Abstract
Pseudomembranes in the large and small intestines are common in hospitalized patients that are immunosuppressed or on certain oral antibiotics. Pseudomembranous enterocolitis, histologically characterized by volcanic-like eruption of inflammatory cellular exudate from the mucosal surface, is mainly attributed to Clostridium difficile toxins and often presents with symptomatic diarrhea. Rarely, there are case reports of similar pseudomembranous lesions limited to the stomach in the absence of intestinal involvement. In this paper, we present a case of localized pseudomembranous gastritis in a 76-year-old patient with personal history limited to prior gastrointestinal bleed, liver cirrhosis, alcohol dependence, diabetes mellitus, and hypertension who was referred to the emergency department from his primary care physician’s office due to low hemoglobin.
Keywords: pseudomembranous gastritis, idiopathic gastritis, hypertensive gastropathy
Introduction
Pseudomembranous enterocolitis, commonly attributed to Clostridium difficile toxins, is not an uncommon occurrence in the large and/or small intestine of hospitalized patients on certain oral antibiotic therapies. Additional risk factors for C. difficile infection include prior C. difficile infection, advanced age, gastric-acid suppressing medications, immunodeficiency, and inflammatory bowel disease [1, 2]. Less commonly, pseudomembranous colitis can be attributed to conditions such as ischemic colitis, inflammatory bowel disease, vasculitis, cytomegalovirus-induced colitis, bacterial and parasitic infection, and toxins [1]. Clinical symptoms of C. difficile-associated pseudomembranous colitis are due to toxins TcdA and TcdB that modulate inflammatory events and disrupt cell function [3]. Patients with pseudomembranous colitis present with diarrhea as the most common symptom. Other symptoms include fever, abdominal pain, and leukocytosis [1]. Histologically, pseudomembranous colitis is described as injured epithelium with a ‘mushroom-like cloud’ or ‘volcanic’ eruption of necrotic and inflammatory cellular debris that overlies the mucosal surface. The adjacent mucosa may be normal or covered in pseudomembrane [4, 5].
Pseudomembranous gastritis with similar gross and histomorphologic findings, without evidence of pseudomembranous enterocolitis, has rarely been described in isolated case studies, occurring as complications in patients with variable clinical and gastrointestinal symptoms [6–8]. Pseudomembranous gastritis has been reported as a complication in patients with Aspergillus infection [6, 7], hepatitis and iritis on carbimazole therapy for Graves’ disease [9], as well as an incidental finding with unknown contributing factors. The gross findings have been described as either diffuse thick exudate overlying the entire gastric mucosa, embedded with Aspergillus species, or a localized endoscopic finding without a discernible contributing causation.
Case presentation
A 76-year-old male was referred to the emergency department from a primary care physician’s office due to low hemoglobin level. The patient’s past medical history was significant for gastrointestinal (GI) bleeding, hepatic cirrhosis, diabetes mellitus, alcohol dependence, and hypertension. The patient otherwise denied significant complaints. Home medications did not list any antibiotics but included: xifaxan tabs, protonix tabs, lovastatin, cephulac, furosemide, ferrous sulfate, lasix, calcium carbonate/vitamin D3, alendronate sodium, and metformin. Physical exam revealed a distended but nontender abdomen and guaiac-positive-stool. Significant laboratory findings included the following: hemoglobin 6.9 g/dL, WBC 3.8 K/mm3, hematocrit 20.8%, platelets 73 K/mm3, alkaline phosphatase 150 IU/L, AST 38 IU/L, and ALT 31 IU/L.
Guaiac positivity and patient’s low hemoglobin level raised concern for possible GI bleed as a source of patient’s anemia. Computed tomography (CT) of the abdomen revealed a cirrhotic liver with possible portal hypertension, splenomegaly, small volume ascites, and a small hiatal hernia.
Esophagogastroduodenoscopy revealed varices in the esophagus as well as the gastric cardia and fundus. Multiple 10–20 mm sessile polyps were also present in the prepyloric and antral regions with some associated pseudomembranous-like exudate (Fig. 1).
Figure 1.
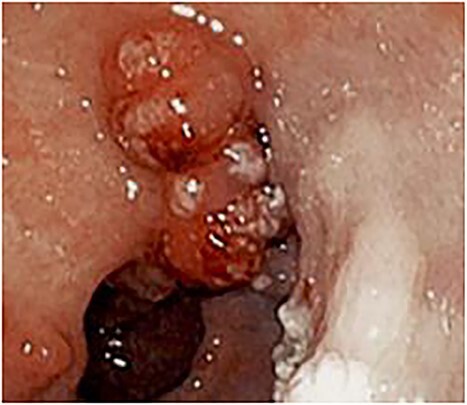
Endoscopy showing 10–20 mm sessile polyps and pseudomembranous-like gray-white exudate overlying the gastric mucosa in the prepyloric and antral regions of stomach.
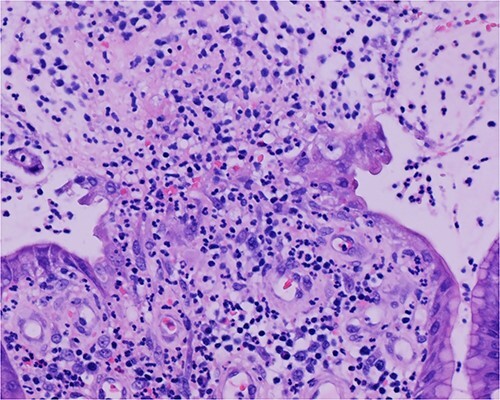
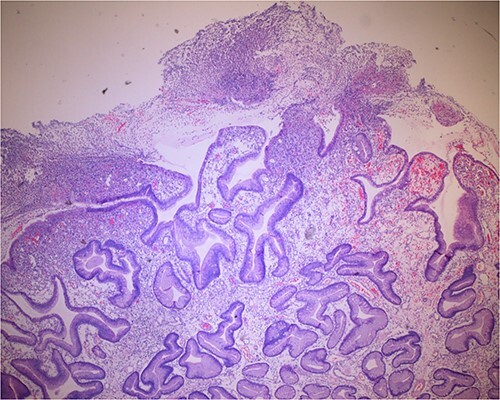
Histologic sections of the polyps demonstrated benign gastric hyperplastic polyps with acute and chronic gastritis (Fig. 2). Portions of the gastric polyps and adjacent mucosa were covered by inflammatory cell exudate (Fig. 3). Higher magnification revealed conspicuous volcanic-like eruption of the exudate, reminiscent of pseudomembranous gastritis (Fig. 4). Alcian yellow and GMS stains were negative for Helicobacter pylori and fungal organisms, respectively.
Figure 2.
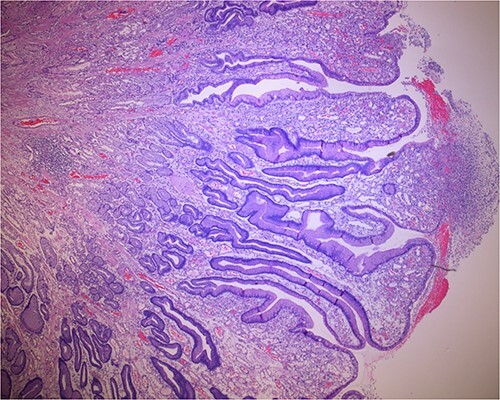
Biopsy of the gastric polyps is consistent with hyperplastic polyps, focally covered by inflammatory volcanic eruption.
Figure 3.
Biopsy of the polyp, covered in part by pseudomembranous inflammatory exudate.
Figure 4.
Volcanic-like eruption of inflammatory exudate, reminiscent of pseudomembranous gastritis.
Discussion
Clostridium difficile toxins are a common cause of extensive pseudomembrane formation, most frequently see in the small and large intestine. Histologically, they are characterized by volcanic-like eruption of inflammatory cell exudate overlying the mucosal surfaces [1, 10]. Pseudomembranous gastritis, on the other hand, is rare and has only been described as isolated case presentations, occurring in patients without evidence of C. difficile as a contributing factor [10]. Though not on antibiotics, our patient presented with a history of chronic alcohol dependence, possibly resulting in disturbances in the gut microbiome [11]. Furthermore, the patient’s history of hepatic cirrhosis may be a contributing factor to an altered immune status, predisposing the patient to an inflammatory or infectious process [12], such as pseudomembranous gastritis.
In conclusion, we describe a case of nonantibiotic-associated localized pseudomembranous gastritis, occurring in a patient with alcohol dependence, hepatic cirrhosis, and reactive/inflammatory gastric polyps, who was otherwise asymptomatic. Based on our study and review of literature, pseudomembranous gastritis may represent a nonspecific finding, seen as an incidental finding or a complication in a variety of nonspecific altered immune disorders, infection, or inflammatory processes.
Conflict of interest statement
None declared.
Funding
None declared.
Contributor Information
Pierre Tran, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, United States.
Rama Sai P, Master of Sciences Department, University of Life Sciences, Lublin, Poland.
Chaya Prasad, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, United States.
Cyrus Parsa, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, United States; Department of Pathology, Beverly Hospital, Montebello, CA 90640, United States .
References
- 1. Salen P, Stankewicz HA. Pseudomembranous Colitis. In: Aboubakr S, (ed). StatPearls. Treasure Island, Florida: StatPearls Publishing, 2023. http://www.ncbi.nlm.nih.gov/books/NBK470319/ (16 October 2023, date last accessed). [PubMed] [Google Scholar]
- 2. Farooq PD, Urrunaga NH, Tang DM, Rosenvinge EC. Pseudomembranous colitis. Dis Mon 2015;61:181–206. 10.1016/j.disamonth.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev 2005;18:247–63. 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gulwani H. Pseudomembranous colitis. Pathology Outlines. Published 1 May 2013. https://www.pathologyoutlines.com/topic/colonaacolitis.html (16 October 2023, date last accessed). [Google Scholar]
- 5. Price AB, Davies DR. Pseudomembranous colitis. J Clin Pathol 1977;30:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yong S, Attal H, Chejfec G. Pseudomembranous gastritis: a novel complication of aspergillus infection in a patient with a bone marrow transplant and graft versus host disease. Arch Pathol Lab Med 2000;124:619–24. 10.5858/2000-124-0619-PG. [DOI] [PubMed] [Google Scholar]
- 7. Sanders DL, Pfeiffer RB, Hashimoto LA, Subramony C, Chen F. Pseudomembranous gastritis: a complication from aspergillus infection. Am Surg 2003;69:536–8. [PubMed] [Google Scholar]
- 8. Kulkarni M, Saxena R, Hammad S, Nicola M, Batke M. Pseudomembranous gastritis of unknown Etiology: 1459. Am J Gastroenterol 2018;113:S837. [Google Scholar]
- 9. Oswald G, Mullen P, Chinyama C. Pseudomembranous gastritis, hepatitis and iritis complicating carbimazole therapy for Graves disease. Endocrine Abstracts 2005;9. https://www.endocrine-abstracts.org/ea/0009/ea0009p168 (30 July 2023, date last accessed). [Google Scholar]
- 10. Mylonakis E, Ryan ET, Calderwood SB. Clostridium difficile–associated Diarrhea: a review. Arch Intern Med 2001;161:525–33. 10.1001/archinte.161.4.525. [DOI] [PubMed] [Google Scholar]
- 11. Sarkar D, Jung MK, Wang HJ. Alcohol and the immune system. Alcohol Res 2015;37:153–5. [Google Scholar]
- 12. Noor MT, Manoria P. Immune dysfunction in cirrhosis. J Clin Transl Hepatol 2017;5:50–8. 10.14218/JCTH.2016.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]


