Abstract
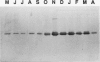
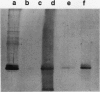
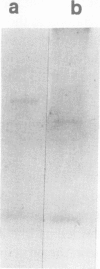
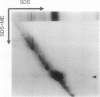
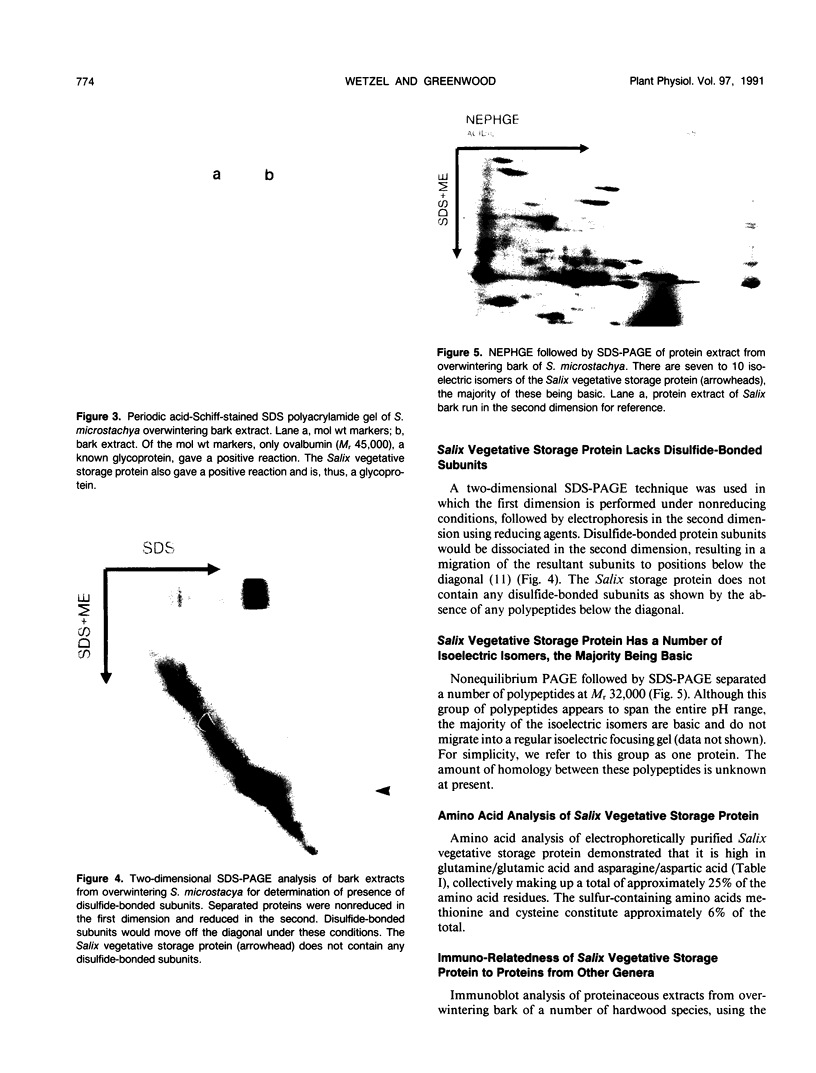
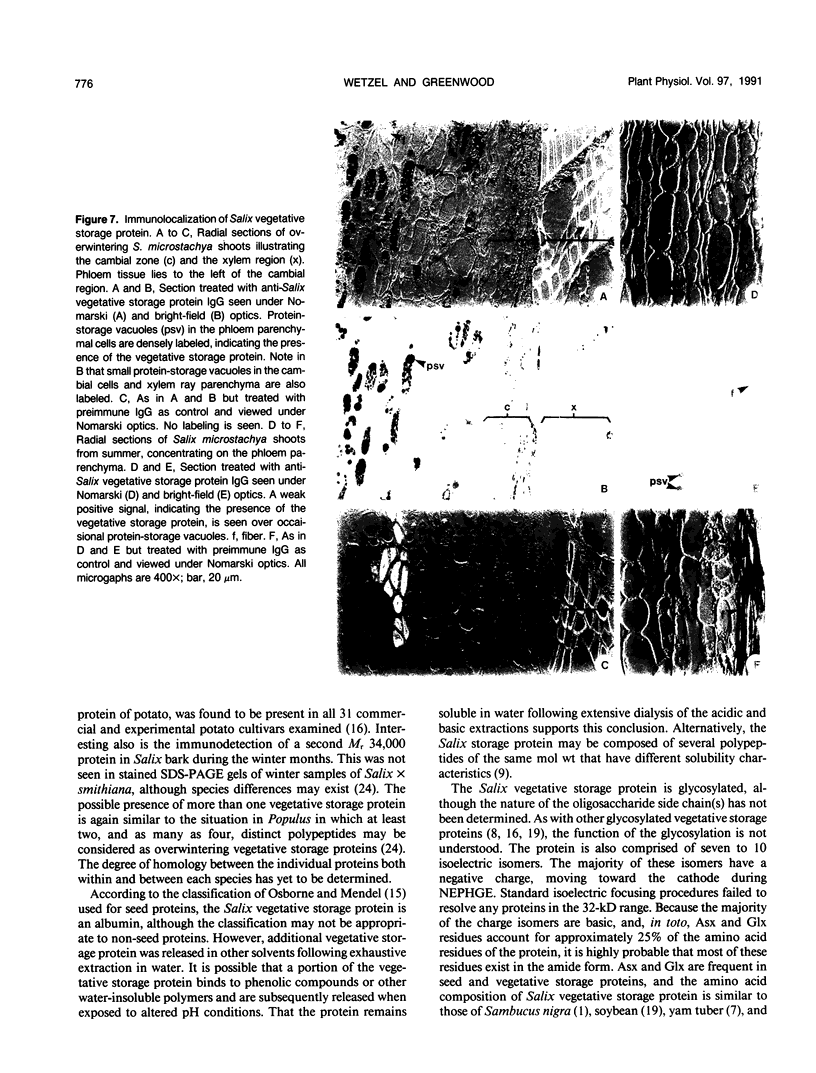
A 32-kilodalton vegetative storage protein, found in Salix microstachya Turz. bark during the overwintering period, was purified and characterized using several polyacrylamide gel electrophoretic procedures. Solubility characteristics and amino acid analyses were also performed. The protein is water soluble, is glycosylated, has no disulfide-bonded subunits, but is composed of a family of isoelectric isomers. The majority of these isomers are basic. Characteristic of storage proteins, the protein is rich in glutamine/glutamate and asparagine/aspartate (28%), the basic nature of the isomers indicating that most of these amino acid residues are in the amide form. The protein was purified using preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis and antibodies raised in chickens. Immunoblot analysis suggested an annual cyclic nature of the accumulation and mobilization of this vegetative storage protein. Immunologically, it is related to a similar molecular weight protein found in the bark of Populus deltoides Marsh. but not to any overwintering storage proteins of the other hardwoods tested. Indirect immunolocalization revealed that the protein was sequestered in protein-storage vacuoles in parenchymatous cells of the inner bark tissues of Salix during the winter months.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broekaert W. F., Nsimba-Lubaki M., Peeters B., Peumans W. J. A lectin from elder (Sambucus nigra L.) bark. Biochem J. 1984 Jul 1;221(1):163–169. doi: 10.1042/bj2210163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman E. M., Hankins C. N., Shannon L. M. Bark and Leaf Lectins of Sophora japonica Are Sequestered in Protein-Storage Vacuoles. Plant Physiol. 1988 Apr;86(4):1027–1031. doi: 10.1104/pp.86.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Esen A. Heterogeneity of soybean seed proteins: one-dimensional electrophoretic profiles of six different solubility fractions. J Agric Food Chem. 1981 May-Jun;29(3):497–501. doi: 10.1021/jf00105a015. [DOI] [PubMed] [Google Scholar]
- Jensenius J. C., Andersen I., Hau J., Crone M., Koch C. Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG. J Immunol Methods. 1981;46(1):63–68. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- Krochko J. E., Bewley J. D. Use of electrophoretic techniques in determining the composition of seed storage proteins in alfalfa. Electrophoresis. 1988 Nov;9(11):751–763. doi: 10.1002/elps.1150091111. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Staswick P. E. Developmental regulation and the influence of plant sinks on vegetative storage protein gene expression in soybean leaves. Plant Physiol. 1989 Jan;89(1):309–315. doi: 10.1104/pp.89.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P. E. Novel Regulation of Vegetative Storage Protein Genes. Plant Cell. 1990 Jan;2(1):1–6. doi: 10.1105/tpc.2.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]