Abstract
Background
Powassan virus (POWV) is an emerging arthropod-borne flavivirus, transmitted by Ixodes spp. ticks, which has been associated with neuroinvasive disease and poor outcomes.
Methods
A retrospective study was conducted at Mayo Clinic from 2013 to 2022. We included clinical and epidemiologic data of probable and confirmed neuroinvasive POWV cases.
Results
Sixteen patients with neuroinvasive POWV were identified; their median age was 63.2 years, and 62.5% were male. Six patients presented with rhombencephalitis, 4 with isolated meningitis, 3 with meningoencephalitis, 2 with meningoencephalomyelitis, and 1 with opsoclonus myoclonus syndrome. A median time of 18 days was observed between symptom onset and diagnosis. Cerebrospinal fluid analysis showed lymphocytic pleocytosis with elevated protein and normal glucose in the majority of patients. Death occurred within 90 days in 3 patients (18.8%), and residual neurologic deficits were seen in 8 survivors (72.7%).
Conclusions
To our knowledge, this is the largest case series of patients with neuroinvasive POWV infection. We highlight the importance of a high clinical suspicion among patients who live in or travel to high-risk areas during the spring to fall months. Our data show high morbidity and mortality rates among patients with neuroinvasive disease.
Keywords: tick-borne diseases, Powassan virus, arbovirus
This large retrospective case series (2013–2022) included 16 patients with neuroinvasive Powassan virus who presented with rhombencephalitis, isolated meningitis, meningoencephalitis, meningoencephalomyelitis, and/or opsoclonus myoclonus syndrome. The 90-day mortality rate was 18.8%, with neurologic deficits seen in 72.7% of survivors.
Powassan virus (POWV) is an RNA tick-borne flavivirus, identified in 1958 in Ontario, Canada [1]. Between 2012 and 2021, the Centers for Disease Control and Prevention reported 202 cases in the United States, among which 189 patients had neuroinvasive disease and 24 cases resulted in death [2]. While most cases occur in the upper Northeast and Midwest regions, the true incidence of POWV infections is unknown owing to limited awareness of this tick-borne pathogen (TBP), low clinical suspicion in cases of mild or nonneuroinvasive disease, and lack of routinely accessible diagnostics.
There are 2 genetically distinct lineages, POWV and deer tick virus, which are serologically indistinguishable [3–5]. We do not differentiate between the 2 lineages in this article and refer to either as “POWV.” The tick species most commonly associated with POWV transmission is Ixodes scapularis [6]. POWV is transmitted within 15 minutes of tick attachment [7], in contrast to Borrelia burgdorferi, which requires 36–72 hours [8, 9]. The prevalence of POWV in ticks ranges from 1% to 3% in I. scapularis, which is less common than other TBPs [10].
POWV diagnoses have been increasing in recent years, with approximately 1 documented case per year from 1958 to 2005, up to 20–40 cases per year between 2016 and 2019 [11]. Given the rise in cases, it is important for clinicians to be familiar with the presentation, diagnosis, and course of POWV infection. Here we describe the clinical features of 16 patients with POWV infection at our institution.
METHODS
This is a retrospective, institutional review board–approved, study of patients evaluated at Mayo Clinic from 2013 to 2022. We collected clinical and epidemiology information from laboratory confirmed POWV cases. The majority of patients (n = 12) were identified using POWV diagnostic results from the Minnesota and Wisconsin departments of health. Four patients were identified through both serologic and molecular testing for POWV at Mayo Clinic following implementation of these tests between February and May 2022. Two patients had metagenomic next-generation sequencing performed through the University of California, San Francisco. We used the Centers for Disease Control and Prevention case definition for arboviral diseases [12] (Supplementary Table 1). It is important to highlight that in the state of Wisconsin, POWV seroreactivity has been reported even in patients without neuroinvasive disease [13].
RESULTS
Patient Cohort
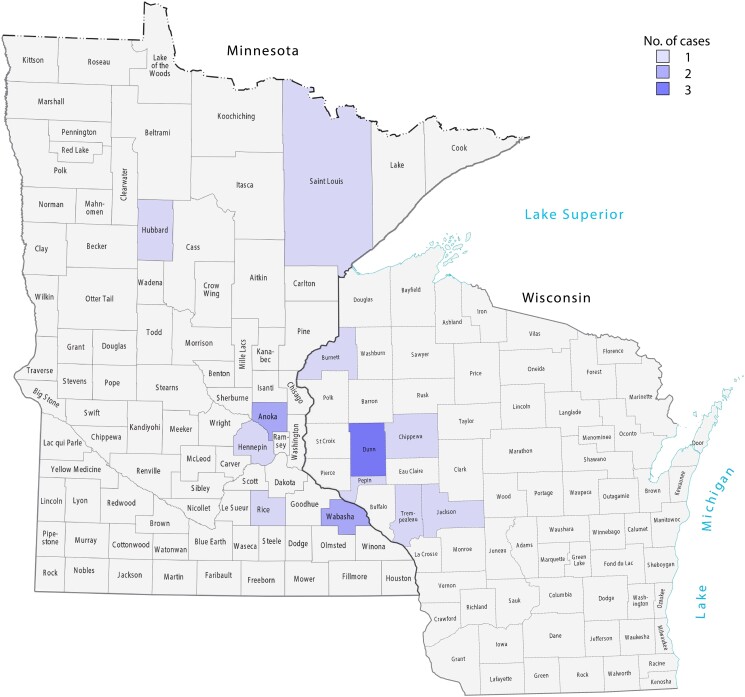
We identified 16 cases; 15 patients met diagnostic criteria for confirmed POWV neuroinvasive disease and 1 for probable neuroinvasive disease (Table 1). Ten patients were male, and their median age was 63.2 years. Four patients were immunosuppressed; 2 were taking ibrutinib for chronic lymphocytic leukemia (patients 1 and 5), 1 was receiving chemotherapy for lung adenocarcinoma (patient 11), and 1 was taking methotrexate for rheumatoid arthritis (patient 13). All patients lived in either Minnesota or Wisconsin according to their zip codes (Figure 1). Seven patients lived in a rural setting, and 10 were routinely active outdoors. Seven patients reported a history of tick bite.
Table 1.
Clinical and Laboratory Presentation of Patients With Diagnosed Powassan Virus
| Characteristic | Patient | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| Sex | M | F | F | M | M | F | M | M | M | M | M | F | F | M | M | F |
| Time of symptom onset, mo/y | 11/2021 | 7/2022 | 6/2020 | 5/2022 | 11/2021 | 6/2022 | 8/2022 | 8/2019 | 11/2019 | 7/2019 | 7/2020 | 8/2013 | 8/2021 | 7/2018 | 10/2021 | 10/2016 |
| Age, y | 75 | 74 | 34 | 0.14 | 68 | 50 | 76 | 76 | 49 | 58 | 74 | 33 | 71 | 78 | 44 | 56 |
| IS(cause) | Yes(CLL) | No | No | No | Yes (CLL) | No | No | No | No | No | Yes (lung cancer) | No | Yes (RA) | No | No | No |
| Neurologic phenotype | RE | RE | Meningitis | ME | MEM | Meningitis | Meningitis | ME | RE | RE | RE | RE | Meningitis | ME | OMS | MEM |
| MR imaging findings | Abn | Nl | NA | Nl | Abn | Nl | Nl | Nl | Abn | Abn | Abn | Abn | Nl | Nl | Nl | Abn |
| Platelet count (cells ×10(9)/L) | 142 | 187 | 186 | 416 | 159 | 143 | 147 | 121 | 198 | 117 | 97 | 168 | 409 | 117 | 239 | 165 |
| CSF findings | ||||||||||||||||
| TNCs (cells/μL) | 6 | 121 | 257 | 480 | 274 | 40 | 55 | 85 | 193 | 66 | 5 | 585 | 43 | NA | 860 | 153 |
| Neutrophils (cells/μL) | 3(50) | 17(14) | 8(3) | 230(48) | 0 | 16.4 (41) | 32(58) | 17.85(21) | 15.44 (8) | 5.3(8) | 0.45 (9) | 6(1) | 0.86 (2) | NA | NA | 53.5(35) |
| Lymphocytes(%) | 2(33) | 87(72) | 242(94) | 62(13) | 236(86) | 16.4(41) | 9(16) | 43(51) | 176(91) | 57(87) | 3(50) | 579(99) | 39(91) | NA | 705.2(82) | 58(38) |
| Diagnostic test | mNGS CSF | Serum IgM and PCR | Serum IgM | Serum IgM | CSF IgM | Serum IgM | Serum IgM | Serum IgM | Serum IgM | Serum IgM, CSF IgM | Serum IgM, CSF IgM | CSF IgM | Serum IgM | Serum IgM | Serum IgM | CSF IgM |
| PRNT result | NA | NA | 1:640 | 1:640 | NA | 1:640 | 1:2560 | 1:640 | 1:40 | 1:160 | <1:10 | 1:32 | 1:1280 | 1:640 | 1:640 | NA |
| Death within 90 da | Yes (at 35 d) | No | No | No | Yes (at 80 d) | No | No | No | No | No | No | No | No | No | No | Yes (at 62 d) |
| Length of hospitalization, d | 30 | 8 | 3 | 6 | 76 | 1 | 7 | 38 | 16 | 5 | 21 | 17 | 12 | 16 | 11 | 28 |
| Modified Rankin score(last follow-up) | 6(NA) | 3(LTF) | 1(2 y) | 0(1 y) | 6(NA) | 0(LTF) | 1(2 mo) | 3(3.5 y) | 0(2 mo) | 1(4 y) | 4(8 mo) | 2(9 y) | 2(1 y) | 0(5 y) | 0(3 mo) | 6(NA) |
Abbreviations: Abn, abnormal; CLL, chronic lymphocytic leukemia; CSF, cerebrospinal fluid; F, female; IgM, immunoglobulin M; IS, immunosuppression; LTF, lost to follow-up; M, male; ME, meningoencephalitis; MEM, meningoencephalomyelitis; mNGS, metagenomic next-generation sequencing; MR, magnetic resonance; NA, not available; Nl, normal; OMS, opsoclonus myoclonus syndrome; PCR, polymerase chain reaction; PRNT, plaque reduction neutralization test; RA, rheumatoid arthritis; RE, rhombencephalitis; TNCs, total nucleated cells,
aDeath within 90 days from symptom onset.
Figure 1.
Geographic location of patients diagnosed with Powassan virus (only 1 patient reported recent travel, to Wyoming 1 month before hospitalization).
Clinical Presentation
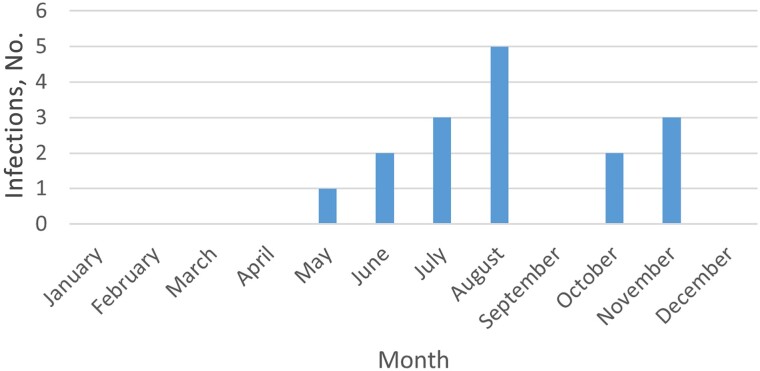
Patients presented for clinical evaluation between May and November (Figure 2), with a median of 4 days after experiencing symptoms. The most common symptom at presentation was fever, in 15 patients (Table 2).
Figure 2.
Number of Powassan infections stratified by month of presentation.
Table 2.
Patient Characteristics and Presentation
| Characteristic | Patients, No. (%)a (n = 16) |
|---|---|
| Age, median (IQR) y | 63.2 (48.2–74.6) |
| Age range | 1.6 mo to 78 y |
| Male sex | 10 (62.5) |
| Immunosuppression | 4 (25) |
| Symptoms at presentation | |
| Fever | 15 (93.8) |
| Rash | 4 (25) |
| Headache | 8 (50) |
| Altered mental status | 9 (56.3) |
| Neurologic phenotype | |
| Rhombencephalitis | 6 (37.5) |
| Meningoencephalitis | 3 (18.8) |
| Meningitis | 4 (25) |
| Meningoencephalomyelitis | 2 (12.5) |
| OMS | 1 (6.3) |
| Laboratory results at admission, median (IQR) | |
| Hemoglobin, g/dL | 12.4 (12.2–13.7) |
| WBC count, ×109 cells/L | 10.9 (7.6–12.3) |
| Absolute neutrophil count, ×109 cell/L | 8.0 (5.8–9.7) |
| Absolute lymphocyte count, ×109 cells/L | 1.27 (0.9–1.8) |
| Platelets, ×109 cells/L | 162 (136.8–189.8)b |
| Creatinine, mg/dL | 1.09 (0.8–1.4) |
| ALT, mg/dL | 24.5 (18.8–35.8) |
| AST, mg/dL | 30 (23.0–41.3) |
| Total bilirubin, mg/dL | 0.6 (0.4–0.7) |
| CSF results, median (IQR) (n = 15) | |
| Protein, mg/dL | 79 (70.5–100.5)c |
| Glucose, mg/dL | 58 (52–67) |
| Glucose/serum ratio | 0.6 (0.5–0.6) |
| Nucleated cells, cells/µL | 121 (49–265.5)c |
| Neutrophils, cells/µL | 11.72 (3.6–17.6) |
| Lymphocytes, cells/µL | 58 (27.8–205.8) |
| Monocytes, cells/µL | 10.73 (3.08–25.33) |
| MR imaging results (n = 15) | |
| Normal | 7 (46.7) |
| Cerebellitis | 3 (20) |
| Leptomeningeal enhancement | 5 (33.3) |
| Basal ganglia involvement | 2 (13.3) |
| POWV diagnosis, no. positive/no. tested) | |
| Serum IgM positivity | 10/13 |
| CSF IgM positivity | 5/6 |
| CSF POWV PCR | 1/1 |
| Serum POWV PCR | 1/1 |
| CSF POWV mNGS | 1/2 |
| Seizures | 3 (18.8) |
| ICU admission | 6 (37.5) |
| Deaths (at 90 d) | 3 (18.8) |
| Neurologic sequelae (n = 11) | 8 (72.7) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CSF, cerebrospinal fluid; ICU, intensive care unit; IgM, immunoglobulin M; IQR, interquartile range; mNGS, metagenomic next-generation sequencing; MR, magnetic resonance; OMS, opsoclonus myoclonus syndrome; PCR, polymerase chain reaction; POWV, Powassan virus; WBC, white blood cell.
aData represent no. (%) of patients unless otherwise specified.
bResults in 7 patients with thrombocytopenia.
cProtein elevated in 100% of patients/>400 cells/uL in 3 patients.
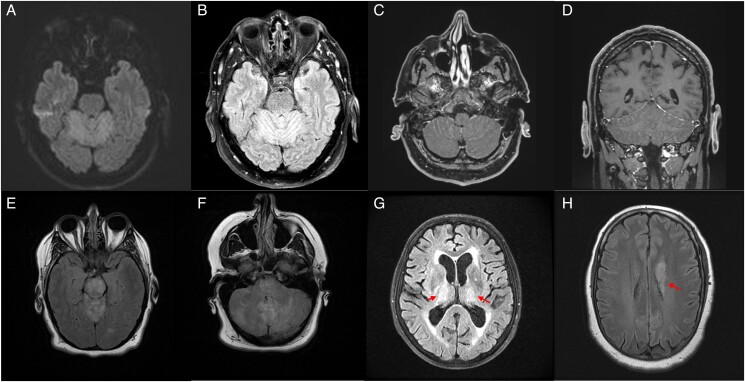
Neurologic presentation was variable but followed certain phenotypes. Six patients presented with rhombencephalitis, 4 with isolated meningitis, 3 with meningoencephalitis, 2 with meningoencephalomyelitis and 1 with opsoclonus myoclonus syndrome (OMS) 7 days after developing a meningitic syndrome (Table 3). Patients presenting with rhombencephalitis had evidence of ataxia, nystagmus, dysarthria, ptosis, cranial nerve palsies, or paresis. Patients with ataxia often had profound truncal ataxia, although appendicular ataxia was also noted. Dysarthria ranged from mild to anarthria. Five patients had cranial nerve palsies on examination. Sixth nerve palsies were most common, but bilateral seventh nerve palsies, third and fourth nerve palsies and bilateral optic perineuritis were also observed. Patients 5 and 16 presented with meningoencephalomyelitis. Patient 16 developed flaccid right upper-extremity monoparesis with electromyographic and nerve conduction study findings consistent with a subacute disorder of anterior horn cells. Patient 5 presented with rapidly progressive quadriparesis and was noted to have subtle ventral cervical cord enhancement. along with T2/fluid-attenuated inversion recovery (FLAIR) hyperintensities in the cerebellum (Figure 3).
Table 3.
Neurologic Summary and Sequelae
| Patient | Neurologic Presentation | MR Imaging Findings | EEG Findings | Neurologic Phenotype | Neurologic Sequelae |
|---|---|---|---|---|---|
| 1 | Encephalopathy, anarthria, quadriparesis, vertical gaze restriction | Cerebellitis with obstructive hydrocephalus | Generalized periodic discharges with triphasic wave morphology and diffuse slowing (3–5-Hz range) | Rhombencephalitis | Persistent encephalopathy and anarthria (expired) |
| 2 | Left-sided weakness, dysarthria, tremor, truncal ataxia | Symmetric T2 hyperintensities in bilateral thalami | Normal | Rhombencephalitis | Lost to follow-up |
| 3 | Headache, fever, nausea, altered mental status | Not performed | Not performed | Meningitis | Cognitive impairment (short-term recall, word finding and concentration) and headaches |
| 4 | Seizures | Normala | Normal | Meningoencephalitis | None |
| 5 | Rapidly progressive quadriparesis, encephalopathy, multiple cranial neuropathies (left 3rd and 4th, bilateral 6th, and bilateral optic perineuritis) | Cerebellitis and ventral cervical cord enhancement | Generalized periodic discharges, rhythmic delta activity with sharp features, and generalized slowing (5–7-Hz range, 2–3 Hz posteriorly) | Meningoencephalomyelitis | Quadriplegia and facial dyskinesias (expired) |
| 6 | Headache, fever, neck pain | Normal | Not performed | Meningitis | Lost to follow-up |
| 7 | Headache, fever, falls | Normal | Not performed | Meningitis | Cognitive impairment (slowed processing speed) |
| 8 | Encephalopathy, multifocal myoclonus | Normalb | Moderate diffuse slowing (4–6-Hz range) | Meningoencephalitis | Cognitive impairment and gait disturbance |
| 9 | Left 6th nerve palsy, dysarthria, upper-extremity dysmetria | Cerebellar and occipital lobe leptomeningeal enhancement | Not performed | Rhombencephalitis | Gait disturbance |
| 10 | Headaches, dysarthria, appendicular and truncal ataxia | Leptomeningeal enhancement at cerebellar folia and cerebral hemispheres | Not performed | Rhombencephalitis | Gait disturbance |
| 11 | Truncal > appendicular ataxia, encephalopathy, anarthria | Diffuse sulcal hyperintensities on postgadolinium FLAIR sequence | Not performed | Rhombencephalitis | Persistent ataxia, dysarthria and deconditioning at last follow-up (8 mo) |
| 12 | Headache, encephalopathy, ataxia and left 6th nerve palsy | Midbrain, pons, and cerebellar FLAIR hyperintensitiesa | Moderate diffuse slowing (4–5-Hz range) | Rhombencephalitis | Postviral levodopa-responsive parkinsonism at last follow-up (9 y) |
| 13 | Headache, fever, meningismus, encephalopathy | Normala | Not performed | Meningitis | Headaches and gait disturbance |
| 14 | Fever, headache, encephalopathy | Normala | Moderate diffuse slowing (4–6 Hz) with intermittent triphasic waveforms | Meningoencephalitis | None |
| 15 | Fever, headache, meningismus, truncal ataxia, tremulousness, opsoclonus | Normal | Not performed | OMS | None |
| 16 | Encephalopathy, truncal ataxia, quadriparesis, dysarthria, left 6th nerve palsy | Leptomeningeal enhancement and left basal ganglia FLAIR hyperintensities | Left temporal seizures | Meningoencephalomyelitis | Parkinsonism and poliomyelitis (expired) |
Abbreviations: EEG, electroencephalography, FLAIR, fluid-attenuated inversion recovery; MR, magnetic resonance; OMS, opsoclonus myoclonus syndrome.
aNo postgadolinium sequences MR imaging obtained.
bMotion degraded, limited study.
Figure 3.
Magnetic resonance imaging findings. A–D, Cerebellitis in patient 5 demonstrated by cerebellar diffusion restriction (A), T2/fluid-attenuated inversion recovery (FLAIR) hyperintensities (B), and leptomeningeal enhancement (C, D). E, F, Extension of T2/FLAIR hyperintensities involving the midbrain (E) and pons (F) in addition to the cerebellum in patient 12. G, Bilateral thalamic T2/FLAIR hyperintensities in patient 2 (two red arrows). H, Left caudate T2/FLAIR hyperintensities in patient 16 (red arrow).
Three patients (4, 5, and 16) experienced seizures, including 1 patient with persistent encephalopathy and frequent focal subclinical seizures. Although all 3 patients responded to initiation of antiseizure medications, 2 ultimately died of their infections
Laboratory Results
A summary of laboratory results is shown in Table 2. The most common abnormalities noted on the cell blood count was thrombocytopenia in 43.8% of cases, followed by lymphopenia. Fifteen of 15 patients underwent a lumbar puncture at presentation (Tables 1 and 2). The majority had mildly elevated protein in cerebrospinal fluid (CSF), with a median of 79 mg/dL. None had hypoglycorrhachia. Ten patients had lymphocytic pleocytosis, with median nucleated, lymphocyte and neutrophil cell counts of 121/µL, 58/µL, and 11.72/µL, respectively. Five patients presented with a neutrophilic/mixed pleocytosis, with an average time of symptom onset to CSF analysis of 4 days, compared with 5.4 days in patients with a predominant lymphocytic pleocytosis. Two of the 5 patients with neutrophilic/mixed pleocytosis (patients 1 and 16) had repeated lumbar punctures 2 and 8 days later, both with conversion to a predominantly lymphocytic profile.
Diagnostic Evaluation
Fifteen patients met diagnostic criteria for confirmed POWV neuroinvasive disease, and 1 met the criterion for probable neuroinvasive disease, owing to concomitant Lyme disease. A median time of 18 days was observed between symptom onset and diagnosis, and a median of 12 days between admission and diagnosis.
Thirteen patients underwent POWV immunoglobulin M (IgM) testing of serum samples, performed at either a public health laboratory (n = 12) or Mayo Clinic (n = 4); 12 (92.3%) had positive results. Six patients underwent CSF POWV IgM evaluation, and 5 tested positive. Plaque reduction neutralization tests (PRNTs) were performed in serum samples from 13 patients, with positive results in 12. Semiquantitative PRNT titers ranged from 1:32 to 1:2560, with higher titers suggesting more recent infection. One patient underwent CSF POWV nucleic acid amplification testing (NAAT), with positive results for deer tick virus lineage 2. Results of CSF metagenomic next-generation sequencing were positive for POWV in 1 of 2 patients tested (Table 1).
Patients were evaluated for other causes of meningoencephalitis. A meningoencephalitis NAAT panel was performed in 12 patients, with negative results in all. Fifteen of the 16 patients were also evaluated for infection with B. burgdorferi in serum, and 7 were assessed for neuroborreliosis using an antibody index assay (AIA) in CSF and serum. Among these patients, patient 5 had an equivocal Lyme AIA result (value of 1.4) with negative CSF polymerase chain reaction and negative serological results in serum; this patient was not considered to have confirmed neuroborreliosis. Patient 11 had a positive Lyme AIA result with a value of 1.8 (values ≥1.5 are considered indicative of intrathecal pathogen-specific antibody synthesis [14]), with positive results for anti–B. burgdorferi IgM in serum. Patients were tested for other endemic mosquito-borne viruses and TBPs, all with negative results (Supplementary Table 3).
Imaging
Fifteen patients underwent magnetic resonance (MR) imaging during their hospitalization, 11 (73%) with postgadolinium sequences included. Eight patients (53.%) had abnormal MR imaging findings related to their infection. Leptomeningeal enhancement was seen in 5 patients. Two patients with rhombencephalitis (patient 1 and 12) and 1 with meningoencephalomyelitis (patient 5) had cerebellar T2/FLAIR hyperintensities, with patient 12 also having T2/FLAIR brainstem hyperintensities in the midbrain and pons, with subsequent development of postencephalitic parkinsonism (Figure 3). T2/FLAIR basal ganglia hyperintensities were seen in 2 patients (patients 2 and 16), with patient 16 going on to have postencephalitic parkinsonism. One patient with meningoencephalomyelitis (patient 5) had evidence of subtle ventral cervical cord enhancement. Seven patients had normal brain imaging findings.
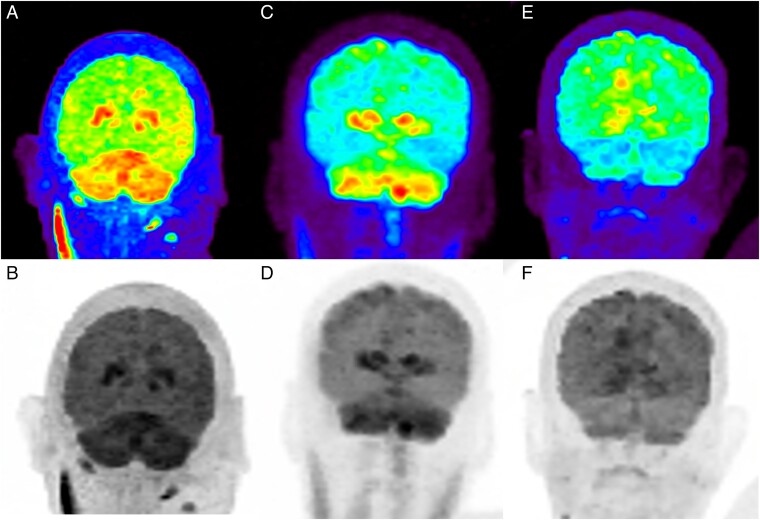
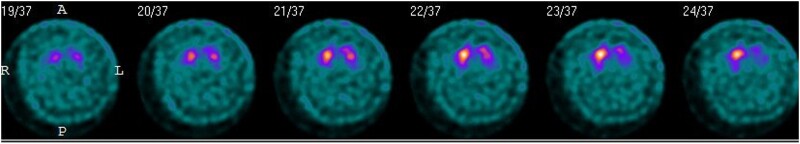
One patient with meningoencephalomyelitis (patient 5) and 1 with rhombencephalitis (patient 11) underwent fluorodeoxyglucose positron emission tomography imaging as part of their diagnostic workup, and both were noted to have basal ganglia and cerebellar hypermetabolism (Figure 4). Neither of these patients had basal ganglia T2/FLAIR hyperintensities, and only 1 had cerebellar T2/FLAIR hyperintensities on MR images. One of the patients with postencephalitic parkinsonism (patient 12) underwent dopamine transporter single-photon emission computed tomography 2 years after infection, which demonstrated marked diminution of tracer uptake in the putamen and caudate bilaterally [15] (Figure 5).
Figure 4.
A–D, Fluorodeoxyglucose positron emission tomography (FDG-PET) scans from patients 5 (A, B) and 11 (C, D), showing hypermetabolism in the basal ganglia and cerebellum. E, F, Partial resolution of hypermetabolism noted in patient 11 on repeated FDG-PET 7 months later.
Figure 5.
Dopamine transporter single-photon emission computed tomographic scan of patient 12, showing marked diminution of tracer uptake in the putamen and caudate bilaterally.
Electroencephalography
Eight patients underwent electroencephalography (EEG), 7 for persistent encephalopathy and 1 after a witnessed self-limited episode of generalized shaking. Six patients had EEG evidence of generalized slowing, 2 of whom also had generalized triphasic waveforms, and 2 had electrographic seizures, 1 with diffuse midline and frontal rhythmic delta activity associated with head jerking (patient 5) and the other with focal left temporal onset associated with persistent encephalopathy (patient 16).
Treatment
Initially, 13 patients received antibiotics for possible bacterial meningitis. Nine patients received immunotherapy in the form of steroids or intravenous immunoglobulin. Eight patients received steroids, 7 during the period of diagnostic uncertainty and 1 for presumed parainfectious OMS. Four patients received intravenous immunoglobulin: patient 1 for his known hypogammaglobulinemia, patient 5 as empiric therapy during the period of diagnostic uncertainty, and patients 8 and 15 specifically for treatment of POWV infection.
Outcomes
Six patients needed intensive care unit level of care, with a median stay of 9 days. Six patients required mechanical intubation (range, 4–14 days), with 1 proceeding to tracheostomy. Seven patients required temporary tube feeding supplementation, with 4 requiring percutaneous endoscopic gastrostomy tube placement. Patient 1 developed obstructive hydrocephalus secondary to cerebellar edema and fourth ventricle effacement, requiring an extraventricular drain for 7 days. Two patients died within 90 days after diagnosis of complications attributable to POWV, and 1 patient died after developing Richter transformation and being transferred to hospice (Supplementary Table 2).
Thirteen of 16 patients (81.3%) survived after 90 days of diagnosis. In 1 patient with meningitis, cerebral venous thrombosis without infarct developed 6 weeks after symptom onset, requiring rehospitalization. Of the 13 patients who survived their infection, 2 were discharged to a rehabilitation facility, 2 to a skilled nursing facility, 1 to a transitional care unit, and 8 to home.
Neurologic Sequela
Of the survivors, 2 were lost to follow-up. Eight patients (72.7%) had evidence of neurologic sequela, defined as neurologic symptoms believed to be a downstream consequence of their infection beyond the infectious period. Patient 12 had partially levodopa-responsive, postviral parkinsonism, which was present at 9-year follow-up [15]. Four patients had persistent gait disturbances, 2 had new headaches, 3 had subjective cognitive impairment (described as poor memory, mental slowing, or decreased concentration), and 1 had persistent ataxia and dysarthria (Table 3). In patients who survived their infection, modified Rankin scores ranged from 0–4 (median, 1) at last follow-up, indicating that most patients had at least some degree of disability after infection.
DISCUSSION
POWV is an emerging infectious disease with an increasing incidence due to multiple factors, including expansion of the geographic range of ticks such as I. scapularis, wider availability of diagnostic methods and heightened clinical awareness. We report a case series of 16 patients, 15 confirmed and 1 with probable Powassan disease from 2013–2022 at a tertiary care center in the Midwest United States. The most commonly found laboratory abnormality was thrombocytopenia, even in the absence of other TBP coinfections, which has been reported in the literature [16, 17]. We found a 90-day mortality rate of 18.8% among patients with diagnosed POWV infection, consistent with previous reports in the literature (10%–15%) [18].
All patients were from Minnesota and Wisconsin, with many clustered in counties bordering major waterways, including the Mississippi and Chippewa rivers. These areas are likely to be at higher risk for human transmission, given their proximity to human population centers, attractiveness for recreational activities, and decreased habitat fragmentation, which may lead to higher densities of ticks and reservoir hosts [19]. Patients presented between May and November, which is longer than the classic presentation between May and September in the northern hemisphere and highlights the importance of having a high clinical suspicion even during the early winter, as reported elsewhere [20]. This wider temporal distribution may be due to the effects of climate change, leading to longer seasonal activity and increased tick populations [21]. In addition, higher temperatures result in people spending longer time outdoors [22].
In our cohort, the majority of the patients presented with encephalitis-related syndromes (fever and altered mental status), such as rhombencephalitis, meningoencephalitis, and isolated meningitis, similar to prior reports [23]. Two patients presented with prominent weakness that could be localized to the ventral spinal cord. One of them presented with rapidly progressive quadriparesis with subtle ventral cervical cord enhancement, and the other with flaccid monoparesis and poliomyelitis demonstrated by electromyography. Other flaviviruses are known to cause a poliomyelitislike flaccid paralysis, and this clinical phenotype has rarely been reported with POWV [24]. Involvement of motor neurons within the ventral horn of the spinal cord has been described in mouse models [25].
One patient presented with OMS, a presumed immune-mediated disorder characterized by chaotic uncontrolled movements of the eyes and involuntary jerklike movements of the body. OMS can be paraneoplastic but has also been described in the setting of infection, including neuroinvasive disease with other flaviviruses [26, 27]. Our patient developed OMS 1 week after presenting with a meningitic syndrome. Given a potential immune-mediated mechanism, immunotherapy may be indicated in cases of OMS, despite the lack of evidence supporting its use in flavivirus meningoencephalitis.
Nearly half of the patients in our cohort had normal head imaging. MR imaging abnormalities, when present, commonly showed evidence of cerebellitis and/or leptomeningeal enhancement. Less commonly observed in our cohort was basal ganglia involvement (12.5%), compared to other case series where basal ganglia involvement was reported to occur in 38%–54% of cases [17, 28]. Interestingly, in 2 patients who underwent fluorodeoxyglucose positron emission tomography as part of a cancer workup, basal ganglia and cerebellum hypermetabolism was found incidentally, possibly suggesting basal ganglia dysfunction that was not evident at MR imaging. One of these patients died of their illness, while the other had persistent ataxia and dysarthria at 8-month follow-up. Neurologic sequelae were observed in 72.7% of our cohort, compared with 50% in previous reports. Notable sequelae included cognitive impairment, headaches, gait disturbance, and parkinsonism, which is similar to previous reports [13].
We also evaluated the incidence of coinfections in patients with POWV. Among the 16 individuals, 1 patient was suspected to have a coinfection with B. burgdorferi; however, it is unclear whether neuroborreliosis would explain his neurologic symptoms. and patients that have had Lyme infection years earlier may have a persistently elevated Lyme AIA results. Another patient was positive for anti–West Nile virus IgM in serum, but confirmatory PRNT titers were <1:10, suggesting that West Nile virus IgM positivity was likely secondary to cross-reactivity [29–31].
We observed that the median time between admission and POWV diagnosis was 12 days, while the time between symptom onset and diagnosis was 18 days. Although there are currently no targeted antiviral treatments, prompt diagnosis of POWV infections is essential to deescalate antibacterial treatment, provide accurate prognostic information, and document the epidemiology of this emerging TBP. There is also a need for more widely available diagnostic testing options, outside of public health laboratories. We were able to appraise how improved accessibility to POWV testing affected the diagnosis at our institution. Four of the 16 cases presented in this case series were diagnosed within 4 months of offering POWV-specific diagnostics in our laboratory, compared with diagnosis of 12 cases between 2018 and 2021.
Alongside test availability, clinicians need to be cognizant of appropriate test use relative to the timing of patient presentation and the limitations associated with currently available diagnostic assays. Importantly, immune response to POWV may not be detectable until 5–7 days after infection, as it has been reported in other tick-borne encephalitis during the initial viremic phase [32, 33], making POWV NAAT preferable during this acute stage of disease, similar to preferences in other arboviral and tick-borne diseases [34], although some reports suggest that specific antibody levels start to increase within 1–2 days [23]. However, a negative NAAT result does not rule out infection as POWV viral loads are transient and low, leading to the potential for clinically false-negative results. As a result, for patients with suspected POWV infection, testing with both NAAT and an anti-POWV IgM assay is recommended. For immunocompromised patients who are unable to mount a strong immune response, serologic results may be false-negative for prolonged periods of time, as seen in one of our patients with chronic lymphocytic leukemia.
Owing to the small sample size, we cannot draw definitive conclusions in terms of risk for mortality and neurologic sequelae. The median age of the 3 patients who died was higher than that of survivors, but this difference was not significant. On the other hand, 2 of the 3 patients who died were immunocompromised owing to chronic lymphocytic leukemia. These 3 patients had abnormal MR imaging findings, consistent with cerebellitis in 2 and left basal involvement in 1. All 3 had abnormal EEG findings, ranging from nonspecific slowing to seizures. Given these cases, we suggest that being immunosuppressed, male, having seizures and cerebellitis on imaging may be associated with a higher mortality risk [17, 28].
Our study has several limitations. First, our sample size was small, which may limit the generalizability of our findings. Second, our study was retrospective, and we could not control for all potential confounding factors. Finally, we were not able to assess the impact of treatment on mortality risk, owing to the limited availability of effective treatments for POWV infections.
In summary, we recommend that POWV infection should be considered in patients who present with acute meningoencephalitis or rhombencephalitis and who live in or travel to endemic areas regardless of tick exposure history. For these patients, we recommend performing lumbar puncture and MR imaging with and without gadolinium contrast. If done early, CSF analysis can demonstrate neutrophilic and lymphocytic mixed pleocytosis, although the most common findings include high protein levels and mild lymphocytic pleocytosis. Normal MR imaging findings do not preclude the diagnosis. Although seizures are relatively rare, subclinical seizures can occur, and EEG should be considered in patients with persistent encephalopathy. Our study provides important insights into the clinical presentation as well as the neurologic profiling of these patients both clinically and by imaging. Our findings underscore the need for increased awareness, surveillance, testing capabilities and prevention of POWV infections, particularly among older patients and those with underlying comorbid conditions.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Maria Alejandra Mendoza, Division of Public Health, Infectious Diseases, and Occupational Medicine News, Mayo Clinic, Rochester, Minnesota, USA.
Reece M Hass, Departement of Neurology, Mayo Clinic, Rochester, Minnesota, USA.
James Vaillant, Division of Public Health, Infectious Diseases, and Occupational Medicine News, Mayo Clinic, Rochester, Minnesota, USA.
Derek R Johnson, Departement of Radiology, Mayo Clinic, Rochester, Minnesota, USA.
Elitza S Theel, Division of Clinical Microbiology, Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, Minnesota, USA.
Michel Toledano, Departement of Neurology, Mayo Clinic, Rochester, Minnesota, USA.
Omar Abu Saleh, Division of Public Health, Infectious Diseases, and Occupational Medicine News, Mayo Clinic, Rochester, Minnesota, USA.
Notes
Financial support. This work was supported by the National Institutes of Health (grant UL1TR002377.).
References
- 1. McLean DM, Donohue WL. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J 1959; 80:708–11. [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Powassan virus. 2023. Available at: https://www.cdc.gov/powassan/statistics.html. Accessed 14 May 2023.
- 3. Beasley DW, Suderman MT, Holbrook MR, Barrett AD. Nucleotide sequencing and serological evidence that the recently recognized deer tick virus is a genotype of Powassan virus. Virus Res 2001; 79:81–9. [DOI] [PubMed] [Google Scholar]
- 4. Hermance ME, Thangamani S. Powassan virus: an emerging arbovirus of public health concern in North America. Vector Borne Zoonotic Dis 2017; 17:453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuno G, Artsob H, Karabatsos N, Tsuchiya KR, Chang GJ. Genomic sequencing of deer tick virus and phylogeny of Powassan-related viruses of North America. Am J Trop Med Hyg 2001; 65:671–6. [DOI] [PubMed] [Google Scholar]
- 6. Telford SR 3rd, Armstrong PM, Katavolos P, et al. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis 1997; 3:165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ebel GD, Kramer LD. Short report: duration of tick attachment required for transmission of Powassan virus by deer ticks. Am J Trop Med Hyg 2004; 71:268–71. [PubMed] [Google Scholar]
- 8. des Vignes F, Piesman J, Heffernan R, Schulze TL, Stafford KC 3rd, Fish D. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J Infect Dis 2001; 183:773–8. [DOI] [PubMed] [Google Scholar]
- 9. Hojgaard A, Eisen RJ, Piesman J. Transmission dynamics of Borrelia burgdorferi s.s. during the key third day of feeding by nymphal Ixodes scapularis (Acari: Ixodidae). J Med Entomol 2008; 45:732–6. [DOI] [PubMed] [Google Scholar]
- 10. Brackney DE, Nofchissey RA, Fitzpatrick KA, Brown IK, Ebel GD. Stable prevalence of Powassan virus in Ixodes scapularis in a northern Wisconsin focus. Am J Trop Med Hyg 2008; 79:971–3. [PMC free article] [PubMed] [Google Scholar]
- 11. Piantadosi A, Solomon IH. Powassan virus encephalitis. Infect Dis Clin North Am 2022; 36:671–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention . Arboviral diseases, neuroinvasive and non-neuroinvasive 2014 case definition. 2023. Available at: https://ndc.services.cdc.gov/case-definitions/arboviral-diseases-neuroinvasive-and-non-neuroinvasive-2014/#:∼:text=A%20clinically%20compatible%20case%20of, signs%20of%20central%20or%20peripheral. Accessed 10 May 2023.
- 13. Frost HM, Schotthoefer AM, Thomm AM, et al. Serologic evidence of Powassan virus infection in patients with suspected Lyme disease. Emerg Infect Dis 2017; 23:1384–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Theel ES, Aguero-Rosenfeld ME, Pritt B, Adem PV, Wormser GP. Limitations and confusing aspects of diagnostic testing for neurologic Lyme disease in the United States. J Clin Microbiol 2019; 57:e01406-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mittal SO, Hassan A, Sanchez J, Robertson C. Powassan virus postencephalitic parkinsonism. Neurol Clin Pract 2017; 7:527–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sung S, Wurcel AG, Whittier S, et al. Powassan meningoencephalitis, New York, New York, USA. Emerg Infect Dis 2013; 19:1549–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piantadosi A, Rubin DB, McQuillen DP, et al. Emerging cases of Powassan virus encephalitis in New England: clinical presentation, imaging, and review of the literature. Clin Infect Dis 2016; 62:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ebel GD. Update on Powassan virus: emergence of a North American tick-borne flavivirus. Annu Rev Entomol 2010; 55:95–110. [DOI] [PubMed] [Google Scholar]
- 19. Estrada-Pena A, de la Fuente J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res 2014; 108:104–28. [DOI] [PubMed] [Google Scholar]
- 20. Piantadosi A, Kanjilal S, Ganesh V, et al. Rapid detection of Powassan virus in a patient with encephalitis by metagenomic sequencing. Clin Infect Dis 2018; 66:789–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Oort BEH, Hovelsrud GK, Risvoll C, Mohr CW, Jore S. A mini-review of Ixodes ticks climate sensitive infection dispersion risk in the Nordic region. Int J Environ Res Public Health 2020; 17:5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bouchard C, Dibernardo A, Koffi J, Wood H, Leighton PA, Lindsay LR. N increased risk of tick-borne diseases with climate and environmental changes. Can Commun Dis Rep 2019; 45:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kemenesi G, Banyai K. Tick-borne flaviviruses, with a focus on Powassan virus. Clin Microbiol Rev 2019; 32:e00106-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jackson AC. Leg weakness associated with Powassan virus infection—Ontario. Can Dis Wkly Rep 1989; 15:123–4. [PubMed] [Google Scholar]
- 25. Santos RI, Hermance ME, Gelman BB, Thangamani S. Spinal cord ventral horns and lymphoid organ involvement in Powassan virus infection in a mouse model. Viruses 2016; 8:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khosla JS, Edelman MJ, Kennedy N, Reich SG. West Nile virus presenting as opsoclonus-myoclonus cerebellar ataxia. Neurology 2005; 64:1095. [DOI] [PubMed] [Google Scholar]
- 27. Klaas JP, Ahlskog JE, Pittock SJ, et al. Adult-onset opsoclonus-myoclonus syndrome. Arch Neurol 2012; 69:1598–607. [DOI] [PubMed] [Google Scholar]
- 28. Khoury MY E, Camargo JF, White JL, et al. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York. U.S.A. Emerg Infect Dis 2013; 19:1926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stiasny K, Aberle JH, Chmelik V, Karrer U, Holzmann H, Heinz FX. Quantitative determination of IgM antibodies reduces the pitfalls in the serodiagnosis of tick-borne encephalitis. J Clin Virol 2012; 54:115–20. [DOI] [PubMed] [Google Scholar]
- 30. Vilibic-Cavlek T, Ferenc T, Vujica Ferenc M, et al. Cross-reactive antibodies in tick-borne encephalitis: case report and literature review. Antibodies (Basel) 2022; 11:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Papa A, Karabaxoglou D, Kansouzidou A. Acute West Nile virus neuroinvasive infections: cross-reactivity with dengue virus and tick-borne encephalitis virus. J Med Virol 2011; 83:1861–5. [DOI] [PubMed] [Google Scholar]
- 32. Madison-Antenucci S, Kramer LD, Gebhardt LL, Kauffman E. Emerging tick-borne diseases. Clin Microbiol Rev 2020; 33:e00083-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taba P, Schmutzhard E, Forsberg P, et al. EAN Consensus review on prevention, diagnosis and management of tick-borne encephalitis. Eur J Neurol 2017; 24:1214-e61. [DOI] [PubMed] [Google Scholar]
- 34. Thomm AM, Schotthoefer AM, Dupuis AP 2nd, et al. Development and validation of a serologic test panel for detection of Powassan virus infection in U.S. patients residing in regions where Lyme disease is endemic. mSphere 2018; 3:e00467-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.