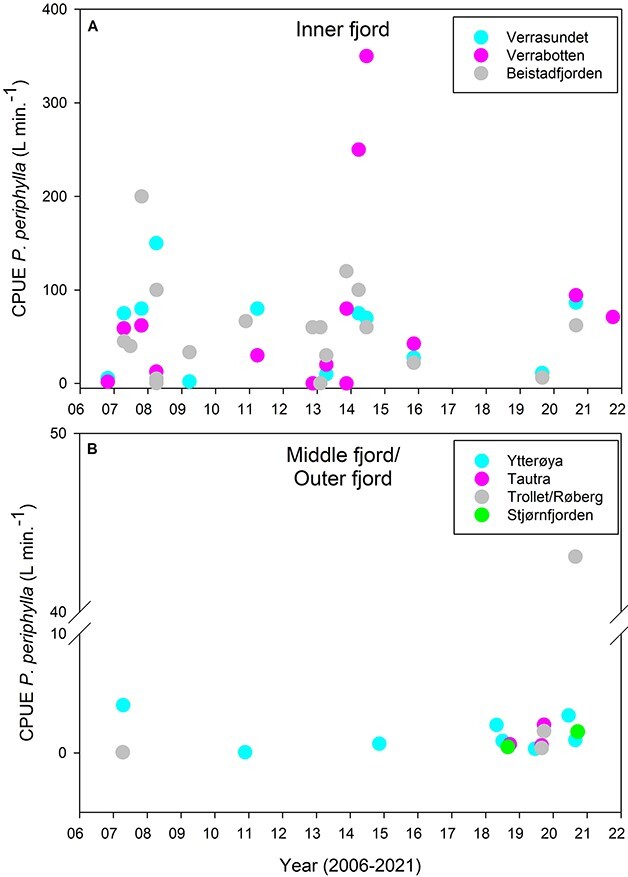
P. periphylla catches (L min.−1) calculated as CPUE during bottom trawl activities in Trondheimsfjorden in the period 2006–2021 in the Inner fjord (stations Verrasundet, Verrabotten and Beistadfjorden) (A) and Middle and Outer fjord (Ytterøya, Tautra, Trollet/Røberg and Stjørnfjorden) (B).
Size and age distribution
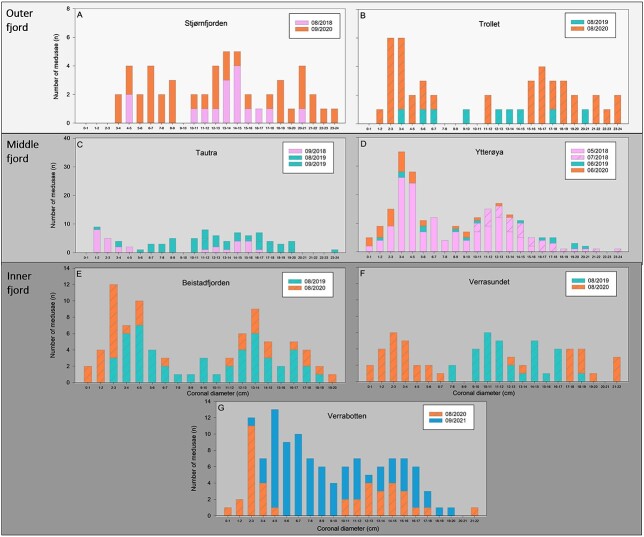
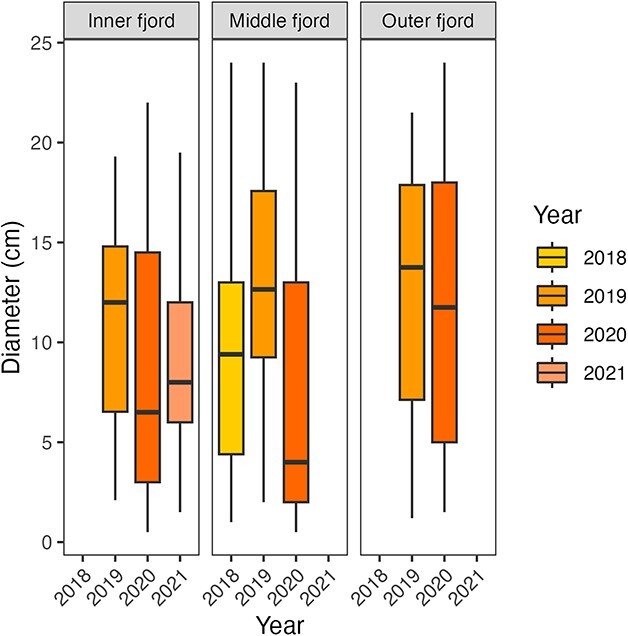
Specimens collected during cruises from 2018 to 2021 in Trondheimsfjorden varied in size between locations and years, with an average size in the category of mature specimen of 9.0 cm ± 5.1 in 2018 (n = 248), 12.1 cm ± 5.3 in 2019 (n = 222) and 9.13 cm ± 6.9 in 2020 (n = 293) (Fig. 3). The outer fjord stations were characterised by a complete lack of individuals < 1 cm corresponding to an age class of < 1 year (young-of-the-year, YOY). Some immature P. periphylla (< 7.5 cm) were found at the outer fjord stations, especially in 2020, while the majority of specimens where mature medusae > 7.5 cm with individuals up to 24 cm CD. Overall, very large P. periphylla (CD > 22 cm) were only found at the middle and outer fjord stations (Fig. 3D–G). The two stations sampled in the middle fjord showed differences in size distribution. While at Tautra, specimens < 1 cm (< 1 year of age) were missing (Fig. 3C), some YOY medusae were found at Ytterøya in 2018 (May) and 2020 (June) (Fig. 3D). While a relative even distribution of size classes from 1 to 20 cm was observed at Tautra, the majority of medusae found at Ytterøya belonged to the embryonal developmental stage or were immature < 7.5 cm (ca. 1–4 years of age). This was particularly true for 2018 (May) and 2020 (June). In 2018, mature specimens (> 7.5 cm) occurred with a majority in the size categories 8–15 cm CD and up to a maximum of 24 cm CD. In the inner fjord, YOY P. periphylla (< 1 cm) were found at all three stations (Fig. 3E–G) but only in 2020 and at low numbers. Overall, the majority of individuals were in the immature/embryonal developmental stage in 2020 with a CD < 7 cm or mature specimens with a CD > 10 cm at all three stations. The intermediate size classes from 7 to 11 cm were entirely missing in 2020 while they were present in 2019 (Beistadfjorden, Verrasundet) and 2021 (Verrabotten). The maximum size of P. periphylla specimens in the inner fjord was 20 (Beistadfjorden) and 22 cm (Verrasundet, Verrabotten).
Fig. 3.
Size distribution of P. periphylla medusae caught in Trondheimsfjorden during bottom trawling activities at the Outer fjord stations Stjørnfjorden (A) and Trollet (B), the Middle fjord stations Tautra (C) and Ytterøya (D), and the Inner fjord stations Beistadfjorden (E), Verrasundet (F) and Verrabotten (G) during the period of spring to autumn (2018–2021).
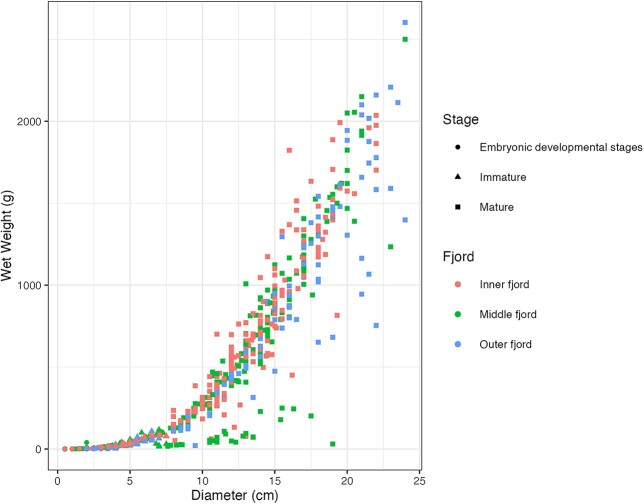
Size–weight relationship
The CD and the wet weight of the P. periphylla medusae sampled in three different areas of Trondheimsfjorden showed an exponential relationship described by the function y = 0.31 x2.92 for individuals in the inner fjord (red color-coded individuals; Fig. 4) and y = 0.58 x2.56 for individuals sampled in the outer and middle fjord (blue and green color-coded individuals; Fig. 4). For both functions, between 89 and 91% of the total variation in weight could be explained by its relation to diameter. Partitioning the data further into fjord location and year did not indicate any population size trend (either decreasing or increasing) over the years (Fig. 4). To investigate the effect of location on the size of P. periphylla, we fitted a random intercept model of scaled and centered P. periphylla diameters with crossed random effects of year and month, using the lmer function from the lme4 package. Our results revealed that 23% of the variance in the data can be explained by the random effect month meanwhile year had negligible effect (< 0.05%; Fig. 5). However, what causes the majority (77%) of the variance is unknown. Our results showed the presence of smaller individuals (β = −0.23 +/− 0.28) in the inner part of the fjord meanwhile the middle and the outer fjord showed the presence of bigger individuals (β = 0.35 +/− 0.14 for the middle fjord and β = 0.32 +/− 0.12 for the outer fjord). These findings confirm our H2 hypothesis indicating that the inner part of the fjord harbors smaller individuals indicating local reproduction.
Fig. 4.
Coronal diameter (CD in cm) to wet weight (g) relationship of P. periphylla medusae sampled during bottom trawling activities in three different areas of Trondheimsfjorden: Inner fjord (left panel), Middle fjord (intermediate panel) and Outer fjord (right panel) in the years 2018–2021. According to size, specimens were categorised as “embryonic developmental stage” (CD < 6 mm; dots), “immature” (CD: 6 to 57.3 ± 17.8 mm; triangles) and “mature” (>75.1 mm; squares) following data provided by Jarms et al. (2002).
Fig. 5.
Coronal diameter (CD in cm) of P. periphylla medusae sampled during bottom trawling activities in three different areas of Trondheimsfjorden (Inner fjord, Middle fjord and Outer fjord) in the years 2018–2021.
Genetic structure and differentiation
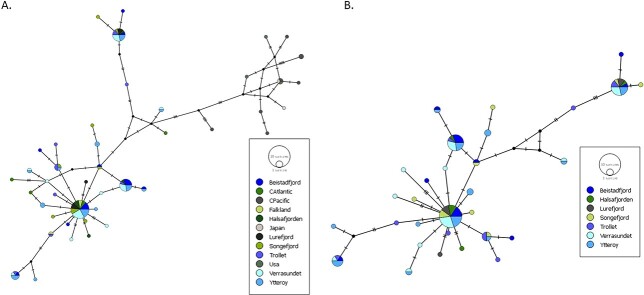
In general, the success rate of COI sequencing was low. The 190 P. periphylla individuals used for molecular analyses resulted in 93 P. periphylla good-quality sequences for the COI region (alignment of 552 bp). On a global scale, 114 specimens with 42 haplotypes defined by 52 segregating sites, of which 32 were parsimony informative, were recorded (Fig. 6A). In Norway, 30 haplotypes defined by 37 segregating sites, of which 20 were parsimony informative, were recorded (Fig. 6B). The haplotype network showed that the most frequently found haplotype occurred at various locations globally and in Norway. Verrasundet, the inner part of Trondheimsfjorden, shared the least haplotypes with other areas. Singletons from the same geographic regions did not cluster in monophyletic groups, while some haplotypes from the Pacific side of Canada and US regions did. In Norway, haplotype richness was high (h = 0.884 ± 0.00046), but differentiation among haplotypes was modest (π = 0.00771, Table II). The highest haplotype richness was calculated in Trollet, Trondheimsfjorden (0.96) and Sognefjorden (0.93) and lowest in Lurefjorden (0.73) whereas the highest COI nucleotide diversity was calculated in Beistadfjorden, Trondheimsfjorden (1%) and the lowest in Lurefjorden (0.68%). The pairwise FST values here indicated significant population differentiation between Trollet and Verrasundet, Ytterøya and Verrasundet in Trondheimsfjorden, and Lurefjorden and Sognefjorden, but after Bonferoni correction, no significant population differentiation was detected (Table III).
Fig. 6.
(A) Median-joining network within P. periphylla showing the relationships between the 42 haplotypes found globally detected by sequencing the mitochondrial DNA cytochrome oxidase I (COI) region and (B) median-joining network within P. periphylla showing the relationships between the 30 haplotypes found in Norway detected by sequencing the mitochondrial DNA COI region. Circle sizes are approximately proportional to haplotype frequency: the smallest circle represents a single individual; the largest circle represents 25 individuals. Each connection represents a single mutation and small open black dots represent missing intermediate haplotypes.
Table II.
Sample sizes and standard diversity indices for COI sequences of P. periphylla medusae sampled in seven different areas in Norway
| Area | Population | N | Nh | H ± sd | π |
|---|---|---|---|---|---|
| Trondheimsfjord | Beistadfjord | 15 | 9 | 0.89 ± 0.06 | 0.99 |
| ¨ | Trollet | 10 | 8 | 0.96 ± 0.06 | 0.82 |
| ¨ | Ytterøy | 24 | 12 | 0.92 ± 0.03 | 0.73 |
| ¨ | Verrasundet | 22 | 10 | 0.87 ± 0.06 | 0.7 |
| Halsafjord | Halsafjord | 6 | 4 | 0.80 ± 0.17 | 0.89 |
| Lurefjord | Lurefjord | 6 | 3 | 0.73 ± 0.16 | 0.68 |
| Sognefjord | Sognefjord | 10 | 8 | 0.93 ± 0.08 | 0.99 |
Seventy-eight sequences from this study and 15 sequences obtained from Bold were analysed. The sample size (N), the number of haplotypes per location (Nh), haplotype diversity (H) and nucleotide diversity (also called heterozygosity, π) are shown.
Table III.
Pairwise FST values between geographic regions based on 10 000 permutations
| Population | Beistadfjorden | Trollet | Ytterøy | Verrasundet | Sognefjorden | Lurefjorden | Halsafjorden |
|---|---|---|---|---|---|---|---|
| Beistadfjorden | |||||||
| Trollet | 0.05 | ||||||
| Ytterøy | 0.05 | 0.07 | |||||
| Verrasundet | −0.01 | 0.12* | 0.13* | ||||
| Sognefjorden | 0.04 | 0.00 | 0.09* | 0.04 | |||
| Lurefjorden | 0.06 | 0.04 | 0.20* | 0.03 | −0.08 | ||
| Halsafjorden | −0.02 | −0.03 | 0.03 | −0.01 | −0.10 | −0.10 |
* indicates significant differentiation uncorrected P < 0.05.
DISCUSSION
Long-term trends in P. periphylla population size
Reliable predictions on jellyfish bloom intensities and frequencies are limited by a lack of knowledge related to their population and life-history dynamics. This is mainly due to a lack of consistent data on jellyfish population size, reproduction rates, mortality and spatiotemporal distributions. Moreover, intra- and inter-annual variations are usually based on snapshot surveys at a low spatiotemporal resolution or are completely missing. In the last decades, progress has been made, especially thanks to recent efforts to collect time series data and citizen science observations (Condon et al., 2013).
In the present study, no significant increase in P. periphylla population size in Trondheimsfjorden was found over the 15 years examined. The CPUE remained at similar levels throughout the period 2006–2021 and no clear trends could be detected thereby rejecting our first hypothesis (H1). This is in contrast to previous studies that reported on a strong decline in CPUE for cod in Trondheimsfjorden during the period 2007–2014, followed by an increase in CPUE for P. periphylla (Tiller et al., 2015). The possibility of mass occurrences of P. periphylla in Trondheimsfjorden raised major concerns as it could potentially lead to a regime shift from a fish- to a jellyfish-dominated ecosystem (Tiller et al., 2014, 2015). These studies focused on the socioeconomic consequences related to the dominance of P. periphylla including declines in fish stocks, challenges for ecosystem management and negative effects on local fisheries, specifically in the innermost parts of the fjord. Based on our CPUE data, strong variation between sampling years and locations in Trondheimsfjorden occurred during the last 15 years with 100-fold higher CPUE obtained in the innermost part of Trondheimsfjorden compared with middle and outer fjord regions. Although this dataset is based on snap-shot sampling and doesn’t tackle entirely patchy and seasonal bloom distribution patterns, some general trends, i.e. on biomass accumulation and life-history dynamics of P. periphylla populations specifically in the innermost parts of the fjord were obtained. The causes and consequences of these findings are discussed in detail in the context of water-mass exchange, fjord topography, advection/retention times and recruitment success in the following discussion sections.
Recruitment and reproduction success of P. Periphylla in Trondheimsfjorden
The helmet jellyfish P. periphylla is characterised by a holopelagic life cycle reproducing sexually and the development from egg to medusa happens directly without including the intermediate planula, polyp and ephyra stages (Jarms et al., 1999, 2002). Little is known about its reproduction rate and lifespan, although a high longevity of this species has previously been assumed (Jarms et al., 1999). Overall, P. periphylla individuals in Trondheimsfjorden showed a wide size distribution with large specimens (CD > 20 cm) found in all three regions and YOY specimens (< 1 cm) found in the middle and inner part of the fjord. Maximum sizes of P. periphylla individuals in Trondheimsfjorden can be considered as large compared with other Norwegian fjords (Bamstedt et al., 2020) and other Atlantic regions (Lucas and Reed, 2010). Since fecundity can be related to female size to some degree (Bamstedt, 2022), the occurrence of large specimens (> 20 cm CD) might provide indication for local reproduction of P. periphylla in Trondheimsfjorden. Young recruits (< 7.5 cm CD) were well represented in all three fjord regions, often even dominating the population. This further supports the assumption of local reproduction and self-sustaining population of P. periphylla in Trondheimsfjorden thus confirming our second hypothesis (H2).
Overall, the size–weight relationship of P. periphylla could be best described by a power function with an exponent of 2.68. This is similar to the equation provided earlier on the size–weight relationship of P. periphylla with an exponent of 2.98 (Bamstedt, 2022). However, size–weight relationships of populations in the middle and outer fjord area deviated significantly from the ones in the inner fjord showing low weights relative to CD size. The size–weight relationships found for inner fjord populations could best be described by a power function with an exponent of 2.92. The findings in this study were in accordance with the relationship previously identified for P. periphylla in Norwegian fjords (Bamstedt, 2022). Between- and within-fjord variations in the size–weight relationship of populations could result from changes in biotic and abiotic conditions that can affect P. periphylla condition and growth. Such alterations in environmental conditions are, for example, considered to induce mass decay in P. periphylla biomass (Bozman et al., 2018) or parasitic infestation (Solheim, 2012).
The inner fjord: a hotspot of P. periphylla reproduction?
Biomass accumulation and retention times of P. periphylla in fjords are considered to be directly related to basin topography, sill depth, water exchange rates, light attenuation and vertical migration behavior (Youngbluth and Bamstedt, 2001; Sornes et al., 2007). Trondheimsfjorden is considered a P. periphylla hotspot with high population size reported from 2002 onwards, especially in the innermost fjord regions (Tiller et al., 2017). Due to a strong accumulation/retention and broad size distribution ranges, the innermost fjord is considered to harbor it’s “mother population” and most recruitment is assumed to happen there (Solheim, 2012). In our study, a size-dependency of P. periphylla in the outer, middle and inner fjord was detected showing a higher proportion of small-sized individuals in the inner fjord. YOY size classes of P. periphylla (< 1 cm, stage 9–11, < 1 year of age) were sampled mainly at the inner fjord stations Verrasundet, Verrasundet and Beistadfjorden and one innermost station in the middle fjord (Ytterøya). The presence of very small specimens (<1 cm) points at a local reproduction of P. periphylla in the inner part of Trondheimsfjorden where populations accumulate, thus confirming hypothesis 2.
Overall, a wide range of P. periphylla size classes with a total size spectrum of medusae ranging from 0 to 24 cm CD could be sampled in Trondheimsfjorden during the period 2018–2021. Despite the fact that the current dataset has its constraints regarding spatiotemporal resolution, it provides snapshots of P. periphylla occurrence, population structure and connectivity from multiple sampling years and fjord locations. The fact that a large proportion of small specimens (size class < 7 cm) was documented in our study (in various years and locations) provides indication for recruitment success of P. periphylla in Trondheimsfjorden. This is in contrast to i.e. Lurefjorden where a 3-year recruitment failure was documented by the lack of young recruits (Bamstedt, 2022). Short intervals of deep-water renewal of fjord basins are considered a possible cause for recruitment failure in P. periphylla (Bamstedt, 2022) due to the fact that eggs and embryonal developmental stages thrive in the deep layers of the fjords. Thus, short intervals of deep-water replacement can lead to a continuous flushing out of young recruits. In Trondheimsfjorden, the intervals of deep-water renewal are subject to seasonal inflow and replacement of bottom water in all three deep basins each spring (inner, middle and outer fjord basins) (Jacobson, 1983). However, the replacement of bottom water in the inner fjord usually lags behind due to the small cross-section and shallow sill at Skarnsund that function as a barrier for water exchange (Jacobson, 1983). The fact that most young recruits (<1 cm, < 1 year) of P. periphylla were found at Beistadfjorden, Verrasundet and Verrabotten in 2020 could point at a reduced deep-water renewal in the inner fjord in this specific year. Further, the overall absence of YOY in the outer fjord regions (incl. Tautra) could be the result of short deep-water renewal intervals that are known to affect local recruitment (Bamstedt, 2022). This could result in a continuous flushing of eggs and young recruits from the outer deep basins and an enhanced dispersal of P. periphylla out of Trondheimsfjorden and into adjacent waters resulting in a northward advection via the Norwegian Coastal Current (Tiller et al., 2017).
Despite strong indication for a continuous reproduction in this coronate species throughout the year (Tiemann and Jarms, 2010; Bamstedt et al., 2020), we found that part of the variation (31%) in CD size could be attributed to the sampling month and deviations in the size–weight relationship for specific age groups. This could point to some degree of seasonality in P. periphylla reproduction in Trondheimsfjorden, similar to what has been observed in Vefsnfjorden in Northern Norway (Bozman et al., 2018). Mortality might also change with season in relation to environmental drivers that can affect P. periphylla growth, survival and performance in specific years and seasons when e.g. mass mortalities (Bozman et al., 2018), parasitic infestation (Solheim, 2012) and nutritional constraints (Sornes et al., 2008) occur. So far, the role of predation by other vertebrate and invertebrate species on P. periphylla and their impact on population regulation are understudied. However, there is indication that intense predation events e.g. by anemones happen, as have been observed in Lurefjorden and Sognefjorden (Jarms and Tiemann, 2004) as well as in Trondheimsfjorden (Jarnegren, pers. comm.).
Population structure and connectivity of P. periphylla in Trondheimsfjorden and adjacent waters
The most consistent result of the COI analyses of P. periphylla is that no clear population structure was detected among the different areas inside Trondheimsfjorden or between Trondheimsfjorden and the more oceanic areas outside the fjord. This finding suggests the presence of mixing populations and is in agreement with H3 of our initial hypotheses. Despite a generally low success rate in sequencing and use of single mitochondrial marker, such a low geographic population structure might be due to strong dispersal and frequent deep-water renewal of the fjord basins that keep populations well-mixed and suggest a high gene flow.
The ecological and biological traits of P. periphylla, such as opportunistic feeding on almost all zooplankton groups, an extended breeding period with solely holoplanktonic life cycle (Fossa, 1992; Jarms et al., 1999; Youngbluth and Bamstedt, 2001) and survival in a wide range of environmental conditions are all factors known to support dispersal and admixture. Similar results have been found for other jellyfish species sharing the same traits, such as Pelagia noctiluca (Stopar et al., 2010). In contrast, more geographically structured intraspecific phylogenies have been detected from jellyfish taxa that show a metagenetic life cycle including a sessile polyp phase (Schroth et al., 2002).
Advective water-mass exchange between the fjord and the open sea has been speculated to be a crucial factor with a strong potential to affect fjord populations. For example, shallow sills at the mouths of fjords have been suggested to restrict gene flow of the mesopelagic fish Benthosema glaciale commonly found in the Norwegian Sea and several west Norwegian fjords (Suneetha and Salvanes, 2001; Kristoffersen and Salvanes, 2009). For P. periphylla, the study by Sørnes et al. (Sornes et al., 2007) presented a model to explain P. periphylla retention in three Norwegian fjords. This model was based on vertical distribution, advection and light attenuation as a governing factor for the vertical distribution. Although the model gives a logical explanation of why the three fjords can have a sustainable population of P. periphylla, it does not explain the mechanism behind the strong population differences in abundance and size distribution between the studied fjords (Sornes et al., 2007). In Trondheimsfjorden, the small genetic diversity and high proportion of small specimens detected in the inner fjord point at a true bloom resulting from local reproduction (Graham et al., 2001), whereas more mixed areas in the outer and middle fjord (e.g. Stjørnfjorden, Trollet, Tautra) promote a mixture of true and apparent aggregations, resulting from the combination of physical advection/retention and local reproduction. This could explain population variations between different areas in the fjords. However, it is important to keep in mind that lack of population structure does not necessarily imply demographic connectivity across the areas (Lowe and Allendorf, 2010; Drake et al., 2022). Hence, more detailed analysis would be needed.
The present study provides new insights into the fine scale population structure and origin of jellyfish blooms within one fjord as well as between different fjord ecosystems. By using a combination of field observation, modeling and molecular tools, the study contributes to a better understanding on the factors that trigger jellyfish bloom formation, population structure and connectivity in fjord ecosystems and adjacent waters, thus enhancing our knowledge on jellyfish bloom dynamics in marine ecosystems.
CONCLUSION
Fjord ecosystems provide unique habitats that are of high ecological and economical relevance and, at the same time, are prone to a variety of anthropogenic stressors (i.e. climate change, pollution, overfishing). Using a combination of approaches including time-series trawl data, sampling at high spatio-temporal resolution during recent years and population genetics provided insights into the dynamics, structure and connectivity of the helmet jellyfish (P. periphylla) in Trondheimsfjorden and adjacent waters. Such knowledge on ecosystem dynamics and environmental status of fjords over longer timescales are essential for the development of reliable and sustainable ecosystem management strategies thus advancing management approaches that meet ecosystems’ and stakeholders’ demands.
ACKNOWLEDGEMENTS
Professor Jarle Mork (1946–2019) is acknowledged for his dedicated and comprehensive research on P. periphylla populations in Trondheimsfjorden and his continuous efforts on data collection during the past decades. Discussions with Jarle stimulated the present study. His hands-on support and recommendations during the initial phase of GoJelly are highly appreciated. We thank the crew of RV Gunnerus for their excellent service and cooperation that enabled the compilation of this dataset.
Contributor Information
Nicole Aberle, Trondhjem Biological Station, Department of Biology Norwegian University of Science and Technology (NTNU), Trondheim 7012, Norway; Institute of Marine Ecosystem and Fishery Science (IMF), Hamburg University, Hamburg 20148, Germany.
Charlotte Volpe, Trondhjem Biological Station, Department of Biology Norwegian University of Science and Technology (NTNU), Trondheim 7012, Norway; Fisheries and New Biomarine Industry, SINTEF Ocean, Trondheim 7465, Norway.
Mari-Ann Østensen, Trondhjem Biological Station, Department of Biology Norwegian University of Science and Technology (NTNU), Trondheim 7012, Norway.
Sanna Majaneva, Trondhjem Biological Station, Department of Biology Norwegian University of Science and Technology (NTNU), Trondheim 7012, Norway; Ecosystems, Akvaplan Niva, Trondheim 7010, Norway.
AUTHOR CONTRIBUTIONS
NA and SM conceptualized and designed the study with input from all co-authors. NA, M-AØ and SM were involved in the planning and performance of field work activities. SM and M-AØ performed the molecular analyses. NA and M-AØ compiled the long-term dataset from hand-written protocols. CV, NA and SM performed data analyses and interpretation. NA wrote the manuscript with contributions from CV, SM and M-AØ. All authors contributed to the article and approved the submitted version.
FUNDING
European Union’s Horizon 2020 research and innovation program (Grant agreement no. 774499) as part of GoJelly (work package 2: “Driving mechanisms and predictions of jellyfish blooms”).
DATA AVAILABILITY
The sequences reported in the study have been deposited in the European Nucleotide Archive repository with accession numbers: OY764966-OY764990.
References
- Abboud, S. S., Gómez Daglio, L. and Dawson, M. N. (2018) A global estimate of genetic and geographic differentiation in macromedusae - implications for identifying the causes of jellyfish blooms. Mar. Ecol. Prog. Ser., 591, 199–216. 10.3354/meps12521. [DOI] [Google Scholar]
- Arai, M. N. (1997) A Functional Biology of Scyphozoa, Vol. 6, Chapman & Hall, London. [Google Scholar]
- Attrill, M. J., Wright, J. and Edwards, M. (2007) Climate-related increases in jellyfish frequency suggest a more gelatinous future for the North Sea. Limnol. Oceanogr., 52, 480–485. 10.4319/lo.2007.52.1.0480. [DOI] [Google Scholar]
- Bakken, T. (2000). Topografien i Trondheimsfjorden. Trondheimsfjorden, Tapir Forlag. 6, 12–18. [Google Scholar]
- Bakken, T., Hårsaker, K. and Daverdin, M. (2023) Marine Invertebrate Collection NTNU University Museum (Version 1.1475) Occurrence dataset In: Technology NUoSa (ed). accessed via GBIF.org on 2023-03-12, Norwegian University of Science and Technology (NTNU, Trondheim: ). [Google Scholar]
- Bamstedt, U. (2022) Life history of a deep-water medusa: first records of size, longevity, growth and recruitment at varying ages. Social Science Research Network (SSRN):0–17. 10.2139/ssrn.4137382. [DOI]
- Bamstedt, U., Sotje, I., Tiemann, H. and Martinussen, M. B. (2020) Fecundity and early life of the deep-water jellyfish Periphylla periphylla. J. Plankton Res., 42, 87–101. 10.1093/plankt/fbz076. [DOI] [Google Scholar]
- Bandelt, H. J., Macaulay, V. and Richards, M. (2000) Median networks: speedy construction and greedy reduction, one simulation, and two case studies from human mtDNA. Mol. Phylogenet. Evol., 16, 8–28. 10.1006/mpev.2000.0792. [DOI] [PubMed] [Google Scholar]
- Bates, D., Machler, M., Bolker, B. M. and Walker, S. C. (2015) Fitting linear mixed-effects models using lme4. J. Stat. Softw., 67, 1–48. 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Baumann, S. and Schernewski, G. (2012) Occurrence and public perception of jellyfish along the German Baltic coastline. J. Coast. Conserv., 16, 555–566. 10.1007/s11852-012-0199-y. [DOI] [Google Scholar]
- Bozman, A., Titelman, J., Kaartvedt, S., Eiane, K. and Aksnes, D. L. (2017) Jellyfish distribute vertically according to irradiance. J. Plankton Res., 39, 280–289. 10.1093/plankt/fbw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozman, A., Aksnes, D. L. and Eiane, K. (2018) First reports of a mass mortality event across multiple life stages in a mesopelagic jellyfish in high latitude coastal waters. Mar. Ecol. (Berl.), 39, 1–11. 10.1111/maec.12498. [DOI] [Google Scholar]
- Condon, R. H., Graham, W. M., Duarte, C. M., Pitt, K. A., Lucas, C. H., Haddock, S. H. D., Sutherland, K. R., Robinson, K. L.et al. (2012) Questioning the rise of gelatinous zooplankton in the World's oceans. Bioscience, 62, 160–169. 10.1525/bio.2012.62.2.9. [DOI] [Google Scholar]
- Condon, R. H., Duarte, C. M., Pitt, K. A.et al. (2013) Recurrent jellyfish blooms are a consequence of global oscillations. Proc. Natl. Acad. Sci. U. S. A., 110, 1000–1005. 10.1073/pnas.1210920110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpadado, P., Ellertsen, B., Melle, W. and Skjoldal, H. R. (1998) Summer distribution patterns and biomass estimates of macrozooplankton and micronekton in the Nordic Seas. Sarsia, 83, 103–116. [Google Scholar]
- Dawson, M. N., Cieciel, K., Decker, M. B., Hays, G. C., Lucas, C. H. and Pitt, K. A. (2015) Population-level perspectives on global change: genetic and demographic analyses indicate various scales, timing, and causes of scyphozoan jellyfish blooms. Biol. Invasions, 17, 851–867. [Google Scholar]
- Dawson, M. N. (2005) Cyanea capillata is not a cosmopolitan jellyfish: morphological and molecular evidence for C. Annaskala and C. Rosea (Scyphozoa: Semaeostomeae: Cyaneidae) in South-Eastern Australia. Invertebr. Syst., 19, 361–370. 10.1071/IS03035. [DOI] [Google Scholar]
- Doney, S. C., Ruckelshaus, M., Duffy, J. E.et al. (2012) Climate change impacts on marine ecosystems. In: Carlson, C. A., Giovannoni, S. J. (eds), published by Annual Reviews, Annual Review of Marine Sciences, Vol4, p 11–37 [DOI] [PubMed] [Google Scholar]
- Dong, Z. (2018) Blooms of the Moon Jellyfish Aurelia: causes, consequences and controls. In World Seas: An Environmental Evaluation, published by Elsevier Ltd. (Academic Press), pp. 163–171. [Google Scholar]
- Drake, J., Lambin, X. and Sutherland, C. (2022) The value of considering demographic contributions to connectivity: a review. Ecography, 2022, e05552. 10.1111/ecog.05552. [DOI] [Google Scholar]
- Duarte, C. M., Pitt, K. A., Lucas, C. H., Purcell, J. E., Uye, S. I., Robinson, K., Brotz, L., Decker, M. B.et al. (2013) Is global ocean sprawl a cause of jellyfish blooms? Front. Ecol. Environ., 11, 91–97. 10.1890/110246. [DOI] [Google Scholar]
- Dupont, N., Klevjer, T. A., Kaartvedt, S. and Aksnes, D. L. (2009) Diel vertical migration of the deep-water jellyfish Periphylla periphylla simulated as individual responses to absolute light intensity. Limnol. Oceanogr., 54, 1765–1775. [Google Scholar]
- Excoffier, L., Smouse, P. E. and Quattro, J. M. (1992) Analysis of molecular variance inferred from metric distances among DNA haplotypes - application to human mitochondrial-DNA restriction data. Genetics, 131, 479–491. 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer, O., Black, M., Hoeh, W., Lutz, R. and Vrijenhoek, R. (1994) DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol., 3, 294–299. [PubMed] [Google Scholar]
- Fossa, J. H. (1992) Mass occurence of Periphylla periphylla (Scyphozoa, Coronatae) in a Norwegian fjord. Sarsia, 77, 237–251. 10.1080/00364827.1992.10413509. [DOI] [Google Scholar]
- GBIF.org (2022), GBIF Home Page. Available from: https://www.gbif.org.
- Geoffroy, M., Berge, J., Majaneva, S., Johnsen, G., Langbehn, T. J., Cottier, F., Mogstad, A. A., Zolich, A.et al. (2018) Increased occurrence of the jellyfish Periphylla periphylla in the European High Arctic. Polar Biol., 41, 2615–2619. 10.1007/s00300-018-2368-4. [DOI] [Google Scholar]
- Granhag, L., Majaneva, S. and Møller, L. (2012) First recordings of the ctenophore Euplokamis sp. (Ctenophora, Cydippida) in Swedish coastal waters and molecular identification of this genus. Aquat. Invasions, 7, 455–463. 10.3391/ai.2012.7.4.002. [DOI] [Google Scholar]
- Graham, W. M., Pages, F. and Hamner, W. M. (2001) A physical context for gelatinous zooplankton aggregations: a review. Hydrobiologia, 451, 199–212. 10.1023/A:1011876004427. [DOI] [Google Scholar]
- Graham, W. M., Gelcich, S., Robinson, K. L., Duarte, C. M., Brotz, L., Purcell, J. E., Madin, L. P., Mianzan, H.et al. (2014) Linking human well-being and jellyfish: ecosystem services, impacts, and societal responses. Front. Ecol. Environ., 12, 515–523. 10.1890/130298. [DOI] [Google Scholar]
- Hall, T. A. (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser., 41, 95–98. [Google Scholar]
- Halsband, C., Majaneva, S., Hosia, A., Emaus, P. A., Gaardsted, F., Zhou, Q., Nøst, O. A. and Renaud, P. E. (2018) Jellyfish summer distribution, diversity and impact on fish farms in a Nordic fjord. Mar. Ecol. Prog. Ser., 591, 267–279. 10.3354/meps12274. [DOI] [Google Scholar]
- Holland, B. S., Dawson, M. N., Crow, G. L. and Hofmann, D. K. (2004) Global phylogeography of Cassiopea (Scyphozoa: Rhizostomeae): molecular evidence for cryptic species and multiple invasions of the Hawaiian islands. Mar. Biol., 145, 1119–1128. 10.1007/s00227-004-1409-4. [DOI] [Google Scholar]
- Jacobson, P. (1983) Physical oceanography of the Trondheimsfjord. Geophys. Astrophys. Fluid Dyn., 26, 3–26. [Google Scholar]
- Jarms, G. and Tiemann, H. (2004) Actinostola callosa (Verrill, 1882) (Actinostolidae, Anthozoa), a medusivorous sea anemone and its mass occurrence in the Lurefjord, Norway. Helgol. Mar. Res., 58, 15–17. 10.1007/s10152-003-0158-y. [DOI] [Google Scholar]
- Jarms, G., Bamstedt, U., Tiemann, H., Martinussen, M. B. and Fossa, J. H. (1999) The holopelagic life cycle of the deep-sea medusa Periphylla periphylla (Scyphozoa, Coronatae). Sarsia, 84, 55–65. 10.1080/00364827.1999.10420451. [DOI] [Google Scholar]
- Jarms, G., Tiemann, H. and Bamstedt, U. (2002) Development and biology of Periphylla periphylla (Scyphozoa: Coronatae) in a Norwegian fjord. Mar. Biol., 141, 647–657. 10.1007/s00227-002-0858-x. [DOI] [Google Scholar]
- Jøssang, I. (2015) The Energy Budget of a Local Jellyfish Proliferation: Periphylla periphylla In the Trondheimsfjord, Master thesis, 1-36, Norwegian University of Science and Technology (NTNU), Norway. [Google Scholar]
- Katoh, K., Rozewicki, J. and Yamada, K. D. (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform., 20, 1160–1166. 10.1093/bib/bbx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartvedt, S., Ugland, K. I., Klevjer, T. A., Rostad, A., Titelman, J. and Solberg, I. (2015) Social behaviour in mesopelagic jellyfish. Sci. Rep., 5, 11310. 10.1038/srep11310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristoffersen, J. B. and Salvanes, A. G. V. (2009) Distribution, growth, and population genetics of the glacier lanternfish (Benthosema glaciale) in Norwegian waters: contrasting patterns in fjords and the ocean. Mar. Biol. Res., 5, 596–604. 10.1080/17451000903042479. [DOI] [Google Scholar]
- Librado, P. and Rozas, J. (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics, 25, 1451–1452. 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- Lowe, W. H. and Allendorf, F. W. (2010) What can genetics tell us about population connectivity? (vol 19, pg 3038, 2010). Mol. Ecol., 19, 5320–5320. 10.1111/j.1365-294X.2010.04878.x. [DOI] [PubMed] [Google Scholar]
- Lucas, C. H. and Reed, A. J. (2010) Gonad morphology and gametogenesis in the deep-sea jellyfish Atolla wyvillei and Periphylla periphylla (Scyphozoa: Coronatae) collected from Cape Hatteras and the Gulf of Mexico. J. Mar. Biol. Assoc. U.K., 90, 1095–1104. 10.1017/S0025315409000824. [DOI] [Google Scholar]
- Mills, C. E. (2001) Jellyfish blooms: are populations increasing globally in response to changing ocean conditions? Hydrobiologia, 451, 55–68. 10.1023/A:1011888006302. [DOI] [Google Scholar]
- Morita, H., Toyokawa, M., Hidaka, K., Nishimoto, A., Sugisaki, H. and Kikuchi, T. (2017) Spatio-temporal structure of the jellyfish community in the transition zone of cold and warm currents in the Northwest Pacific. Plankton Benthos Res, 12, 266–284. 10.3800/pbr.12.266. [DOI] [Google Scholar]
- Posada, D. (2008) jModelTest: phylogenetic model averaging. Mol. Biol. Evol., 25, 1253–1256. 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- Prieto, L., Armani, A. and Macías, D. (2013) Recent strandings of the giant jellyfish Rhizostoma luteum Quoy and Gaimard, 1827 (Cnidaria: Scyphozoa: Rhizostomeae) on the Atlantic and Mediterranean coasts. Mar. Biol., 160, 3241–3247. 10.1007/s00227-013-2293-6. [DOI] [Google Scholar]
- Ramos-Onsins, S. E. and Rozas, J. (2002) Statistical properties of new neutrality tests against population growth. Mol. Biol. Evol., 19, 2092–2100. 10.1093/oxfordjournals.molbev.a004034. [DOI] [PubMed] [Google Scholar]
- Ronquist, F., et al. (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol., 61, 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth, W., Jarms, G., Streit, B. and Schierwater, B. (2002) Speciation and phylogeography in the cosmopolitan marine moon jelly, Aurelia sp. BMC Evol. Biol., 2, 1. 10.1186/1471-2148-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim, H. (2012) Population Trend of Periphylla periphylla in Inner Trondheimsfjord, Master Thesis, 1-37, Norwegian University of Science and Technology (NTNU), Norway. [Google Scholar]
- Sornes, T. A., Aksnes, D. L., Bamstedt, U. and Youngbluth, M. J. (2007) Causes for mass occurrences of the jellyfish Periphylla periphylla: a hypothesis that involves optically conditioned retention. J. Plankton Res., 29, 157–167. [Google Scholar]
- Sornes, T. A., Hosia, A., Bamstedt, U. and Aksnes, D. L. (2008) Swimming and feeding in Periphylla periphylla (Scyphozoa, Coronatae). Mar. Biol., 153, 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopar, K., Ramsak, A., Trontelj, P. and Malej, A. (2010) Lack of genetic structure in the jellyfish Pelagia noctiluca (Cnidaria: Scyphozoa: Semaeostomeae) across European seas. Mol. Phylogen. Evol., 57, 417–428. [DOI] [PubMed] [Google Scholar]
- Suneetha, K. B. and Salvanes, A. G. V. (2001) Population genetic structure of the glacier lanternfish, Benthosema glaciale (Myctophidae) in Norwegian waters. Sarsia, 86, 203–212. 10.1080/00364827.2001.10420476. [DOI] [Google Scholar]
- Tiemann, H. and Jarms, G. (2010) Organ-like gonads, complex oocyte formation, and long-term spawning in Periphylla periphylla (Cnidaria, Scyphozoa, Coronatae). Mar. Biol., 157, 527–535. 10.1007/s00227-009-1338-3. [DOI] [Google Scholar]
- Tiller, R. G., Mork, J., Richards, R., Eisenhauer, L., Liu, Y., Nakken, J. F. and Borgersen, A. L. (2014) Something fishy: assessing stakeholder resilience to increasing jellyfish (Periphylla periphylla) in Trondheimsfjord, Norway. Mar. Policy, 46, 72–83. 10.1016/j.marpol.2013.12.006. [DOI] [Google Scholar]
- Tiller, R. G., Mork, J., Liu, Y. J., Borgersen, A. L. and Richards, R. (2015) To adapt or not adapt: assessing the adaptive capacity of artisanal fishers in the Trondheimsfjord (Norway) to jellyfish (Periphylla periphylla) bloom and purse seiners. Mar. Coast. Fish., 7, 260–273. 10.1080/19425120.2015.1037873. [DOI] [Google Scholar]
- Tiller, R. G., Borgersen, A. L., Knutsen, O., Bailey, J., Bjelland, H. V., Mork, J., Eisenhauer, L. and Liu, Y. (2017) Coming soon to a fjord near you: future jellyfish scenarios in a changing climate. Coast Manage., 45, 1–23. 10.1080/08920753.2017.1237239. [DOI] [Google Scholar]
- Youngbluth, M. J. and Bamstedt, U. (2001) Distribution, abundance, behavior and metabolism of Periphylla periphylla, a mesopelagic coronate medusa in a Norwegian fjord. Hydrobiologia, 451, 321–333. 10.1023/A:1011874828960. [DOI] [Google Scholar]
- Zwickl, D. J. (2006) Genetic Algorithm Approaches for the Phylogenetic Analysis of Large Biological Sequence Datasets under the Maximum Likelihood Criterion, The University of Texas at; Austin, USA, p. 115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The sequences reported in the study have been deposited in the European Nucleotide Archive repository with accession numbers: OY764966-OY764990.