Abstract
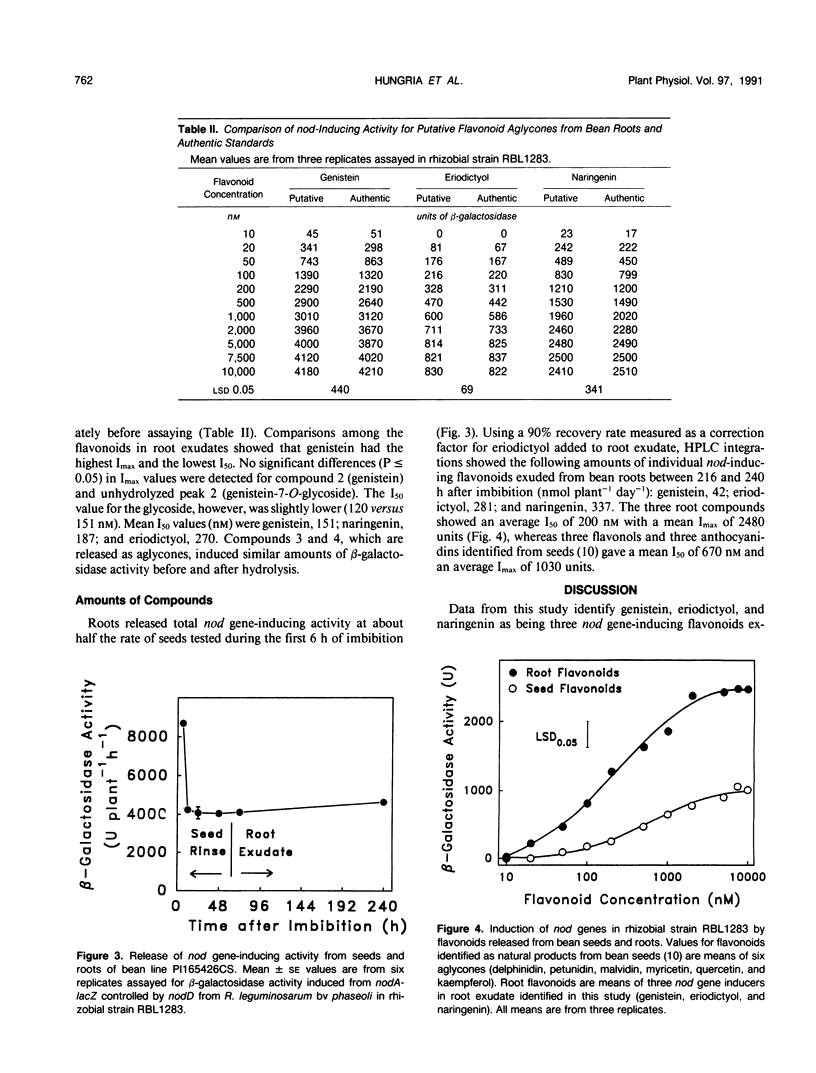
Four compounds exuded from young roots of a black-seeded bean (Phaseolus vulgaris L., cv PI165426CS) induce transcription of nod genes in Rhizobium leguminosarum biovar phaseoli. The three most active nod gene inducers were identified by spectroscopic methods (ultraviolet/visible absorbance, proton nuclear magnetic resonance, and mass spectrometry) as being eriodictyol (5,7,3′,4′ -tetrahydroxyflavanone), naringenin (5,7,4′ -trihydroxyflavanone), and a 7-O-glycoside of genistein (5,7,4′ -trihydroxyisoflavone). Comparisons with authentic standards verified the chemical structures of the aglycones and their capacity to induce β-galactosidase activity in R. leguminosarum strains containing nodA-lacZ or nodC-lacZ fusions controlled by R. leguminosarum biovar phaseoli nodD genes. Roots of 9-day-old seedlings released 42, 281, and 337 nanomoles per plant per day of genistein, eriodictyol, and naringenin, respectively. Genistein and naringenin induced higher maximum β-galactosidase activities and required lower concentrations for half-maximum induction than eriodictyol. Comparing the nod gene-inducing activity of seed rinses with root exudate from PI165426CS bean showed that root flavonoids were released at about 6% the rate of those from seeds on a molar basis, but on average the individual compounds from roots were approximately three times more active than nod gene inducers from seeds.
Full text
PDF
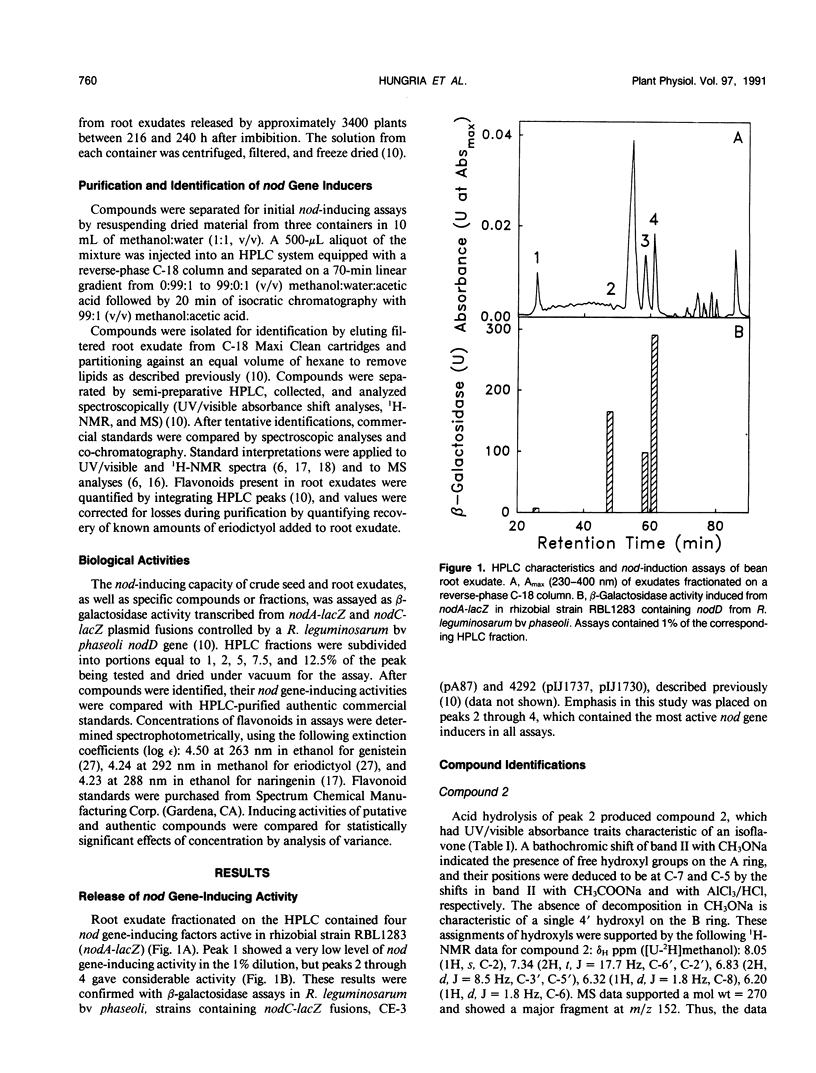



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis E. O., Johnston A. W. Regulatory functions of the three nodD genes of Rhizobium leguminosarum biovar phaseoli. Mol Microbiol. 1990 Jun;4(6):933–941. doi: 10.1111/j.1365-2958.1990.tb00666.x. [DOI] [PubMed] [Google Scholar]
- Hartwig U. A., Joseph C. M., Phillips D. A. Flavonoids Released Naturally from Alfalfa Seeds Enhance Growth Rate of Rhizobium meliloti. Plant Physiol. 1991 Mar;95(3):797–803. doi: 10.1104/pp.95.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig U. A., Maxwell C. A., Joseph C. M., Phillips D. A. Chrysoeriol and Luteolin Released from Alfalfa Seeds Induce nod Genes in Rhizobium meliloti. Plant Physiol. 1990 Jan;92(1):116–122. doi: 10.1104/pp.92.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig U. A., Maxwell C. A., Joseph C. M., Phillips D. A. Effects of alfalfa nod gene-inducing flavonoids on nodABC transcription in Rhizobium meliloti strains containing different nodD genes. J Bacteriol. 1990 May;172(5):2769–2773. doi: 10.1128/jb.172.5.2769-2773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungria M., Joseph C. M., Phillips D. A. Anthocyanidins and Flavonols, Major nod Gene Inducers from Seeds of a Black-Seeded Common Bean (Phaseolus vulgaris L.). Plant Physiol. 1991 Oct;97(2):751–758. doi: 10.1104/pp.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bookland R., Barkei J., Paaren H. E., Appelbaum E. R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Joshi R. S., Bowen B. A., Paaren H. E., Appelbaum E. R. Strain-Specific Inhibition of nod Gene Induction in Bradyrhizobium japonicum by Flavonoid Compounds. Appl Environ Microbiol. 1990 May;56(5):1333–1341. doi: 10.1128/aem.56.5.1333-1341.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- Maxwell C. A., Hartwig U. A., Joseph C. M., Phillips D. A. A Chalcone and Two Related Flavonoids Released from Alfalfa Roots Induce nod Genes of Rhizobium meliloti. Plant Physiol. 1989 Nov;91(3):842–847. doi: 10.1104/pp.91.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Peters N. K., Long S. R. Alfalfa Root Exudates and Compounds which Promote or Inhibit Induction of Rhizobium meliloti Nodulation Genes. Plant Physiol. 1988 Oct;88(2):396–400. doi: 10.1104/pp.88.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recourt K., Schripsema J., Kijne J. W., van Brussel A. A., Lugtenberg B. J. Inoculation of Vicia sativa subsp. nigra roots with Rhizobium leguminosarum biovar viciae results in release of nod gene activating flavanones and chalcones. Plant Mol Biol. 1991 May;16(5):841–852. doi: 10.1007/BF00015076. [DOI] [PubMed] [Google Scholar]
- Tsai S. M., Phillips D. A. Flavonoids released naturally from alfalfa promote development of symbiotic glomus spores in vitro. Appl Environ Microbiol. 1991 May;57(5):1485–1488. doi: 10.1128/aem.57.5.1485-1488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vázquez M., Dávalos A., de las Peñas A., Sánchez F., Quinto C. Novel organization of the common nodulation genes in Rhizobium leguminosarum bv. phaseoli strains. J Bacteriol. 1991 Feb;173(3):1250–1258. doi: 10.1128/jb.173.3.1250-1258.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


