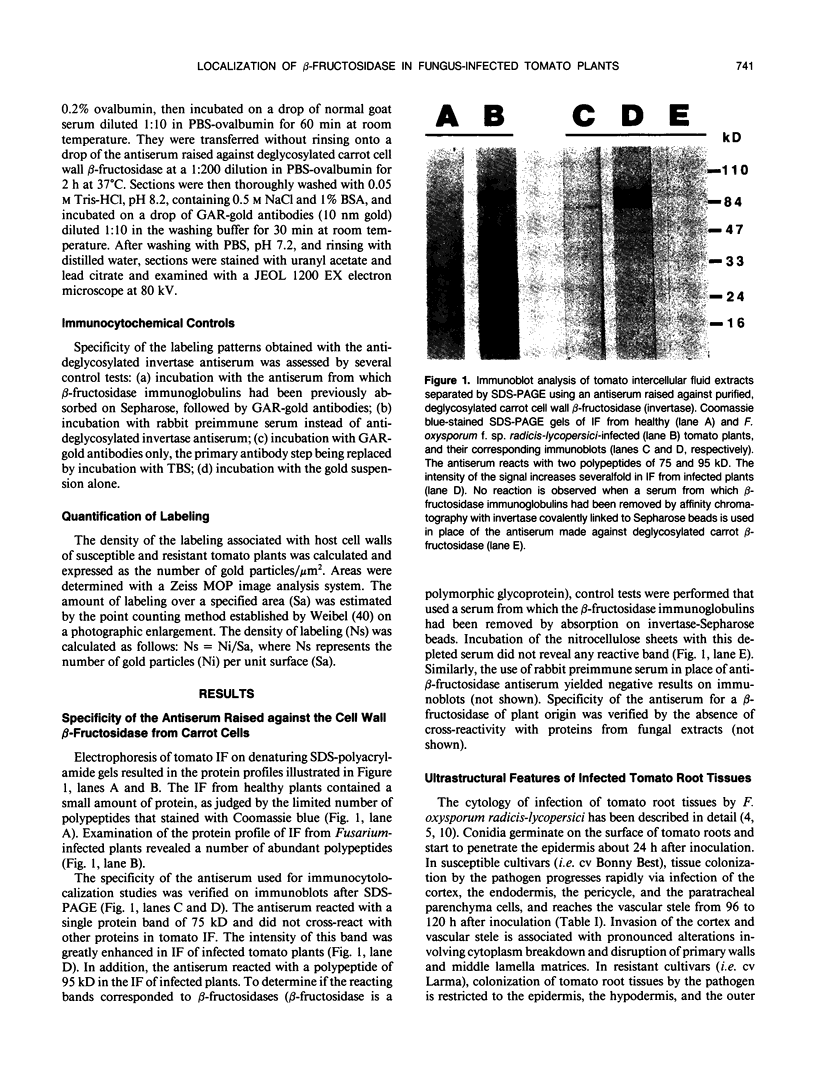
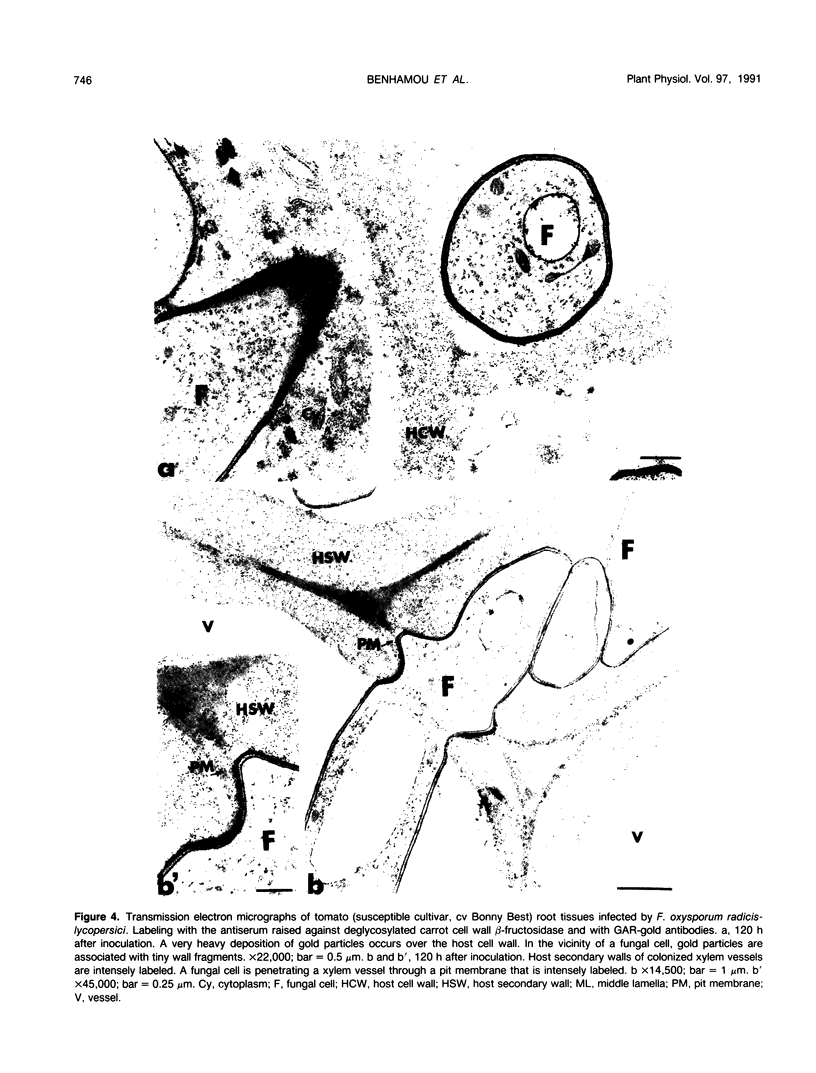
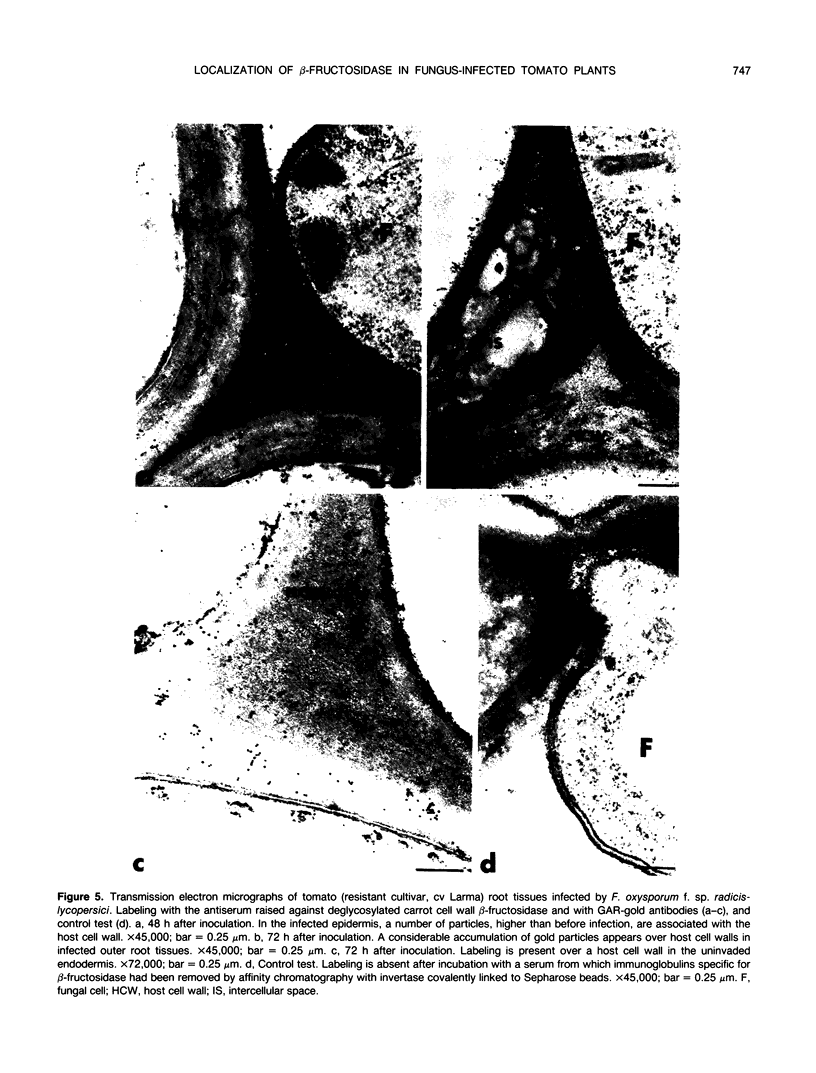
Abstract
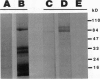
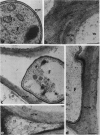
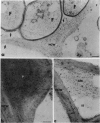
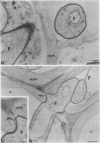
Active defense in plants is associated with marked metabolic alterations, but little is known about the exact role of the reported changes in specific activity of several enzymes in infected plant tissues. β-Fructosidase (invertase), the enzyme that converts sucrose into glucose and fructose, increases upon infection by fungi and bacteria. To understand the relationship between fungal growth and β-fructosidase accumulation, we used an antiserum raised against a purified deglycosylated carrot cell wall β-fructosidase to study by immunogold labeling the spatial and temporal distribution of the enzyme in susceptible and resistant tomato (Lycopersicon esculentum) root tissues infected with the necrotrophic fungus, Fusarium oxysporum f. sp. racidis-lycopersici. In susceptible plants, the enzyme started to accumulate in host cell walls about 72 hours after inoculation. Accumulation occurred only in colonized cells and was mainly restricted to areas where the walls of both partners contacted each other. In resistant plants, accumulation of β-fructosidase was noticeable as soon as 48 hours after inoculation and appeared to reach an optimum by 72 hours after inoculation. Increase in wall-bound β-fructosidase was not restricted to infected cells but occurred also, to a large extent, in tissues that remained uncolonized during the infection process. The enzyme also accumulated in wall appositions (papillae) and intercellular spaces. This pattern of enzyme distribution suggests that induction of β-fructosidase upon fungal infection is part of the plant's defense response. The possible physiological role(s) of this enzyme in infected tomato plants is discussed in relation to the high demand in energy and carbon sources during pathogenesis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benhamou N., Chamberland H., Pauzé F. J. Implication of Pectic Components in Cell Surface Interactions between Tomato Root Cells and Fusarium oxysporum f. sp. radicis-lycopersici: A Cytochemical Study by Means of a Lectin with Polygalacturonic Acid-Binding Specificity. Plant Physiol. 1990 Apr;92(4):995–1003. doi: 10.1104/pp.92.4.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou N., Grenier J., Asselin A., Legrand M. Immunogold localization of beta-1,3-glucanases in two plants infected by vascular wilt fungi. Plant Cell. 1989 Dec;1(12):1209–1221. doi: 10.1105/tpc.1.12.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamou N., Joosten M. H., De Wit P. J. Subcellular Localization of Chitinase and of Its Potential Substrate in Tomato Root Tissues Infected by Fusarium oxysporum f. sp. radicis-lycopersici. Plant Physiol. 1990 Apr;92(4):1108–1120. doi: 10.1104/pp.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye L., Mouatassim B., Ghorbel A. Cell Wall and Cytoplasmic Isozymes of Radish beta-Fructosidase Have Different N-Linked Oligosaccharides. Plant Physiol. 1986 Jan;80(1):27–33. doi: 10.1104/pp.80.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurière M., Laurière C., Chrispeels M. J., Johnson K. D., Sturm A. Characterization of a xylose-specific antiserum that reacts with the complex asparagine-linked glycans of extracellular and vacuolar glycoproteins. Plant Physiol. 1989 Jul;90(3):1182–1188. doi: 10.1104/pp.90.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K., Uritani I. Change in invertase activity of sweet potato in response to wounding and purification and properties of its invertases. Plant Physiol. 1974 Jul;54(1):60–66. doi: 10.1104/pp.54.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch F., Hadwiger L. A., Boller T. Antifungal Hydrolases in Pea Tissue : I. Purification and Characterization of Two Chitinases and Two beta-1,3-Glucanases Differentially Regulated during Development and in Response to Fungal Infection. Plant Physiol. 1988 Jun;87(2):325–333. doi: 10.1104/pp.87.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon L. M., Uritani I., Imaseki H. De novo synthesis of peroxidase isozymes in sweet potato slices. Plant Physiol. 1971 Apr;47(4):493–498. doi: 10.1104/pp.47.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A., Chrispeels M. J. cDNA cloning of carrot extracellular beta-fructosidase and its expression in response to wounding and bacterial infection. Plant Cell. 1990 Nov;2(11):1107–1119. doi: 10.1105/tpc.2.11.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- von Schaewen A., Stitt M., Schmidt R., Sonnewald U., Willmitzer L. Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 1990 Oct;9(10):3033–3044. doi: 10.1002/j.1460-2075.1990.tb07499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]