Abstract
O-linked β-N-acetylglucosamine (O-GlcNAcylation) is a dynamic post-translational modification that regulates thousands of proteins and almost all cellular processes. Aberrant O-GlcNAcylation has been associated with numerous diseases, including cancer, neurodegenerative diseases, cardiovascular diseases, and type 2 diabetes. O-GlcNAcylation is highly nutrient-sensitive since it is dependent on UDP-GlcNAc, the end product of the hexosamine biosynthetic pathway (HBP). We previously observed daily rhythmicity of protein O-GlcNAcylation in a Drosophila model that is sensitive to the timing of food consumption. We showed that the circadian clock is pivotal in regulating daily O-GlcNAcylation rhythms given its control of the feeding-fasting cycle and hence nutrient availability. Interestingly, we reported that the circadian clock also modulates daily O-GlcNAcylation rhythm by regulating molecular mechanisms beyond the regulation of food consumption time. A large body of work now indicates that O-GlcNAcylation is likely a generalized cellular status effector as it responds to various cellular signals and conditions, such as ER stress, apoptosis, and infection. In this review, we summarize the metabolic regulation of protein O-GlcNAcylation through nutrient availability, HBP enzymes, and O-GlcNAc processing enzymes. We discuss the emerging roles of circadian clocks in regulating daily O-GlcNAcylation rhythm. Finally, we provide an overview of other cellular signals or conditions that impact O-GlcNAcylation. Many of these cellular pathways are themselves regulated by the clock and/or metabolism. Our review highlights the importance of maintaining optimal O-GlcNAc rhythm by restricting eating activity to the active period under physiological conditions and provides insights into potential therapeutic targets of O-GlcNAc homeostasis under pathological conditions.
Keywords: circadian clock, Drosophila melanogaster, metabolism, signal transduction, hexosamine biosynthetic pathway, glutamine fructose-6-phosphate aminotransferase, O-GlcNAc processing enzymes, OGT, OGA
Protein O-linked β-N-acetylglucosamine (O-GlcNAcylation) is a unique type of glycosylation, where N-acetylglucosamine (GlcNAc) dynamically cycles on serine and threonine residues of proteins. Since its first discovery in the 1980s in lymphocytes (1), O-GlcNAcylation has been widely found in over 16,000 proteins to date in all domains of life (2). These O-GlcNAcylated proteins are involved in a wide range of fundamental biological processes (3, 4, 5, 6, 7, 8), including transcription, translation, nutrient sensing, immune response, cell signaling, cell cycle, and circadian clocks. Importantly, aberrant O-GlcNAcylation has become the hallmark of many diseases (5, 9, 10, 11, 12), such as cancer, neurodegenerative diseases, cardiovascular diseases, insulin resistance, and type 2 diabetes.
Given the indispensable role of O-GlcNAcylation under both physiological and pathological conditions, it is critical to understand the mechanisms that regulate the cycling of O-GlcNAcylation. O-GlcNAcylation is conventionally considered nutrient-sensitive because it is dependent on the substrate UDP-GlcNAc, the end product of the hexosamine biosynthetic pathway (HBP). HBP is a critical pathway that integrates glucose, amino acid, lipid, and nucleotide metabolism (5). After UDP-GlcNAc is produced, O-GlcNAc transferase (OGT) catalyzes the addition of GlcNAc groups onto proteins, i.e. O-GlcNAcylation (13, 14, 15), while O-GlcNAcase (OGA) catalyzes the removal of GlcNAc group (16, 17). Indeed, metabolic input determines substrate availability of O-GlcNAcylation (8, 18, 19, 20, 21). Decades of investigations also uncovered the regulation of O-GlcNAcylation by metabolic input via the modulation of HBP enzymes and O-GlcNAc processing enzymes.
Accumulating evidence showed that many other cellular signals and conditions also contribute to the regulation of protein O-GlcNAcylation. Notably, the circadian clock has been shown to shape the daily rhythm of protein O-GlcNAcylation (18). The circadian clock is an endogenous biochemical timer that allows organisms to anticipate predictable environmental changes over the 24-h day-night cycles, such as daily light-dark cycles and temperature cycles (22, 23, 24, 25). In animals, daily oscillations of physiological, metabolic, and behavioral processes are strongly regulated by circadian clocks (22, 23, 24, 25). Therefore, the regulation of protein O-GlcNAcylation by circadian clocks could be imposed at multiple levels. At the behavioral level, the circadian clock controls daily feeding-fasting behavior to rhythmically provide metabolic input for UDP-GlcNAc production and O-GlcNAcylation (18). Indeed, under physiological conditions, the daily feeding-fasting cycle is shown to correlate with daily O-GlcNAcylation rhythm in mouse hearts (26) and Drosophila tissues (18). At the cellular level, circadian clocks can orchestrate daily rhythms in mRNA, protein abundance, and/or enzymatic activities of HBP enzymes and O-GlcNAc processing enzymes (18, 27, 28, 29). Finally, a number of other factors also influence O-GlcNAcylation, such as endoplasmic reticulum (ER) stress, oxidative stress, infection, apoptosis, development, and aging, to name a few (30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40).
In this review, we will provide a broad overview of the metabolic, circadian, and cellular signals that regulate protein O-GlcNAcylation. We will summarize the effect of nutrient availability on HBP and O-GlcNAcylation, highlight the metabolic regulation of protein O-GlcNAcylation through impacting HBP and O-GlcNAc processing enzymes, discuss the evidence on the regulation of daily O-GlcNAcylation rhythms by the circadian clock, and outline other cellular signals that modulate protein O-GlcNAcylation.
The regulation of protein O-GlcNAcylation by nutrient availability
Depending on the cell type and metabolic status of the cell, 0.003% to 3% of the glucose in cells feeds into the HBP pathway (41, 42, 43). In primary cultured adipocytes, Marshall et al. (41) indirectly estimated that 2% to 3% of glucose feeds into the HBP by measuring the relative glucosamine versus glucose utilization of the cells. Gibb et al. (42) concluded that the HBP in cultured neonatal cardiomyocytes utilizes more glucose than the pentose phosphate pathway by measuring the overall levels of isotope-labeled UDP-HexNAc. Their study suggested that previous studies severely underestimated glucose flux into the HBP. Olson et al. (43) directly monitored glucose flux through the HBP using isotope tracking in an isolated working mouse heart and observed that only 0.003% to 0.006% of glucose is metabolized through the HBP. Future investigations are warranted to determine the glucose fluxes into the HBP in different cell types, organs, and species and investigate how glucose fluxes are differentially regulated in different cell types and organs.
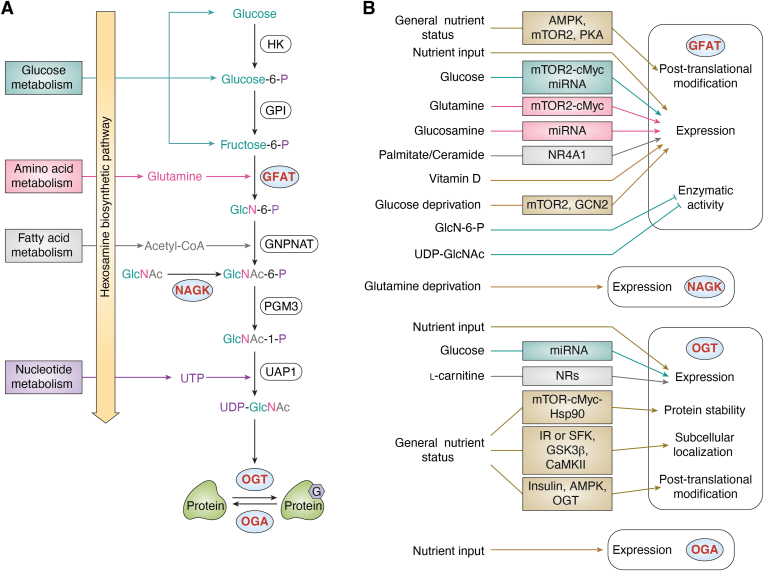
The HBP then converts metabolites from amino acid, lipid, and nucleotide metabolism to produce UDP-GlcNAc (Fig. 1A). Since the activity of OGT is highly responsive to UDP-GlcNAc concentration with multiple Km values (44), the effect of nutrient availability on UDP-GlcNAc level has been of great interest. Generally, UDP-GlcNAc levels in many tissue culture experiments have been observed to change in accordance with the levels of various nutrients, including glucose, glutamine, glucosamine, acetylglucosamine, uridine, palmitate, and stearate (19, 20, 21, 45, 46, 47, 48, 49) (Fig. 1B). Moreover, the correlation between nutrient availability and UDP-GlcNAc has also been investigated in animals. The UDP-GlcNAc level has been shown to increase in rat skeletal muscles after infusion of lipid emulsion, uridine, or GlcN for 7 h (50). In Drosophila, the daily UDP-GlcNAc level correlates with feeding rhythm under ad libitum conditions (18). This correlation has been confirmed by time-restricted feeding paradigms. When the fly feeding activity was restricted to different 6-h windows within a day-night cycle, the timing of the peak of the daily UDP-GlcNAc rhythm shifted according to the timing of food consumption.
Figure 1.
Metabolic regulation of protein O-GlcNAcylation.A, nutrient input from feeding activity or cell culture media provides essential metabolites for hexosamine biosynthetic pathway (HBP). The end product of HBP, UDP-GlcNAc, is the substrate for protein O-GlcNAcylation. The key enzymes that are under additional metabolic regulation are highlighted in blue circle. B, nutrient level regulates the activities of glutamine:fructose-6-phosphate amidotransferase (GFAT) (13, 44, 54, 60, 61, 62, 63, 64, 66, 67, 68, 69, 70, 71, 72, 73, 76, 77), N-acetylglucosamine kinase (NAGK) (78), O-GlcNAc transferase (OGT) (13, 14, 55, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91) and O-GlcNAcase (OGA) (13). These enzymes represent hubs for metabolic regulation of O-GlcNAcylation. The expression denotes mRNA and/or protein expression of indicated enzymes. Functional groups of each metabolite are color-coded to highlight the processes that contribute to their metabolism. Other metabolites or nutrient status that contribute to protein O-GlcNAcylation are illustrated in brown. O-GlcNAc is depicted as G on protein. AMPK, AMP-activated protein kinase; CaMKII, calcium–calmodulin (CaM)-dependent protein kinase II; GCN2, general control nonderepressible 2; GFAT, glutamine–fructose-6-phosphate aminotransferase; GlcN-6-P, glucosamine-6-phosphate; GlcNAc, N-acetylglucosamine; GlcNAc-1-P, N acetylglucosamine-1-phosphate; GlcNAc-6-P, N-acetylglucosamine-6-phosphate; GNPNAT, glucosamine-phosphate N-acetyltransferase; GPI, phosphoglucose isomerase; GSK3β, glycogen synthase kinase-3 β; HK, Hexokinase; Hsp90, Heat shock protein 90; IR, insulin receptor; miRNA, microRNA; mTOR, mechanistic target of rapamycin; NR, nuclear receptor; NR4A1, nuclear subfamily four group A member one; OGA, O-GlcNAcase; OGT, O-GlcNAc transferase; PGM3, phosphoacetylglucosamine mutase; PKA, protein kinase A; PTM, post-translational modification; SFK, Src family kinases, UAP1, UDP-N-acetyl glucosamine pyrophosphorylase one; UDP-GlcNAc, uridine diphosphate N-acetylglucosamine; UTP, uridine triphosphate.
Experiments to determine whether protein O-GlcNAcylation level corresponds to nutrient availability have produced mixed results so far. In some cell lines or ex vivo tissues, O-GlcNAcylation levels increase as the level of glucose, glucosamine, and glutamine increases (51, 52, 53, 54) and vice versa (19, 55). Similarly, under pathological conditions, excessive glucose availability during hyperglycemia results in higher O-GlcNAcylation levels (11, 56, 57, 58). In vivo studies in mouse heart and fly tissues also reported that daily O-GlcNAcylation rhythm is correlated with feeding-fasting cycles and nutrient availability (18, 26). Nevertheless, glucose starvation has been shown to increase O-GlcNAcylation in several studies (19, 59, 60). In addition, when flies are fed at unnatural feeding time (i.e. when flies are awake but normally fasting), O-GlcNAcylation rhythm does not shift its peak time to coincide with the shifted time of food consumption, but rather feeding at unnatural time results in dampened O-GlcNAcylation rhythm (18). On one hand, glucose starvation or mistimed eating could modulate OGT, OGA, and glutamine fructose-6-phosphate aminotransferase (GFAT), the rate-limiting enzyme of HBP (See sections below) (19, 59, 60), thus creating more complex scenarios rather strict correlation between nutrient availability and O-GlcNAcylation level. Indeed, mistimed eating alters ogt and oga mRNA levels, as well as GFAT enzymatic activity (18). On the other hand, it is very likely that the response of protein O-GlcNAcylation to glucose starvation or mistimed eating could be cell type or tissue-specific, as Pham et al. (55) demonstrated that nutrient deprivation differentially influences protein O-GlcNAcylation in a number of cell lines.
In conclusion, despite that nutrient availability mostly determines UDP-GlcNAc production in cell lines and tissues, cellular O-GlcNAcylation level does not always correspond to nutrient availability. This suggests that additional cellular factors regulate protein O-GlcNAcylation. Future investigations into conservation and differences in the regulation of UDP-GlcNAc production and O-GlcNAcylation by nutrient availability in different organs and species, especially diurnal versus nocturnal animals, will certainly provide new insights into the regulation of protein O-GlcNAcylation by nutrient availability.
Metabolic regulation of protein O-GlcNAcylation through regulation of HBP enzymes
In addition to nutrient availability, the activity of HBP enzymes also plays critical roles in UDP-GlcNAc production. Four key enzymes are involved in HBP: GFAT, glucosamine-phosphate N-acetyltransferase (GNPNAT), GlcNAc phosphomutase (PGM3/AGM1), and UDP-N-acetylglucosamine pyrophosphorylase (UAP1/AGX1) (Fig. 1A).
As the rate-limiting enzyme of HBP (61), GFAT is the most studied to date. In animals, two GFAT paralogs, GFAT1 and GFAT2, share 75% to 80% amino acid sequence. They are functionally equivalent but distributed in different tissues or cell types (62, 63, 64). At the mRNA level, gfat is responsive to metabolic signals (Fig. 1B). Many macronutrients stimulate gfat mRNA expression, including glucose (65, 66, 67), glutamine (67), glucosamine (65), palmitate (49, 68), stearate (49), and ceramide (68) (Fig. 1B). The effect of glucose and glutamine on gfat1 expression is mediated by mTOR2-cMYC axis (67), while nuclear receptor subfamily four group A member 1 (NR4A1) transcription factor can respond to palmitate and ceramide levels to increase gfat2 mRNA (68). Moreover, glucose and glucosamine can upregulate gfat mRNA levels by inhibiting microRNA (miR)-27b-3p (65) (Fig. 1B). In addition to evidence from cell culture experiments described above, we showed that gfat2 mRNA in fly tissues, but not gfat1 mRNA, is strongly responsive to nutrient input via feeding activity (18). On the contrary, nutrient deprivation also increases gfat1 mRNA expression. This could be mediated by mTOR2 (69) and/or general control nonderepressible 2 (GCN2)-activating transcription factor 4 (ATF4) pathways (59) (Fig. 1B). Although these observations are counterintuitive considering the nutrient sensitivity of HBP, the increased gfat1 expression is speculated to have protective roles upon moderate nutrient deprivation (59, 69). In addition to macronutrients, vitamin D can inhibit gfat expression in rat hearts through an unknown mechanism (70) (Fig. 1B).
At the posttranslational level, GFAT is phosphorylated by nutrient-sensing kinases, including AMP-activated protein kinase (AMPK), mTOR complex 2 (mTORC2), and protein kinase A (PKA) (Fig. 1B). AMPK, an energy sensor, phosphorylates GFAT1 at serine 243 (S243) (71, 72). mTORC2 also phosphorylates S243 in response to glucose and glutamine concentration in cell media (73). Furthermore, nucleoside diphosphate kinase B (NDPKB) counteracts S243 phosphorylation through an unknown mechanism (74). Despite that the kinases involved are characterized, how S243 phosphorylation modulates GFAT activity remains controversial. Moloughney et al. (73) reported that S243 phosphorylation increases GFAT1 activity, while Eguchi et al. (71) showed that S243 phosphorylation downregulates GFAT1 activity. This could be due to different cell lines utilized in these studies (mouse embryonic fibroblasts and HeLa cells in (73), Chinese hamster ovary cells overexpressing insulin receptors in (71)). Finally, under fasting or starvation conditions, GFAT exhibits lower activity (75, 76), which could be mediated by PKA (75) (Fig. 1B). PKA-directed S205 phosphorylation reduces GFAT1 activity (75, 77). The function of PKA-dependent GFAT1(S235) phosphorylation remains unknown (75). On the contrary, PKA is also shown to increase GFAT activity in two other studies (78, 79). Given that PKA also phosphorylates GFAT2(S202) and increases GFAT2 activity by 2.2-fold (80), increased GFAT2 activity may counteract the PKA-dependent reduction of GFAT1 activity (79). Therefore, the effect of PKA on GFAT activity could exhibit cell type specificity depending on relative expression levels of GFAT1 and GFAT2.
Finally, HBP metabolites can feedback to regulate GFAT activity. In the early 2000s, in vitro studies showed that glucosamine-6-phosphate (GlcN-6-P), the immediate product of the GFAT-catalyzed reaction, and UDP-GlcNAc both inhibit GFAT activity (78, 81) (Fig. 1B). Follow-up structural biology analysis showed that UDP-GlcNAc interacts with an interdomain linker to allosterically downregulate GFAT activity (82).
In summary, significant progress has been made to uncover the metabolic regulation of gfat mRNA and GFAT protein expression as well as its enzymatic activity. In comparison, studies that examined the metabolic regulation of other HBP enzymes are limited. One recent study revealed that glutamine deprivation increases UDP-GlcNAc levels via N-acetylglucosamine kinase (NAGK)-dependent salvage of GlcNAc, another precursor of UDP-GlcNAc (83). Future investigations of other HBP enzymes will be important to provide a comprehensive understanding of the metabolic regulation of protein O-GlcNAcylation.
Metabolic regulation of protein O-GlcNAcylation through regulation of O-GlcNAc processing enzymes
OGT utilizes UDP-GlcNAc to modify proteins, while OGA reverses this modification. In this section, we discuss the mechanistic regulation of OGT and OGA by nutrient availability and nutrient sensing pathways. Nutrient availability and nutrient sensing pathways can regulate OGT protein expression (Fig. 1B). Feeding activity and hence nutrient availability have been shown to regulate daily OGT expression rhythm in Drosophila animals (18). When flies are subjected to time-restricted feeding (TRF) at different time windows, robust daily oscillation of OGT protein is observed. The peak time of OGT protein rhythm coincides well with the timing of food consumption (18). A similar phenomenon has been observed at the ogt mRNA level under TRF conditions, except for two minor differences: (a) the amplitude of ogt mRNA rhythm remains the same when the timing of food consumption is altered and (b) the peak time of ogt mRNA is delayed by a few hours after feeding time (18).
A number of other studies investigated the effect of glucose on OGT expression, producing somewhat contradictory conclusions. Under high glucose conditions, glucose can promote ogt mRNA and subsequently protein levels through inhibiting miR-200a/b (84) (Fig. 1B). Interestingly, ogt mRNA and OGT protein levels have also been shown to be elevated under glucose deprivation (19, 60), although the mechanism is unknown. More recently, L-carnitine has been shown to regulate ogt mRNA expression through the activity of nuclear receptors (85) (Fig. 1B). Finally, it has been reported that mTOR nutrient-sensing pathway can promote OGT protein levels via the cMYC-Hsp90 axis (86, 87, 88) (Fig. 1B).
Regulation of mRNA and protein levels is not the only mechanism by which O-GlcNAc processing enzymes are regulated by metabolic signals. OGT subcellular localization is modulated by nutrient-sensing pathways to achieve temporal O-GlcNAcylation of proteins in different cellular compartments. OGT contains a nuclear localization sequence (NLS) (residue 451–453). Upon O-GlcNAcylation of OGT(S389), importin α5 recognizes the NLS and imports OGT into the nucleus (89) (Fig. 1B). Given that O-GlcNAcylation is responsive to metabolic signals as described in previous sections, OGT(S389) O-GlcNAcylation could be one of the mechanisms by which metabolic signals regulate OGT. In addition, in response to the glucose level in cell media, AMPK also modulates the nuclear translocation of OGT by OGT(T444) phosphorylation, probably through importin α5-dependent mechanisms (90) (Fig. 1B). Finally, the insulin signaling pathway has been shown to recruit OGT to the cytoplasm (91, 92). Upon insulin stimulation, PI(3,4,5)P3 binds the PIP-binding domain of OGT and promotes cytoplasmic localization (92) (Fig. 1B).
Nutrient-sensitive OGT phosphorylation can directly control its enzymatic activity. Insulin signaling promotes phosphorylation of yet uncharacterized tyrosine residue(s) on OGT, possibly by insulin receptor and/or Src protein-tyrosine kinase (91) (Fig. 1B). Downstream of insulin signaling, glycogen synthase kinase 3β (GSK3β) phosphorylates OGT(S3/4) (93) (Fig. 1B). Wang et al. (94) further showed that inhibiting GSK3β alters OGT substrate selectivity, adding an additional layer of OGT regulation by insulin signaling. During nutrient deprivation, calcium-calmodulin-dependent protein kinase II (CaMKII) phosphorylates OGT(S20) and increases OGT activity (95, 96) (Fig. 1B).
Similar to OGT, OGA protein levels are highly dependent on the timing of food consumption in Drosophila animals (18). At the transcript level, oga mRNA is rhythmic when flies feed at natural feeding time but not at unnatural feeding time when they are normally fasting (18), indicating oga/OGT is regulated by both nutrient input via feeding and circadian clocks (see next section). At present, the molecular mechanisms by which oga mRNA and OGA protein are regulated by nutrient availability and/or nutrient sensing pathways are largely unknown. One potential mechanism is that nutrient-sensitive O-GlcNAcylation can modify OGA (97). However the functional outcome of OGA O-GlcNAcylation remains a gap in knowledge. In conclusion, OGT can be regulated by metabolic signals in multiple ways, and more studies are warranted to investigate the metabolic regulation of OGA.
Regulation of daily protein O-GlcNAcylation rhythm by the circadian clock
Given the nutrient sensitivity of protein O-GlcNAcylation discussed in the previous sections, it is perhaps not surprising that protein O-GlcNAcylation oscillates over the 24-h day–night cycle due to rhythmic feeding-fasting behaviors in animals. Indeed, both mice and flies display daily protein O-GlcNAcylation rhythms, primarily peaking during their feeding windows (18, 26) (Fig. 2). Interestingly, several lines of evidence suggest circadian clocks regulate protein O-GlcNAcylation through other mechanisms in addition to the regulation of rhythmic feeding-fasting behavior. First, when flies are forced to feed at unnatural feeding time, protein O-GlcNAcylation rhythm dampens significantly. This indicates circadian clocks may provide buffering mechanisms that prevent protein O-GlcNAcylation from peaking at nonoptimal time. Second, in flies with defective circadian clocks, protein O-GlcNAcylation rhythm also dampens significantly and the levels remain constant throughout a 24-h period (18). This is not purely due to disrupted feeding-fasting activities, given that restricting the feeding time of clock mutant flies at natural feeding time does not rescue O-GlcNAcylation rhythms. We devote this section to reviewing the evidence on the regulation of daily O-GlcNAcylation rhythm by the circadian clock.
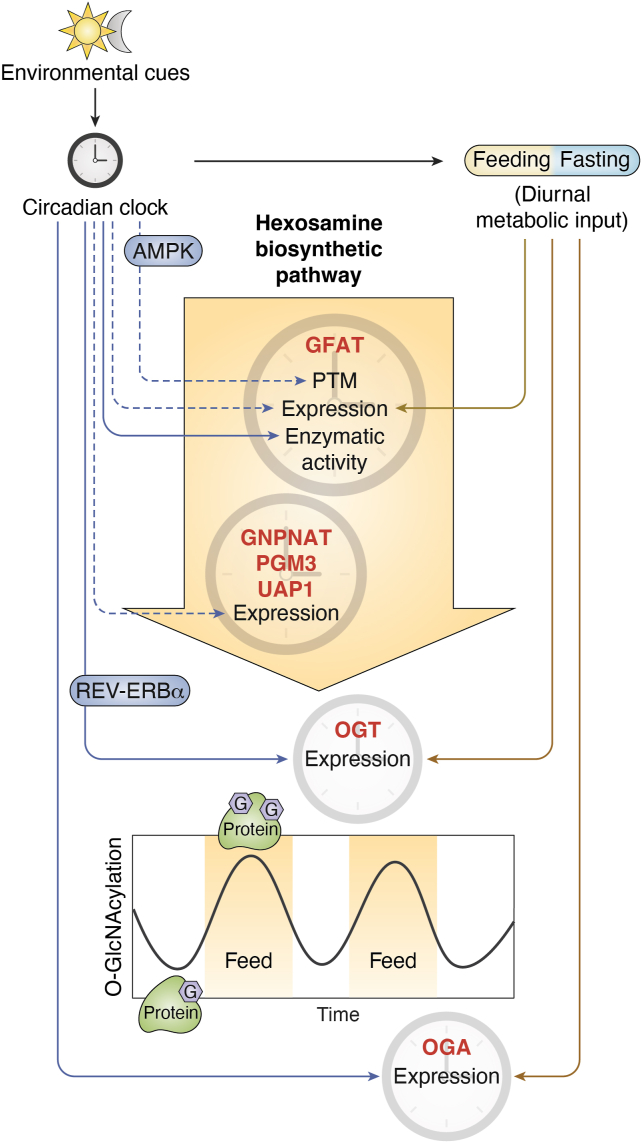
Figure 2.
The circadian clock and metabolic signals from the feeding-fasting cycle shape the daily oscillation of protein O-GlcNAcylation. The circadian clock receives environmental signals, such as light-dark cycles, and regulates the daily feeding-fasting cycle. Feeding-fasting cycle rhythmically provides nutrient input for the hexosamine biosynthetic pathway (HBP) and contributes to the daily rhythmic production of UDP-GlcNAc and protein O-GlcNAcylation rhythms (18). The circadian clock and feeding-fasting cycle also regulate protein O-GlcNAcylation rhythm by modulating the activity of HBP enzymes (13, 22, 23, 24, 93, 94) and O-GlcNAc processing enzymes (13, 23, 24, 88, 93, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105). Enzymes under daily rhythmic regulation are annotated with clock cartoons. The expression denotes mRNA and/or protein expression of indicated enzymes. Solid arrows indicate evidence from targeted studies of individual proteins, while dashed arrows indicate evidence gathered from omics studies. Line color differentiates clock-driven regulation (blue) and feeding-driven regulation (brown). AMPK and REV-ERBα are potential molecular mediators of clock-driven regulation on GFAT (71, 72, 98) and OGT (99), respectively. AMPK, AMP-activated protein kinase; GFAT, glutamine–fructose-6-phosphate aminotransferase; GNPNAT, glucosamine-phosphate N-acetyltransferase; OGA, O-GlcNAcase; OGT, O-GlcNAc transferase; PGM3, phosphoacetylglucosamine mutase; UAP1, UDP-N-acetyl glucosamine pyrophosphorylase one.
In addition to rhythmic UDP-GlcNAc production driven by clock-controlled feeding activity (18), the activity of HBP enzymes could also be under clock control. Based on publicly available circadian transcriptomic datasets in flies and mice, we found that the transcripts encoding all four HBP enzymes oscillate over a day-night cycle in at least one study (Fig. 2 and Table 1). At the protein level, published proteomic datasets suggest that GFAT, GNPNAT, and UAP1 proteins exhibit daily oscillation (Fig. 2 and Table 1). As for the enzymatic activity of HBP enzymes, our recent study reported daily oscillation of GFAT enzymatic activity in wild type (WT) fly tissues, but this rhythmicity is diminished in arrhythmic clock mutant flies (per0; period null mutant fly) (18). Interestingly, restricting food consumption of clock mutant flies at natural feeding time of WT flies failed to rescue rhythmic GFAT enzymatic activity. This suggests that a functional circadian clock is essential to maintain GFAT activity rhythm beyond the regulation of feeding-fasting cycles. Given our observation that the peak of daily GFAT activity rhythm is always fixed right after the natural feeding time window in WT flies, even when flies were fed at unnatural feeding time (18), we concluded that circadian clocks impose a stronger regulation on GFAT activity rhythm as compared to nutrient input (Fig. 2). Clock-controlled GFAT regulation likely happens at posttranscriptional and/or posttranslational levels (18). At present, it remains unknown whether the clock-controlled daily feeding-fasting cycle shapes daily GFAT activity rhythm through metabolic regulations (See previous sections). Intriguingly, phosphoproteomics in mouse liver tissue detected rhythmic GFAT1(S259) phosphorylation over a 24-h period and occupancy of this phosphorylation peaks right after mouse feeding phase (98) (Fig. 2). Mouse GFAT1(S259) is equivalent to human GFAT1(S243), the AMPK-directed phosphorylation with still debatable effects on GFAT1 activity as discussed above. Finally, whether rhythmic GFAT1(S259) phosphorylation leads to rhythmic GFAT activity remains to be investigated.
Table 1.
Circadian regulation of hexosamine biosynthetic pathway and O-GlcNAc cycling enzymes in fly or mouse tissues based on published data
| Enzymes | Mouse |
Fly |
Reference | |||
|---|---|---|---|---|---|---|
| Transcripts | Protein | Phospho-peptide | Transcripts | Protein | ||
| GFAT/GFPT | Cycling in liver, SCN, BAT, kidney, and aorta | Cycling in macrophages | Cycling in liver | Cycling in head and body | Non-cycling in head | (18, 28, 29, 98, 173, 197, 198, 199, 200, 201, 202) |
| GNPNAT | Cycling in liver | Cycling in macrophages | N/A | Cycling in head | Cycling in head | (27, 29, 197, 202, 203, 204) |
| PGM3 | Cycling in liver, urinary bladder, lung, and AG | Cycling in macrophages | N/A | Non-cycling in head | Cycling in head | (27, 28, 29, 197, 199, 202, 205, 206, 207, 208, 209) |
| UAP1 | Cycling in kidney, lung and WAT | Cycling in liver and macrophages | N/A | Non-cycling in head | Cycling in head | (27, 28, 29, 197, 198, 199, 202, 206, 207, 209, 210) |
| OGT | Cycling in liver, SCN and DC | Cycling in macrophages | N/A | Cycling in body | Cycling in body | (18, 27, 28, 29, 93, 173, 197, 199, 202, 206, 210, 211, 212, 213) |
| OGA | Cycling in liver | Cycling in liver and macrophages | N/A | Cycling in head and body | Cycling in head and body | (18, 27, 28, 29, 198, 202, 203, 205, 211, 214, 215, 216) |
Abbreviations: AG, adrenal gland; BAT, brown adipose tissue; DC, distal colon; GFAT/GFPT, Glutamine--fructose-6-phosphate aminotransferase; GNPNAT, Glucosamine-phosphate N-acetyltransferase; N/A, Undetected; OGA, O-GlcNAcase; OGT, O-GlcNAc transferase; PGM3, Phosphoacetylglucosamine mutase; SCN, suprachiasmatic nucleus; UAP1, UDP-N-Acetyl glucosamine pyrophosphorylase one; WAT, white adipose tissue.
Other than HBP enzymes, OGT and OGA are also under circadian clock regulation. Combing through published circadian transcriptomic and proteomic datasets, we found that both transcript and protein levels of oga and ogt are rhythmic in mouse and fly tissues (Fig. 2 and Table 1). In our TRF experiments, the respective mRNA and protein of OGT and OGA are all rhythmic in fly body tissues (18) (Fig. 2). More importantly, the rhythmicity of OGT and OGA protein expression is diminished in arrhythmic clock mutant flies (per0) even under natural feeding time (18), suggesting the requirement of an intact circadian clock to properly regulate the rhythm of OGT and OGA proteins. Finally, in human hepatoma HepG2 cells, REV-ERBα, a core component of the molecular clock, can bind and stabilize OGT (99) (Fig. 2). As REV-ERBα translocates between nucleus and cytoplasm over a day-night cycle, OGT stability in different cellular compartments can also oscillate (99).
In summary, the expression of HBP enzymes and O-GlcNAc processing enzymes are highly regulated by circadian clocks. The phosphorylation and enzymatic activity of GFAT are also rhythmic over a 24-h period. Therefore, under physiological conditions, it is essential to maintain a proper feeding-fasting cycle as established by circadian clocks to maintain an O-GlcNAcylation homeostasis. The regulation of daily O-GlcNAcylation rhythm by the circadian clock is likely to be more extensive than discussed here. Currently, it is unknown whether other HBP enzymes and O-GlcNAc processing enzymes are also rhythmically phosphorylated or rhythmically modified by other posttranslational modifications (PTMs) and whether the activities of other HBP enzymes and O-GlcNAc processing enzymes oscillate over 24-h day-night cycles.
A wide range of cellular signals regulate protein O-GlcNAcylation
In addition to metabolic and environmental signals communicated through the circadian clock, protein O-GlcNAcylation is also responsive to various other cellular signals, including ER stress, oxidative stress, infection, apoptosis, and cell division (31, 32, 33, 35, 36, 38, 39) (Fig. 3). Significantly, elevated O-GlcNAcylation under many stress conditions facilitates cell survival (31, 32, 36, 39). Cellular signals are known to regulate protein O-GlcNAcylation by modulating the activity of HBP enzymes, specifically GFAT, and O-GlcNAc processing enzymes. In this section, we will discuss the cellular signals and relevant mechanisms that target GFAT, OGT, and OGA to regulate protein O-GlcNAcylation. The examples provided in this section are mostly from mammalian cell culture studies.
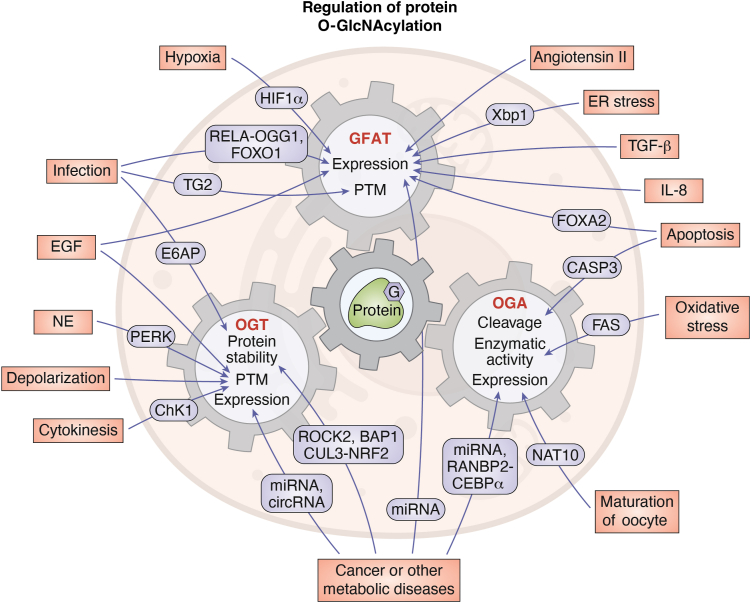
Figure 3.
The regulation of protein O-GlcNAcylation by cellular signals and conditions. Protein O-GlcNAcylation can respond to a wide range of cellular signals and conditions, mediated by GFAT (61, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 143), OGT (117, 118, 119, 120, 121, 122, 123, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 139, 140, 141, 142, 146, 147) and OGA (117, 124, 125, 137, 138, 139, 144). The expression denotes mRNA and/or protein expression of indicated enzymes. Known regulators involved are shown and discussed in the text. BAP1, BRCA1 associated protein-1; CASP3, caspase-3; CEBPα, CCAAT/enhancer-binding protein α; ChK1, checkpoint kinase one; circRNA, circular RNA; CUL3, cullin three; E6AP, ubiquitin ligase E6AP; EGF, epidermal growth factor; FAS, fatty acid synthase; FOXA2, forkhead box A2; FOXO1, forkhead box protein O1; HIF1α, hypoxia-inducible Factor; IL-8, interleukin-8; miRNA, microRNA; NAT10, N-acetyltransferase 10; NE, Norepinephrine; NRF2, nuclear factor E2-related factor-2; OGG1, 8-oxoguanine DNA glycosylase one; PERK, protein kinase R-like ER kinase; RANBP2, RAN-binding protein two; RELA, nuclear factor-κB p65; ROCK2, Rho-associated coiled-coil forming protein kinase two; TG2, transglutaminase two; TGF-β, transforming growth factor β; Xbp1, spliced X-box binding protein one.
Multiple humoral signals, environmental stimuli, and stress conditions influence the expression and PTM of GFAT. Angiotensin II (100), epidermal growth factor (EGF) (66), transforming growth factor β (TGF-β) (101), and interleukin-8 (IL-8) (102) can promote GFAT protein expression via yet uncharacterized mechanisms (Fig. 3). Other cellular signals can modify transcription factors that are responsible for gfat mRNA expression (103, 104, 105, 106, 107, 108, 109) (Fig. 3). For example, hypoxia can stimulate gfat1 expression through hypoxia inducible factor 1α (HIF1α) (104). Forkhead box A2 (FOXA2) can up-regulate gfat1 expression during apoptosis (106). Unfolded protein response can trigger the expression of multiple transcripts that encode HBP enzymes through the activity of spliced X-box binding protein 1 (Xbp1s) (103). Finally, infection increases GFAT enzymatic activity by transglutaminase 2 (TG2)-dependent PTM on GFAT (110) (Fig. 3).
OGT protein and enzymatic activity also respond to various stimuli and stress conditions. Oxidative stress (111), virus infection (112, 113), and cytokinesis (114) have been shown to regulate OGT protein levels (Fig. 3). The E6 protein from human papillomavirus can interact with E6AP E3 ligase to enhance OGT ubiquitination and promote OGT degradation (113) (Fig. 3). During cytokinesis, checkpoint kinase 1 (CHK1) phosphorylates OGT(S20), which stabilizes OGT by decreasing OGT ubiquitination (114) (Fig. 3). Other OGT phosphorylation events have also been reported to regulate OGT activity in response to cellular signals. EGF signaling stimulates OGT(Y976) phosphorylation and alters its substrate selectivity (115) (Fig. 3). Norepinephrine (NE) can promote OGT phosphorylation by protein kinase R-like ER kinase (PERK) and increase OGT activity on casein kinase 2α (CK2α) (116) (Fig. 3). Depolarization of neuronal cells can stimulate OGT activity through CaMKIV-dependent phosphorylation (117) (Fig. 3).
Similar to OGT, OGA is also regulated by a range of cellular conditions. OGA expression and activity have been shown to be responsive to apoptosis (118), oxidative stress (111), and maturation of oocytes (119) (Fig. 3). Apoptosis induces the cleavage of OGA protein by caspase-3 (CASP3), yet interestingly no effect on OGA activity was observed (118) (Fig. 3). Under oxidative stress, OGA activity is diminished by interacting with fatty acid synthase (FAS) (111) (Fig. 3). During in vitro maturation of mouse oocytes, N-acetyltransferase 10 (NAT10) increases OGA protein levels by stabilizing oga mRNA via mRNA N4-acetylcytidine (ac4C) (119) (Fig. 3).
In addition to cellular signals and conditions described above, GFAT, OGT and OGA are also altered under pathophysiological conditions (120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141) (Fig. 3). The misregulation of O-GlcNAcylation has been of great interest in the study of cancers. For example, several microRNAs (123, 124, 125, 126, 127, 128, 129, 130, 135, 140, 141) and a circular RNA (122) are found to negatively regulate OGT activity, while Rho-associated coiled-coil forming protein kinase 2 (ROCK2) (120) and BRCA1 associated protein-1 (BAP1) (121) inhibit OGT ubiquitination and stabilize OGT (Fig. 3). OGA expression could be downregulated by RAN-binding protein 2 (RANBP2), a SUMO E3 ligase, which cause degradation of CCAAT/enhancer-binding protein α (CEBPα), a transcription factor of OGA (131) (Fig. 3).
In summary, O-GlcNAcylation is responsive to a wide range of cellular signals and/or conditions, suggesting the role of O-GlcNAcylation as a more generalized cellular status effector. Interestingly, cellular signals and/or conditions mentioned in this section also respond to metabolic and circadian signals, forming an intertwined network of regulation on O-GlcNAcylation (Table 2). Given the ubiquitous function of O-GlcNAcylation in biological processes and the importance of maintaining O-GlcNAcylation homeostasis, it is worth noting that OGT and OGA levels generally correlate with each other in many different cell types or conditions (18, 60, 132, 133, 142, 143). Also, general cellular O-GlcNAcylation status can feedback to regulate OGT and/or OGA expression (134, 144, 145). The detailed molecular mechanism that maintains cellular O-GlcNAcylation homeostasis has been summarized in other review articles (5, 6).
Table 2.
Some cellular signals that regulate protein O-GlcNAcylation are themselves under circadian and/or metabolic regulation
| Cellular signals | Enzymes that regulate protein O-GlcNAcylation | Under circadian control? | Under metabolic control? |
|---|---|---|---|
| ER stress | Xbp1s | (217) | (218) |
| TGF-β | (184, 185) | (219, 220, 221, 222) | |
| IL-8 | (223) | (224, 225) | |
| Angiotensin II | (180, 181) | (226) | |
| Apoptosis | FOXA2 | - | (227) |
| CASP3 | (228) | (229) | |
| Oxidative stress | FAS | - | (230, 231) |
| Maturation of oocyte | NAT10 | - | - |
| Cancer or other metabolic diseases | RANBP2-CEBPα | (98) | - |
| ROCK2 | (232) | (233) | |
| BAP1 | - | - | |
| CUL3-NRF2 | (234) | - | |
| miRNA | (235) | (236) | |
| cirRNA | - | - | |
| Cytokinesis | ChK1 | (237, 238) | - |
| Depolarization | CaMKIV | - | - |
| Norepinephrine | PERK | (239) | (240, 241) |
| EGF | (182, 183) | - | |
| Infection | RELA-OGG1 | (242) | (243, 244) |
| FOXO1 | - | (245) | |
| TG2 | - | - | |
| E6AP | - | (246) | |
| Hypoxia | HIF1α | (247, 248, 249, 250, 251) | (252) |
Abbreviations: BAP1, BRCA1 associated protein-1; CASP3, caspase-3; CEBPα, CCAAT/enhancer-binding protein α; ChK1, checkpoint kinase one; circRNA, circular RNA; CUL3, cullin three; E6AP, ubiquitin ligase E6AP; EGF, epidermal growth factor; FAS, fatty acid synthase; FOXA2, forkhead box A2; FOXO1, forkhead box protein O1; HIF1α, hypoxia inducible factor 1α; IL-8, interleukin-8; miRNA, microRNA; NAT10, N-acetyltransferase 10; NRF2, nuclear factor E2-related factor-2; OGG1, 8-oxoguanine DNA glycosylase one; PERK, Protein Kinase RNA-like ER kinase; RANBP2, RAN-binding protein two; RELA, nuclear factor-κB p65; ROCK2, Rho-associated coiled-coil forming protein kinase two; TG2, transglutaminase two; TGF-β, transforming growth factor β; Xbp1, spliced X-box binding protein one.
Conclusion and future prospects
Over 3 decades of investigations have advanced our understanding of the functions of O-GlcNAcylation in various fundamental cellular processes and pathological development of many diseases (5, 9, 10, 11, 12). Many studies have shown that O-GlcNAcylation is regulated by nutrient availability and nutrient sensing pathways (Fig. 1). In addition, there is rapidly accumulating evidence indicating that many other cellular signals and molecular pathways modulate protein O-GlcNAcylation (Figs. 2 and 3), suggesting the role of O-GlcNAcylation as a generalized cellular status effector. In this review, we discuss the metabolic regulation of protein O-GlcNAcylation through HBP and O-GlcNAc processing enzymes, summarize the mechanisms by which circadian clocks shape daily O-GlcNAcylation rhythms, and highlight other cellular signals and associated molecular mechanisms that influence cellular O-GlcNAcylation level. Our review emphasizes the importance of maintaining proper daily O-GlcNAcylation rhythm by controlling mealtime. O-GlcNAcylation is tightly regulated by daily nutrient input and circadian clocks and is expected to provide time-of-day specific regulation on cellular processes to mediate clock and metabolic control of daily rhythmic physiology and behavior (8). This review also provides insights into potential targets for the development of therapeutics to alleviate disruption of O-GlcNAc homeostasis in metabolic diseases. As metabolic input is the primary regulatory factor for O-GlcNAcylation, we propose that O-GlcNAcylation could partially mediate the beneficial effects of time-restricted eating in patients with metabolic syndrome, which limits the eating time to under 10 h during the active period (146). Notably, many regulatory factors of O-GlcNAcylation described in this review, such as ROCK2 and BAP1, have been already used as therapeutic targets in patients (147, 148).
In this review, we largely focused on the cellular signals and pathways that regulate overall protein O-GlcNAcylation. Besides the gaps in knowledge we have already highlighted in the previous sections, there remain many questions regarding the regulation of O-GlcNAcylation that warrant future investigations. One interesting question is how OGT and OGA achieve substrate specificity (149, 150), given there are thousands of protein substrates for O-GlcNAcylation. So far, it has been uncovered that mechanisms underlying substrate specificity include phosphorylation (94, 115, 151), subcellular localization (89, 90, 92), dimerization (152, 153, 154), structural divergence (111, 155, 156, 157, 158, 159, 160, 161), and adaptor proteins (6, 162, 163, 164, 165, 166). Another interesting question is how do other PTMs on target proteins, such as phosphorylation (167), acetylation (168, 169, 170), and ubiquitination (171, 172, 173) interact with O-GlcNAcylation in individual proteins to regulate structure and function. Among these PTMs, phosphorylation is the most well-studied PTM that can facilitate or inhibit protein O-GlcNAcylation depending on the position of amino acid residues (167, 174, 175, 176). Future studies examining the interactions between O-GlcNAcylation and other PTMs in response to metabolic and cellular signals are critical to provide a comprehensive understanding of the regulation of biological processes via multiple PTMs.
Beyond the cellular level, there have been more and more studies that investigated the impact of environmental, physiological, and systemic changes on protein O-GlcNAcylation in whole animal models. Development (30), aging (37), maternal stress (34), exercise (40, 141, 143, 177), cold exposure (116), fear conditioning (178) and rapid eye movement (REM) sleep deprivation (179) are all shown to influence protein O-GlcNAcylation. However, our knowledge of the underlying mechanisms is rather limited. In mouse skeletal muscle, exercise-induced lactate and hypoxia can signal to HIF1α, which increases OGT expression and O-GlcNAcylation (141). In mouse brown adipose tissue, cold exposure can trigger OGT phosphorylation by PERK and promote OGT activity (116). Perhaps not surprisingly, the O-GlcNAcylation response appears to be tissue-specific. For example, exercise upregulates O-GlcNAcylation in skeletal muscle (141, 177), but downregulates it in the heart (143). Therefore, more studies are warranted to investigate the mechanisms of O-GlcNAcylation regulation by various physiological and environmental conditions in animal models.
Finally, although the circadian clock has been shown to regulate daily O-GlcNAcylation rhythm (18, 26), the molecular mediator(s) are just starting to be explored (Fig. 2). Many regulators of O-GlcNAcylation mentioned in this review are under clock control, including angiotensin II (180, 181), EGF (182, 183), and TGF-β (184, 185) (Table 2). Many of them could play important roles in regulating rhythmic O-GlcNAcylation to control daily biological rhythms. It is important to point out that the daily O-GlcNAcylation rhythm is likely to be different in diurnal versus nocturnal animals, given their distinct feeding windows. In the same species, O-GlcNAcylation rhythm and its response to nutrient availability may display organ specificity, due to distinct nutrient sensitivity and metabolic properties of individual organs. For example, the blood-brain barrier renders the brain tissue less sensitive to the fluctuation of nutrients in peripheries, while the heart is extremely metabolically active so that it might be highly impacted by daily feeding activity. It is also interesting to investigate the impact of metabolic diseases on daily O-GlcNAcylation rhythm. As metabolic diseases, or even high-fat diet, are known to disrupt daily rhythms in the transcriptome, sleep/wake cycle, and feeding/fasting cycle (186, 187, 188, 189, 190, 191), O-GlcNAcylation might mediate the disruptive effect of metabolic diseases on circadian physiology. Finally, to characterize the function of O-GlcNAcylation in circadian physiology, it is critical to identify the proteins that are rhythmically modified by O-GlcNAcylation using proteomic approaches. Comparative O-GlcNAcome is warranted to reveal tissue-specific and/or pathologically disrupted rhythm of O-GlcNAc proteins. Additionally, since phosphorylation (29, 98, 192, 193), acetylation (194, 195), and ubiquitination (196) has been shown to oscillate over a 24-h period at the proteome level, proteome-wide interaction between rhythmic O-GlcNAcylation and other PTMs will also shed light on the regulation of O-GlcNAcylation rhythm on individual proteins.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
This work is supported by NIH R01 DK124068 and R56 DK124068 to J. C. C. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
X. L., Y. D. C., and J. C. C. conceptualization; X. L. and Y. D. C. writing–original draft; X. L., Y. D. C., and J. C. C. writing–reviewing and editing.
Reviewed by members of the JBC Editorial Board. Edited by Robert Haltiwanger
References
- 1.Torres C.R., Hart G.W. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-linked GlcNAc. J. Biol. Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 2.Wulff-Fuentes E., Berendt R.R., Massman L., Danner L., Malard F., Vora J., et al. The human O-GlcNAcome database and meta-analysis. Sci. Data. 2021;8:25. doi: 10.1038/s41597-021-00810-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hart G.W. Nutrient regulation of signaling and transcription. J. Biol. Chem. 2019;294:2211–2231. doi: 10.1074/jbc.AW119.003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y., Fang X., Wang S., Wang B., Chu F., Tian Z., et al. The role of O-GlcNAcylation in innate immunity and inflammation. J. Mol. Cell Biol. 2023;14:mjac065. doi: 10.1093/jmcb/mjac065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatham J.C., Zhang J., Wende A.R. Role of O -linked N -acetylglucosamine protein modification in cellular (patho)physiology. Physiol. Rev. 2021;101:427–493. doi: 10.1152/physrev.00043.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Qian K. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat. Rev. Mol. Cell Biol. 2017;18:452–465. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan E.P., Duncan F.E., Slawson C. The sweet side of the cell cycle. Biochem. Soc. Trans. 2017;45:313–322. doi: 10.1042/BST20160145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X., Chiu J.C. Nutrient-sensitive protein O-GlcNAcylation shapes daily biological rhythms. Open Biol. 2022;12 doi: 10.1098/rsob.220215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenzel D.M., Olivier-Van Stichelen S. The O-GlcNAc cycling in neurodevelopment and associated diseases. Biochem. Soc. Trans. 2022;50:1693–1702. doi: 10.1042/BST20220539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciraku L., Esquea E.M., Reginato M.J. O-GlcNAcylation regulation of cellular signaling in cancer. Cell. Signal. 2022;90 doi: 10.1016/j.cellsig.2021.110201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolanle I.O., Palmer T.M. Targeting protein O-GlcNAcylation, a link between type 2 diabetes mellitus and inflammatory disease. Cells. 2022;11:705. doi: 10.3390/cells11040705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H.-F., Wang Y.-X., Zhou Y.-P., Wei Y.-P., Yan Y., Zhang Z.-J., et al. Protein O-GlcNAcylation in cardiovascular diseases. Acta Pharmacol. Sin. 2023;44:8–18. doi: 10.1038/s41401-022-00934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haltiwanger R.S., Holt G.D., Hart G.W. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide beta-N-acetylglucosaminyltransferase. J. Biol. Chem. 1990;265:2563–2568. [PubMed] [Google Scholar]
- 14.Haltiwanger R.S., Blomberg M.A., Hart G.W. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide beta-N-acetylglucosaminyltransferase. J. Biol. Chem. 1992;267:9005–9013. [PubMed] [Google Scholar]
- 15.Kreppel L.K., Blomberg M.A., Hart G.W. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem. 1997;272:9308–9315. doi: 10.1074/jbc.272.14.9308. [DOI] [PubMed] [Google Scholar]
- 16.Dong D.L., Hart G.W. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. J. Biol. Chem. 1994;269:19321–19330. [PubMed] [Google Scholar]
- 17.Gao Y., Wells L., Comer F.I., Parker G.J., Hart G.W. Dynamic O-glycosylation of nuclear and cytosolic proteins: cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J. Biol. Chem. 2001;276:9838–9845. doi: 10.1074/jbc.M010420200. [DOI] [PubMed] [Google Scholar]
- 18.Liu X., Blaženović I., Contreras A.J., Pham T.M., Tabuloc C.A., Li Y.H., et al. Hexosamine biosynthetic pathway and O-GlcNAc-processing enzymes regulate daily rhythms in protein O-GlcNAcylation. Nat. Commun. 2021;12:4173. doi: 10.1038/s41467-021-24301-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor R.P., Geisler T.S., Chambers J.H., McClain D.A. Up-regulation of O-GlcNAc transferase with glucose deprivation in HepG2 cells is mediated by decreased hexosamine pathway flux. J. Biol. Chem. 2009;284:3425–3432. doi: 10.1074/jbc.M803198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wellen K.E., Lu C., Mancuso A., Lemons J.M.S., Ryczko M., Dennis J.W., et al. The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 2010;24:2784–2799. doi: 10.1101/gad.1985910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swamy M., Pathak S., Grzes K.M., Damerow S., Sinclair L.V., van Aalten D.M.F., et al. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat. Immunol. 2016;17:712–720. doi: 10.1038/ni.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo V.D., Panda S. Fasting, circadian rhythms, and time-restricted feeding in Healthy Lifespan. Cell Metab. 2016;23:1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finger A.-M., Dibner C., Kramer A. Coupled network of the circadian clocks: a driving force of rhythmic physiology. FEBS Lett. 2020;594:2734–2769. doi: 10.1002/1873-3468.13898. [DOI] [PubMed] [Google Scholar]
- 24.Dubowy C., Sehgal A. Circadian rhythms and sleep in Drosophila melanogaster. Genetics. 2017;205:1373–1397. doi: 10.1534/genetics.115.185157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patke A., Young M.W., Axelrod S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020;21:67–84. doi: 10.1038/s41580-019-0179-2. [DOI] [PubMed] [Google Scholar]
- 26.Durgan D.J., Pat B.M., Laczy B., Bradley J.A., Tsai J.-Y., Grenett M.H., et al. O-GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J. Biol. Chem. 2011;286:44606–44619. doi: 10.1074/jbc.M111.278903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Shi L., Zhang K., Wei W., Liu Q., Mao F., et al. CirGRDB: a database for the genome-wide deciphering circadian genes and regulators. Nucleic Acids Res. 2018;46:D64–D70. doi: 10.1093/nar/gkx944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez J., Tang C.-H.A., Khodor Y.L., Vodala S., Menet J.S., Rosbash M. Nascent-Seq analysis of Drosophila cycling gene expression. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E275–E284. doi: 10.1073/pnas.1219969110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C., Shui K., Ma S., Lin S., Zhang Y., Wen B., et al. Integrated omics in Drosophila uncover a circadian kinome. Nat. Commun. 2020;11:2710. doi: 10.1038/s41467-020-16514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lefebvre T., Baert F., Bodart J.-F., Flament S., Michalski J.-C., Vilain J.-P. Modulation of O-GlcNAc glycosylation during Xenopus oocyte maturation. J. Cell. Biochem. 2004;93:999–1010. doi: 10.1002/jcb.20242. [DOI] [PubMed] [Google Scholar]
- 31.Zachara N.E., Molina H., Wong K.Y., Pandey A., Hart G.W. The dynamic stress-induced “O-GlcNAc-ome” highlights functions for O-GlcNAc in regulating DNA damage/repair and other cellular pathways. Amino Acids. 2011;40:793–808. doi: 10.1007/s00726-010-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zachara N.E., O’Donnell N., Cheung W.D., Mercer J.J., Marth J.D., Hart G.W. Dynamic O-GlcNAc modification of nucleocytoplasmic proteins in response to stress: a survival response OF mammalian cells. J. Biol. Chem. 2004;279:30133–30142. doi: 10.1074/jbc.M403773200. [DOI] [PubMed] [Google Scholar]
- 33.Drougat L., Olivier-Van Stichelen S., Mortuaire M., Foulquier F., Lacoste A.-S., Michalski J.-C., et al. Characterization of O-GlcNAc cycling and proteomic identification of differentially O-GlcNAcylated proteins during G1/S transition. Biochim. Biophys. Acta. 2012;1820:1839–1848. doi: 10.1016/j.bbagen.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Howerton C.L., Morgan C.P., Fischer D.B., Bale T.L. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc. Natl. Acad. Sci. U. S. A. 2013;110:5169–5174. doi: 10.1073/pnas.1300065110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y., Yao R.-Z., Lian S., Liu P., Hu Y.-J., Shi H.-Z., et al. O-GlcNAcylation: the “stress and nutrition receptor” in cell stress response. Cell Stress Chaperones. 2021;26:297–309. doi: 10.1007/s12192-020-01177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groves J.A., Lee A., Yildirir G., Zachara N.E. Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones. 2013;18:535–558. doi: 10.1007/s12192-013-0426-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fülöp N., Feng W., Xing D., He K., Nőt L.G., Brocks C.A., et al. Aging leads to increased levels of protein O-linked N-acetylglucosamine in heart, aorta, brain and skeletal muscle in Brown-Norway rats. Biogerontology. 2008;9:139–151. doi: 10.1007/s10522-007-9123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins H.E., Chatham J.C. Regulation of cardiac O-GlcNAcylation: more than just nutrient availability. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ngoh G.A., Watson L.J., Facundo H.T., Jones S.P. Augmented O-GlcNAc signaling attenuates oxidative stress and calcium overload in cardiomyocytes. Amino Acids. 2011;40:895–911. doi: 10.1007/s00726-010-0728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox E.J., Marsh S.A. Exercise and diabetes have opposite effects on the assembly and O-GlcNAc modification of the mSin3A/HDAC1/2 complex in the heart. Cardiovasc. Diabetol. 2013;12:101. doi: 10.1186/1475-2840-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marshall S., Bacote V., Traxinger R.R. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J. Biol. Chem. 1991;266:4706–4712. [PubMed] [Google Scholar]
- 42.Gibb A.A., Lorkiewicz P.K., Zheng Y.-T., Zhang X., Bhatnagar A., Jones S.P., et al. Integration of flux measurements to resolve changes in anabolic and catabolic metabolism in cardiac myocytes. Biochem. J. 2017;474:2785–2801. doi: 10.1042/BCJ20170474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson A.K., Bouchard B., Zhu W.Z., Chatham J.C., Des Rosiers C. First characterization of glucose flux through the hexosamine biosynthesis pathway (HBP) in ex vivo mouse heart. J. Biol. Chem. 2020;295:2018–2033. doi: 10.1074/jbc.RA119.010565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kreppel L.K., Hart G.W. Regulation of a cytosolic and nuclear O-GlcNAc transferase. J. Biol. Chem. 1999;274:32015–32022. doi: 10.1074/jbc.274.45.32015. [DOI] [PubMed] [Google Scholar]
- 45.Nakajima K., Kitazume S., Angata T., Fujinawa R., Ohtsubo K., Miyoshi E., et al. Simultaneous determination of nucleotide sugars with ion-pair reversed-phase HPLC. Glycobiology. 2010;20:865–871. doi: 10.1093/glycob/cwq044. [DOI] [PubMed] [Google Scholar]
- 46.Abdel Rahman A.M., Ryczko M., Pawling J., Dennis J.W. Probing the hexosamine biosynthetic pathway in human tumor cells by multitargeted tandem mass spectrometry. ACS Chem. Biol. 2013;8:2053–2062. doi: 10.1021/cb4004173. [DOI] [PubMed] [Google Scholar]
- 47.Marshall S., Nadeau O., Yamasaki K. Dynamic actions of glucose and glucosamine on hexosamine biosynthesis in isolated adipocytes. J. Biol. Chem. 2004;279:35313–35319. doi: 10.1074/jbc.M404133200. [DOI] [PubMed] [Google Scholar]
- 48.Grigorian A., Lee S.-U. Control of T cell-mediated autoimmunity by metabolite flux to N-glycan biosynthesis. J. Biol. Chem. 2007;282:20027–20035. doi: 10.1074/jbc.M701890200. [DOI] [PubMed] [Google Scholar]
- 49.Weigert C., Klopfer K., Kausch C., Brodbeck K., Stumvoll M., Häring H.U., et al. Palmitate-induced activation of the hexosamine pathway in human Myotubes. Diabetes. 2003;52:650–656. doi: 10.2337/diabetes.52.3.650. [DOI] [PubMed] [Google Scholar]
- 50.Hawkins M., Barzilai N., Liu R., Hu M., Chen W., Rossetti L. Role of the glucosamine pathway in fat-induced insulin resistance. J. Clin. Invest. 1997;99:2173–2182. doi: 10.1172/JCI119390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu J., Marchase R.B., Chatham J.C. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J. Mol. Cell. Cardiol. 2007;42:177–185. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasconcelos-dos-Santos A., Loponte H.F.B.R., Mantuano N.R., Oliveira I.A., de Paula I.F., Teixeira L.K., et al. Hyperglycemia exacerbates colon cancer malignancy through hexosamine biosynthetic pathway. Oncogenesis. 2017;6 doi: 10.1038/oncsis.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palorini R., Cammarata F.P., Balestrieri C., Monestiroli A., Vasso M., Gelfi C., et al. Glucose starvation induces cell death in K-ras-transformed cells by interfering with the hexosamine biosynthesis pathway and activating the unfolded protein response. Cell Death Dis. 2013;4:e732. doi: 10.1038/cddis.2013.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hamiel C.R., Pinto S., Hau A., Wischmeyer P.E. Glutamine enhances heat shock protein 70 expression via increased hexosamine biosynthetic pathway activity. Am. J. Physiol. Cell Physiol. 2009;297:C1509–C1519. doi: 10.1152/ajpcell.00240.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pham L.V., Bryant J.L., Mendez R., Chen J., Tamayo A.T., Xu-Monette Z.Y., et al. Targeting the hexosamine biosynthetic pathway and O-linked N-acetylglucosamine cycling for therapeutic and imaging capabilities in diffuse large B-cell lymphoma. Oncotarget. 2016;7:80599–80611. doi: 10.18632/oncotarget.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chomphoo S., Kunprom W., Thithuan K., Sorin S., Khawkhiaw K., Kamkaew A., et al. Hyperglycemia alters O-GlcNAcylation Patterns of hepatocytes in mice treated with Hepatoxic Carcinogen. In Vivo. 2023;37:685–695. doi: 10.21873/invivo.13129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliveri L.M., Buzaleh A.M., Gerez E.N. An increase in O-GlcNAcylation of Sp1 down-regulates the gene expression of pi class glutathione S-transferase in diabetic mice. Biochem. Biophys. Rep. 2021;27 doi: 10.1016/j.bbrep.2021.101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen X., Zhang L., He H., Sun Y., Shen Q., Shi L. Increased O-GlcNAcylation induces myocardial hypertrophy. In Vitro Cell. Dev. Biol. Anim. 2020;56:735–743. doi: 10.1007/s11626-020-00503-z. [DOI] [PubMed] [Google Scholar]
- 59.Chaveroux C., Sarcinelli C., Barbet V., Belfeki S., Barthelaix A., Ferraro-Peyret C., et al. Nutrient shortage triggers the hexosamine biosynthetic pathway via the GCN2-ATF4 signalling pathway. Sci. Rep. 2016;6 doi: 10.1038/srep27278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou L., Zhu-Mauldin X., Marchase R.B., Paterson A.J., Liu J., Yang Q., et al. Glucose deprivation-induced increase in protein O-GlcNAcylation in cardiomyocytes is calcium-dependent. J. Biol. Chem. 2012;287:34419–34431. doi: 10.1074/jbc.M112.393207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paneque A., Fortus H., Zheng J., Werlen G., Jacinto E. The hexosamine biosynthesis pathway: regulation and function. Genes (Basel). 2023;14:933. doi: 10.3390/genes14040933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oki T., Yamazaki K., Kuromitsu J., Okada M., Tanaka I. cDNA cloning and mapping of a novel subtype of glutamine:fructose-6-phosphate amidotransferase (GFAT2) in human and mouse. Genomics. 1999;57:227–234. doi: 10.1006/geno.1999.5785. [DOI] [PubMed] [Google Scholar]
- 63.Yamazaki K. In: Handbook of Glycosyltransferases and Related Genes. Taniguchi N., Honke K., Fukuda M., Narimatsu H., Yamaguchi Y., Angata T., editors. Springer; Japan, Tokyo: 2014. Glutamine–Fructose-6-Phosphate Transaminase 1,2 (GFPT1,2) pp. 1465–1479. [Google Scholar]
- 64.Nabeebaccus A.A., Verma S., Zoccarato A., Emanuelli G., Santos C.X., Streckfuss-Bömeke K., et al. Cardiomyocyte protein O-GlcNAcylation is regulated by GFAT1 not GFAT2. Biochem. Biophys. Res. Commun. 2021;583:121–127. doi: 10.1016/j.bbrc.2021.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng H., Huang J., Zhang M., Zhao H.-J., Chen P., Zeng Z.-H. miR-27b-3p Improved high glucose-induced Spermatogenic cell damage via regulating Gfpt1/HBP signaling. Eur. Surg. Res. 2022;63:64–76. doi: 10.1159/000518960. [DOI] [PubMed] [Google Scholar]
- 66.Paterson A.J., Kudlow J.E. Regulation of glutamine:fructose-6-phosphate amidotransferase gene transcription by epidermal growth factor and glucose. Endocrinology. 1995;136:2809–2816. doi: 10.1210/endo.136.7.7789306. [DOI] [PubMed] [Google Scholar]
- 67.Liu B., Huang Z.-B., Chen X., See Y.-X., Chen Z.-K., Yao H.-K. Mammalian target of rapamycin 2 (MTOR2) and C-MYC modulate glucosamine-6-phosphate synthesis in glioblastoma (GBM) cells through glutamine: fructose-6-phosphate aminotransferase 1 (GFAT1) Cell. Mol. Neurobiol. 2019;39:415–434. doi: 10.1007/s10571-019-00659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dai W., Dierschke S.K., Toro A.L., Dennis M.D. Consumption of a high fat diet promotes protein O-GlcNAcylation in mouse retina via NR4A1-dependent GFAT2 expression. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:3568–3576. doi: 10.1016/j.bbadis.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moloughney J.G., Kim P.K., Vega-Cotto N.M., Wu C.-C., Zhang S., Adlam M., et al. mTORC2 responds to glutamine catabolite levels to modulate the hexosamine biosynthesis enzyme GFAT1. Mol. Cell. 2016;63:811–826. doi: 10.1016/j.molcel.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Derakhshanian H., Djazayery A., Javanbakht M.H., Eshraghian M.R., Mirshafiey A., Jahanabadi S., et al. Vitamin D downregulates key genes of diabetes complications in cardiomyocyte. J. Cell. Physiol. 2019;234:21352–21358. doi: 10.1002/jcp.28743. [DOI] [PubMed] [Google Scholar]
- 71.Eguchi S., Oshiro N., Miyamoto T., Yoshino K., Okamoto S., Ono T., et al. AMP-activated protein kinase phosphorylates glutamine: fructose-6-phosphate amidotransferase 1 at Ser243 to modulate its enzymatic activity. Genes Cells. 2009;14:179–189. doi: 10.1111/j.1365-2443.2008.01260.x. [DOI] [PubMed] [Google Scholar]
- 72.Zibrova D., Vandermoere F., Göransson O., Peggie M., Mariño K.V., Knierim A., et al. GFAT1 phosphorylation by AMPK promotes VEGF-induced angiogenesis. Biochem. J. 2017;474:983–1001. doi: 10.1042/BCJ20160980. [DOI] [PubMed] [Google Scholar]
- 73.Moloughney J.G., Vega-Cotto N.M., Liu S., Patel C., Kim P.K., Wu C., et al. mTORC2 modulates the amplitude and duration of GFAT1 Ser-243 phosphorylation to maintain flux through the hexosamine pathway during starvation. J. Biol. Chem. 2018;293:16464–16478. doi: 10.1074/jbc.RA118.003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chatterjee A., Eshwaran R., Poschet G., Lomada S., Halawa M., Wilhelm K., et al. Involvement of NDPK-B in glucose metabolism-mediated endothelial damage via activation of the hexosamine biosynthesis pathway and suppression of O-GlcNAcase activity. Cells. 2020;9:2324. doi: 10.3390/cells9102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang Q., Su K., Baker J.R., Yang X., Paterson A.J., Kudlow J.E. Phosphorylation of human glutamine:fructose-6-phosphate amidotransferase by cAMP-dependent protein kinase at serine 205 blocks the enzyme activity. J. Biol. Chem. 2000;275:21981–21987. doi: 10.1074/jbc.M001049200. [DOI] [PubMed] [Google Scholar]
- 76.Nelson B.A., Robinson K.A., Koning J.S., Buse M.G. Effects of exercise and feeding on the hexosamine biosynthetic pathway in rat skeletal muscle. Am. J. Physiol. 1997;272:E848–E855. doi: 10.1152/ajpendo.1997.272.5.E848. [DOI] [PubMed] [Google Scholar]
- 77.Ruegenberg S., Mayr F.A.M.C., Atanassov I., Baumann U., Denzel M.S. Protein kinase a controls the hexosamine pathway by tuning the feedback inhibition of GFAT-1. Nat. Commun. 2021;12:2176. doi: 10.1038/s41467-021-22320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Graack H.R., Cinque U., Kress H. Functional regulation of glutamine:fructose-6-phosphate aminotransferase 1 (GFAT1) of Drosophila melanogaster in a UDP-N-acetylglucosamine and cAMP-dependent manner. Biochem. J. 2001;360:401–412. doi: 10.1042/0264-6021:3600401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou J., Huynh Q.K., Hoffman R.T., Crook E.D., Daniels M.C., Gulve E.A., et al. Regulation of glutamine:fructose-6-phosphate amidotransferase by cAMP-dependent protein kinase. Diabetes. 1998;47:1836–1840. doi: 10.2337/diabetes.47.12.1836. [DOI] [PubMed] [Google Scholar]
- 80.Hu Y., Riesland L., Paterson A.J., Kudlow J.E. Phosphorylation of mouse glutamine-fructose-6-phosphate amidotransferase 2 (GFAT2) by cAMP-dependent protein kinase increases the enzyme activity. J. Biol. Chem. 2004;279:29988–29993. doi: 10.1074/jbc.M401547200. [DOI] [PubMed] [Google Scholar]
- 81.Broschat K.O., Gorka C., Page J.D., Martin-Berger C.L., Davies M.S., Huang H., et al. Kinetic characterization of human glutamine-fructose-6-phosphate amidotransferase I. J. Biol. Chem. 2002;277:14764–14770. doi: 10.1074/jbc.M201056200. [DOI] [PubMed] [Google Scholar]
- 82.Ruegenberg S., Horn M., Pichlo C., Allmeroth K., Baumann U., Denzel M.S. Loss of GFAT-1 feedback regulation activates the hexosamine pathway that modulates protein homeostasis. Nat. Commun. 2020;11:687. doi: 10.1038/s41467-020-14524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell S., Mesaros C., Izzo L., Affronti H., Noji M., Schaffer B.E., et al. Glutamine deprivation triggers NAGK-dependent hexosamine salvage. Elife. 2021;10 doi: 10.7554/eLife.62644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lo W.-Y., Yang W.-K., Peng C.-T., Pai W.-Y., Wang H.-J. MicroRNA-200a/200b modulate high glucose-induced endothelial inflammation by targeting O-linked N-acetylglucosamine transferase expression. Front. Physiol. 2018;9:355. doi: 10.3389/fphys.2018.00355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Förster L., Indra D., Rosenberger K., Zver L., Hofbauer R. L-carnitine exerts a nutrigenomic effect via direct modulation of nuclear receptor signaling in adipocytes, hepatocytes and SKMC, demonstrating its nutritional impact. Nutr. Res. 2021;85:84–98. doi: 10.1016/j.nutres.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 86.Sodi V.L., Khaku S., Krutilina R., Schwab L.P., Vocadlo D.J., Seagroves T.N., et al. mTOR/MYC Axis regulates O-GlcNAc transferase expression and O-GlcNAcylation in breast cancer. Mol. Cancer Res. 2015;13:923–933. doi: 10.1158/1541-7786.MCR-14-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang F., Snead C.M., Catravas J.D. Hsp90 regulates O -linked β- N -acetylglucosamine transferase: a novel mechanism of modulation of protein O -linked β- N -acetylglucosamine modification in endothelial cells. Am. J. Physiol. Cell Physiol. 2012;302:C1786–C1796. doi: 10.1152/ajpcell.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park S., Pak J., Jang I., Cho J. Inhibition of mTOR affects protein stability of OGT. Biochem. Biophys. Res. Commun. 2014;453:208–212. doi: 10.1016/j.bbrc.2014.05.047. [DOI] [PubMed] [Google Scholar]
- 89.Seo H.G., Kim H.B., Kang M.J., Ryum J.H., Yi E.C., Cho J.W. Identification of the nuclear localisation signal of O-GlcNAc transferase and its nuclear import regulation. Sci. Rep. 2016;6 doi: 10.1038/srep34614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bullen J.W., Balsbaugh J.L., Chanda D., Shabanowitz J., Hunt D.F., Neumann D., et al. Cross-talk between two essential nutrient-sensitive enzymes. J. Biol. Chem. 2014;289:10592–10606. doi: 10.1074/jbc.M113.523068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whelan S.A., Lane M.D., Hart G.W. Regulation of the O-linked β-N-acetylglucosamine transferase by insulin signaling. J. Biol. Chem. 2008;283:21411–21417. doi: 10.1074/jbc.M800677200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang X., Ongusaha P.P., Miles P.D., Havstad J.C., Zhang F., So W.V., et al. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 93.Kaasik K., Kivimäe S., Allen J.J., Chalkley R.J., Huang Y., Baer K., et al. Glucose sensor O-GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell Metab. 2013;17:291–302. doi: 10.1016/j.cmet.2012.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Z., Pandey A., Hart G.W. Dynamic interplay between O-linked N-Acetylglucosaminylation and glycogen synthase kinase-3-dependent phosphorylation. Mol. Cell. Proteomics. 2007;6:1365–1379. doi: 10.1074/mcp.M600453-MCP200. [DOI] [PubMed] [Google Scholar]
- 95.Huttlin E.L., Jedrychowski M.P., Elias J.E., Goswami T., Rad R., Beausoleil S.A., et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruan H.-B., Ma Y., Torres S., Zhang B., Feriod C., Heck R.M., et al. Calcium-dependent O-GlcNAc signaling drives liver autophagy in adaptation to starvation. Genes Dev. 2017;31:1655–1665. doi: 10.1101/gad.305441.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hornbeck P.V., Zhang B., Murray B., Kornhauser J.M., Latham V., Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512–520. doi: 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robles M.S., Humphrey S.J., Mann M. Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell Metab. 2017;25:118–127. doi: 10.1016/j.cmet.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 99.Berthier A., Vinod M., Porez G., Steenackers A., Alexandre J., Yamakawa N., et al. Combinatorial regulation of hepatic cytoplasmic signaling and nuclear transcriptional events by the OGT/REV-ERBα complex. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E11033–E11042. doi: 10.1073/pnas.1805397115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.James L.R., Ingram A., Ly H., Thai K., Cai L., Scholey J.W. Angiotensin II activates the GFAT promoter in mesangial cells. Am. J. Physiol. Renal Physiol. 2001;281:F151–F162. doi: 10.1152/ajprenal.2001.281.1.F151. [DOI] [PubMed] [Google Scholar]
- 101.Lucena M.C., Carvalho-Cruz P., Donadio J.L., Oliveira I.A., de Queiroz R.M., Marinho-Carvalho M.M., et al. Epithelial Mesenchymal transition induces aberrant glycosylation through hexosamine biosynthetic pathway activation. J. Biol. Chem. 2016;291:12917–12929. doi: 10.1074/jbc.M116.729236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shimizu M., Tanaka N. IL-8-induced O-GlcNAc modification via GLUT3 and GFAT regulates cancer stem cell-like properties in colon and lung cancer cells. Oncogene. 2019;38:1520–1533. doi: 10.1038/s41388-018-0533-4. [DOI] [PubMed] [Google Scholar]
- 103.Wang Z.V., Deng Y., Gao N., Pedrozo Z., Li D.L., Morales C.R., et al. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell. 2014;156:1179–1192. doi: 10.1016/j.cell.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Manzari B., Kudlow J.E., Fardin P., Merello E., Ottaviano C., Puppo M., et al. Induction of macrophage glutamine: fructose-6-phosphate amidotransferase expression by hypoxia and by Picolinic acid. Int. J. Immunopathol. Pharmacol. 2007;20:47–58. doi: 10.1177/039463200702000106. [DOI] [PubMed] [Google Scholar]
- 105.Al-Mukh H., Baudoin L., Bouaboud A., Sanchez-Salgado J.-L., Maraqa N., Khair M., et al. Lipopolysaccharide induces GFAT2 expression to promote O -linked β- N -acetylglucosaminylation and attenuate inflammation in macrophages. J. Immunol. 2020;205:2499–2510. doi: 10.4049/jimmunol.2000345. [DOI] [PubMed] [Google Scholar]
- 106.Huang H., Wang Y., Huang T., Wang L., Liu Y., Wu Q., et al. FOXA2 inhibits doxorubicin-induced apoptosis via transcriptionally activating HBP rate-limiting enzyme GFPT1 in HCC cells. J. Physiol. Biochem. 2021;77:625–638. doi: 10.1007/s13105-021-00829-6. [DOI] [PubMed] [Google Scholar]
- 107.Yamazaki K., Mizui Y., Oki T., Okada M., Tanaka I. Cloning and characterization of mouse glutamine:fructose-6-phosphate amidotransferase 2 gene promoter. Gene. 2000;261:329–336. doi: 10.1016/s0378-1119(00)00497-2. [DOI] [PubMed] [Google Scholar]
- 108.Chao D., Ariake K., Sato S., Ohtsuka H., Takadate T., Ishida M., et al. Stomatin-like protein 2 induces metastasis by regulating the expression of a rate-limiting enzyme of the hexosamine biosynthetic pathway in pancreatic cancer. Oncol. Rep. 2021;45:90. doi: 10.3892/or.2021.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu X., Qiao D., Pan L., Boldogh I., Zhao Y., Brasier A.R. RELA∙8-Oxoguanine DNA glycosylase1 is an epigenetic regulatory complex coordinating the hexosamine biosynthetic pathway in RSV infection. Cells. 2022;11:2210. doi: 10.3390/cells11142210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maffei B., Laverrière M., Wu Y., Triboulet S., Perrinet S., Duchateau M., et al. Infection-driven activation of transglutaminase 2 boosts glucose uptake and hexosamine biosynthesis in epithelial cells. EMBO J. 2020;39 doi: 10.15252/embj.2019102166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Groves J.A., Maduka A.O., O’Meally R.N., Cole R.N., Zachara N.E. Fatty acid synthase inhibits the O-GlcNAcase during oxidative stress. J. Biol. Chem. 2017;292:6493–6511. doi: 10.1074/jbc.M116.760785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zeng Q., Zhao R.-X., Chen J., Li Y., Li X.-D., Liu X.-L., et al. O-linked GlcNAcylation elevated by HPV E6 mediates viral oncogenesis. Proc. Natl. Acad. Sci. U. S. A. 2016;113:9333–9338. doi: 10.1073/pnas.1606801113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peng K., Liu R., Jia C., Wang Y., Jeong G.H., Zhou L., et al. Regulation of O-linked N-acetyl glucosamine transferase (OGT) through E6 stimulation of the ubiquitin ligase activity of E6AP. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li Z., Li X., Nai S., Geng Q., Liao J., Xu X., et al. Checkpoint kinase 1–induced phosphorylation of O-linked β-N-acetylglucosamine transferase regulates the intermediate filament network during cytokinesis. J. Biol. Chem. 2017;292:19548–19555. doi: 10.1074/jbc.M117.811646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Y., Shu H., Liu J., Jin X., Wang L., Qu Y., et al. EGF promotes PKM2 O-GlcNAcylation by stimulating O-GlcNAc transferase phosphorylation at Y976 and their subsequent association. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.102340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Latorre-Muro P., O’Malley K.E., Bennett C.F., Perry E.A., Balsa E., Tavares C.D.J., et al. A cold-stress-inducible PERK/OGT axis controls TOM70-assisted mitochondrial protein import and cristae formation. Cell Metab. 2021;33:598–614.e7. doi: 10.1016/j.cmet.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song M., Kim H.-S., Park J.-M., Kim S.-H., Kim I.-H., Ryu S.H., et al. o-GlcNAc transferase is activated by CaMKIV-dependent phosphorylation under potassium chloride-induced depolarization in NG-108-15 cells. Cell. Signal. 2008;20:94–104. doi: 10.1016/j.cellsig.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 118.Butkinaree C., Cheung W.D., Park S., Park K., Barber M., Hart G.W. Characterization of β-N-acetylglucosaminidase cleavage by caspase-3 during apoptosis. J. Biol. Chem. 2008;283:23557–23566. doi: 10.1074/jbc.M804116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin J., Xiang Y., Huang J., Zeng H., Zeng Y., Liu J., et al. NAT10 maintains OGA mRNA stability through ac4C modification in regulating oocyte maturation. Front. Endocrinol. (Lausanne) 2022;13 doi: 10.3389/fendo.2022.907286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deng X., Yi X., Huang D., Liu P., Chen L., Du Y., et al. ROCK2 mediates osteosarcoma progression and TRAIL resistance by modulating O-GlcNAc transferase degradation. Am. J. Cancer Res. 2020;10:781–798. [PMC free article] [PubMed] [Google Scholar]
- 121.Dey A., Seshasayee D., Noubade R., French D.M., Liu J., Chaurushiya M.S., et al. Loss of the tumor suppressor BAP1 causes myeloid transformation. Science. 2012;337:1541–1546. doi: 10.1126/science.1221711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li C.-J., Li D.-G., Liu E.-J., Jiang G.-Z. Circ8199 encodes a protein that inhibits the activity of OGT by JAK2-STAT3 pathway in esophageal squamous cell carcinoma. Am. J. Cancer Res. 2023;13:1107–1117. [PMC free article] [PubMed] [Google Scholar]
- 123.Sun M.-X., An H.-Y., Sun Y.-B., Sun Y.-B., Bai B. LncRNA EBLN3P attributes methotrexate resistance in osteosarcoma cells through miR-200a-3p/O-GlcNAc transferase pathway. J. Orthop. Surg. Res. 2022;17:557. doi: 10.1186/s13018-022-03449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang L., Feng Y., Zhang C., Chen X., Huang H., Li W., et al. Upregulation of OGT by Caveolin-1 promotes hepatocellular carcinoma cell migration and invasion. Cell Biol. Int. 2021;45:2251–2263. doi: 10.1002/cbin.11673. [DOI] [PubMed] [Google Scholar]
- 125.Huang W., Chen L., Zhu K., Wang D. Oncogenic microRNA-181d binding to OGT contributes to resistance of ovarian cancer cells to cisplatin. Cell Death Discov. 2021;7:379. doi: 10.1038/s41420-021-00715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang N., Yu M., Fu Y., Ma Z. Blocking ATM attenuates SKOV3 cell proliferation and migration by disturbing OGT/OGA expression via hsa-miR-542-5p. Front. Oncol. 2022;12 doi: 10.3389/fonc.2022.839508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Herzog K., Bandiera S., Pernot S., Fauvelle C., Jühling F., Weiss A., et al. Functional microRNA screen uncovers O-linked N-acetylglucosamine transferase as a host factor modulating hepatitis C virus morphogenesis and infectivity. Gut. 2020;69:380–392. doi: 10.1136/gutjnl-2018-317423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kalantzakos T.J., Sullivan T.B., Sebel L.E., Canes D., Burks E.J., Moinzadeh A., et al. MicroRNAs MiR-15a and MiR-26a cooperatively regulate O-GlcNAc-transferase to control proliferation in clear cell renal cell carcinoma. Cancer Biomark. 2021;30:343–351. doi: 10.3233/CBM-200553. [DOI] [PubMed] [Google Scholar]
- 129.Kalantzakos T.J., Sullivan T.B., Gloria T., Canes D., Moinzadeh A., Rieger-Christ K.M. MiRNA-424-5p suppresses proliferation, migration, and invasion of clear cell renal cell carcinoma and attenuates expression of O-GlcNAc-Transferase. Cancers (Basel) 2021;13:5160. doi: 10.3390/cancers13205160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ning D., Chen J., Du P., Liu Q., Cheng Q., Li X., et al. The crosstalk network of XIST/miR-424-5p/OGT mediates RAF1 glycosylation and participates in the progression of liver cancer. Liver Int. 2021;41:1933–1944. doi: 10.1111/liv.14904. [DOI] [PubMed] [Google Scholar]
- 131.Liu X., Chen X., Xiao M., Zhu Y., Gong R., Liu J., et al. RANBP2 activates O-GlcNAcylation through inducing CEBPα-dependent OGA downregulation to promote hepatocellular carcinoma Malignant Phenotypes. Cancers (Basel). 2021;13:3475. doi: 10.3390/cancers13143475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vaidyanathan K., Niranjan T., Selvan N., Teo C.F., May M., Patel S., et al. Identification and characterization of a missense mutation in the O-linked β-N-acetylglucosamine (O-GlcNAc) transferase gene that segregates with X-linked intellectual disability. J. Biol. Chem. 2017;292:8948–8963. doi: 10.1074/jbc.M116.771030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qian K., Wang S., Fu M., Zhou J., Singh J.P., Li M.-D., et al. Transcriptional regulation of O-GlcNAc homeostasis is disrupted in pancreatic cancer. J. Biol. Chem. 2018;293:13989–14000. doi: 10.1074/jbc.RA118.004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin C.-H., Liao C.-C., Chen M.-Y., Chou T.-Y. Feedback regulation of O-GlcNAc transferase through translation control to maintain Intracellular O-GlcNAc homeostasis. Int. J. Mol. Sci. 2021;22:3463. doi: 10.3390/ijms22073463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang M., Xu B., Li X., Shang Y., Chu Y., Wang W., et al. O-GlcNAcylation promotes colorectal cancer metastasis via the miR-101-O-GlcNAc/EZH2 regulatory feedback circuit. Oncogene. 2019;38:301–316. doi: 10.1038/s41388-018-0435-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Li X., Zhang Z., Li L., Gong W., Lazenby A.J., Swanson B.J., et al. Myeloid-derived cullin 3 promotes STAT3 phosphorylation by inhibiting OGT expression and protects against intestinal inflammation. J. Exp. Med. 2017;214:1093–1109. doi: 10.1084/jem.20161105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Young M.E., Yan J., Razeghi P., Cooksey R.C., Guthrie P.H., Stepkowski S.M., et al. Proposed regulation of gene expression by glucose in Rodent heart. Gene Regul. Syst. Bio. 2007;1:251–262. doi: 10.4137/grsb.s222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Muthusamy S., DeMartino A.M., Watson L.J., Brittian K.R., Zafir A., Dassanayaka S., et al. MicroRNA-539 is up-regulated in failing heart, and suppresses O-GlcNAcase expression. J. Biol. Chem. 2014;289:29665–29676. doi: 10.1074/jbc.M114.578682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang C., Lian H., Xie L., Yin N., Cui Y. LncRNA ELFN1-AS1 promotes esophageal cancer progression by up-regulating GFPT1 via sponging miR-183-3p. Biol. Chem. 2020;401:1053–1061. doi: 10.1515/hsz-2019-0430. [DOI] [PubMed] [Google Scholar]
- 140.Liu R., Ma X., Chen L., Yang Y., Zeng Y., Gao J., et al. MicroRNA-15b suppresses Th17 differentiation and is associated with pathogenesis of multiple Sclerosis by targeting O -GlcNAc transferase. J. Immunol. 2017;198:2626–2639. doi: 10.4049/jimmunol.1601727. [DOI] [PubMed] [Google Scholar]
- 141.Yan W., Cao M., Ruan X., Jiang L., Lee S., Lemanek A., et al. Cancer-cell-secreted miR-122 suppresses O-GlcNAcylation to promote skeletal muscle proteolysis. Nat. Cell Biol. 2022;24:793–804. doi: 10.1038/s41556-022-00893-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Muthusamy S., Hong K.U., Dassanayaka S., Hamid T., Jones S.P. E2F1 transcription factor regulates O-linked N-acetylglucosamine (O-GlcNAc) transferase and O-GlcNAcase expression. J. Biol. Chem. 2015;290:31013–31024. doi: 10.1074/jbc.M115.677534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Belke D.D. Swim-exercised mice show a decreased level of protein O -GlcNAcylation and expression of O -GlcNAc transferase in heart. J. Appl. Physiol. 2011;111:157–162. doi: 10.1152/japplphysiol.00147.2011. [DOI] [PubMed] [Google Scholar]
- 144.Zhang Z., Tan E.P., VandenHull N.J., Peterson K.R., Slawson C. O-GlcNAcase expression is sensitive to changes in O-GlcNAc homeostasis. Front. Endocrinol. (Lausanne) 2014;5:206. doi: 10.3389/fendo.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Park S.-K., Zhou X., Pendleton K.E., Hunter O.V., Kohler J.J., O’Donnell K.A., et al. A conserved splicing silencer dynamically regulates O-GlcNAc transferase intron retention and O-GlcNAc homeostasis. Cell Rep. 2017;20:1088–1099. doi: 10.1016/j.celrep.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wilkinson M.J., Manoogian E.N.C., Zadourian A., Lo H., Fakhouri S., Shoghi A., et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31:92–104.e5. doi: 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kim S., Kim S.A., Han J., Kim I.-S. Rho-kinase as a target for cancer therapy and its immunotherapeutic potential. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222312916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kobrinski D.A., Yang H., Kittaneh M. BAP1: role in carcinogenesis and clinical implications. Transl Lung Cancer Res. 2020;9:S60–S66. doi: 10.21037/tlcr.2019.11.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stephen H.M., Adams T.M., Wells L. Regulating the regulators: mechanisms of substrate selection of the O-GlcNAc cycling enzymes OGT and OGA. Glycobiology. 2021;31:724–733. doi: 10.1093/glycob/cwab005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.King D.T., Males A., Davies G.J., Vocadlo D.J. Molecular mechanisms regulating O-linked N-acetylglucosamine (O-GlcNAc)–processing enzymes. Curr. Opin. Chem. Biol. 2019;53:131–144. doi: 10.1016/j.cbpa.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 151.Cheung W.D., Sakabe K., Housley M.P., Dias W.B., Hart G.W. O-linked β-N-acetylglucosaminyltransferase substrate specificity is regulated by myosin phosphatase targeting and other interacting proteins. J. Biol. Chem. 2008;283:33935–33941. doi: 10.1074/jbc.M806199200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jínek M., Rehwinkel J., Lazarus B.D., Izaurralde E., Hanover J.A., Conti E. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin alpha. Nat. Struct. Mol. Biol. 2004;11:1001–1007. doi: 10.1038/nsmb833. [DOI] [PubMed] [Google Scholar]
- 153.Kohler J.J. Carb cutting works better with a partner. Nat. Struct. Mol. Biol. 2017;24:433–435. doi: 10.1038/nsmb.3405. [DOI] [PubMed] [Google Scholar]
- 154.Elsen N.L., Patel S.B., Ford R.E., Hall D.L., Hess F., Kandula H., et al. Insights into activity and inhibition from the crystal structure of human O-GlcNAcase. Nat. Chem. Biol. 2017;13:613–615. doi: 10.1038/nchembio.2357. [DOI] [PubMed] [Google Scholar]
- 155.Joiner C.M., Levine Z.G., Aonbangkhen C., Woo C.M., Walker S. Aspartate residues far from the active site drive O-GlcNAc transferase substrate selection. J. Am. Chem. Soc. 2019;141:12974–12978. doi: 10.1021/jacs.9b06061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vocadlo D.J. O-GlcNAc processing enzymes: catalytic mechanisms, substrate specificity, and enzyme regulation. Curr. Opin. Chem. Biol. 2012;16:488–497. doi: 10.1016/j.cbpa.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 157.März P., Stetefeld J., Bendfeldt K., Nitsch C., Reinstein J., Shoeman R.L., et al. Ataxin-10 interacts with O-linked beta-N-acetylglucosamine transferase in the brain. J. Biol. Chem. 2006;281:20263–20270. doi: 10.1074/jbc.M601563200. [DOI] [PubMed] [Google Scholar]
- 158.Levine Z.G., Walker S. The Biochemistry of O -GlcNAc transferase: which functions Make it essential in mammalian cells? Annu. Rev. Biochem. 2016;85:631–657. doi: 10.1146/annurev-biochem-060713-035344. [DOI] [PubMed] [Google Scholar]
- 159.Stephen H.M., Praissman J.L., Wells L. Generation of an interactome for the tetratricopeptide repeat domain of O-GlcNAc transferase indicates a role for the enzyme in intellectual disability. J. Proteome Res. 2021;20:1229–1242. doi: 10.1021/acs.jproteome.0c00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martin J.C., Fadda E., Ito K., Woods R.J. Defining the structural origin of the substrate sequence independence of O-GlcNAcase using a combination of molecular docking and dynamics simulation. Glycobiology. 2014;24:85–96. doi: 10.1093/glycob/cwt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schimpl M., Schüttelkopf A.W., Borodkin V.S., van Aalten D.M.F. Human OGA binds substrates in a conserved peptide recognition groove. Biochem. J. 2010;432:1–7. doi: 10.1042/BJ20101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ruan H.-B., Han X., Li M.-D., Singh J.P., Qian K., Azarhoush S., et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1α stability. Cell Metab. 2012;16:226–237. doi: 10.1016/j.cmet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cheung W.D., Hart G.W. AMP-activated protein kinase and p38 MAPK activate O-GlcNAcylation of neuronal proteins during glucose deprivation. J. Biol. Chem. 2008;283:13009–13020. doi: 10.1074/jbc.M801222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Whisenhunt T.R., Yang X., Bowe D.B., Paterson A.J., Van Tine B.A., Kudlow J.E. Disrupting the enzyme complex regulating O-GlcNAcylation blocks signaling and development. Glycobiology. 2006;16:551–563. doi: 10.1093/glycob/cwj096. [DOI] [PubMed] [Google Scholar]
- 165.Lai C.-Y., Liu H., Tin K.X., Huang Y., Yeh K.-H., Peng H.W., et al. Identification of UAP1L1 as a critical factor for protein O-GlcNAcylation and cell proliferation in human hepatoma cells. Oncogene. 2019;38:317–331. doi: 10.1038/s41388-018-0442-6. [DOI] [PubMed] [Google Scholar]
- 166.Ito R., Katsura S., Shimada H., Tsuchiya H., Hada M., Okumura T., et al. TET3-OGT interaction increases the stability and the presence of OGT in chromatin. Genes Cells. 2014;19:52–65. doi: 10.1111/gtc.12107. [DOI] [PubMed] [Google Scholar]
- 167.Laarse S.A.M., Leney A.C., Heck A.J.R. Crosstalk between phosphorylation and O-Glc NA cylation: friend or foe. FEBS J. 2018;285:3152–3167. doi: 10.1111/febs.14491. [DOI] [PubMed] [Google Scholar]
- 168.Allison D.F., Wamsley J.J., Kumar M., Li D., Gray L.G., Hart G.W., et al. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-κB acetylation and transcription. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16888–16893. doi: 10.1073/pnas.1208468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ma Z., Chalkley R.J., Vosseller K. Hyper-O-GlcNAcylation activates nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) signaling through interplay with phosphorylation and acetylation. J. Biol. Chem. 2017;292:9150–9163. doi: 10.1074/jbc.M116.766568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kronlage M., Dewenter M., Grosso J., Fleming T., Oehl U., Lehmann L.H., et al. O-GlcNAcylation of histone deacetylase 4 protects the diabetic heart from failure. Circulation. 2019;140:580–594. doi: 10.1161/CIRCULATIONAHA.117.031942. [DOI] [PubMed] [Google Scholar]
- 171.Fujiki R., Hashiba W., Sekine H., Yokoyama A., Chikanishi T., Ito S., et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature. 2011;480:557–560. doi: 10.1038/nature10656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ruan H.-B., Nie Y., Yang X. Regulation of protein degradation by O-GlcNAcylation: crosstalk with ubiquitination. Mol. Cell. Proteomics. 2013;12:3489–3497. doi: 10.1074/mcp.R113.029751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li M.-D., Ruan H.-B., Hughes M.E., Lee J.-S., Singh J.P., Jones S.P., et al. O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab. 2013;17:303–310. doi: 10.1016/j.cmet.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yao H., Li A., Wang M. Systematic analysis and prediction of in Situ Cross talk of O-GlcNAcylation and phosphorylation. Biomed. Res. Int. 2015;2015:279823. doi: 10.1155/2015/279823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Leney A.C., El Atmioui D., Wu W., Ovaa H., Heck A.J.R. Elucidating crosstalk mechanisms between phosphorylation and O-GlcNAcylation. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E7255–E7261. doi: 10.1073/pnas.1620529114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Aulak K.S., Barnes J.W., Tian L., Mellor N.E., Haque M.M., Willard B., et al. Specific O-GlcNAc modification at Ser-615 modulates eNOS function. Redox Biol. 2020;36 doi: 10.1016/j.redox.2020.101625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hortemo K.H., Lunde P.K., Anonsen J.H., Kvaløy H., Munkvik M., Rehn T.A., et al. Exercise training increases protein O-GlcNAcylation in rat skeletal muscle. Physiol. Rep. 2016;4 doi: 10.14814/phy2.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Park J., Jung S., Kim S.-M., Park I.Y., Bui N.A., Hwang G.-S., et al. Repeated hypoxia exposure induces cognitive dysfunction, brain inflammation, and amyloidβ/p-Tau accumulation through reduced brain O-GlcNAcylation in zebrafish. J. Cereb. Blood Flow Metab. 2021;41:3111–3126. doi: 10.1177/0271678X211027381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kim S.-M., Zhang S., Park J., Sung H.J., Tran T.-D.T., Chung C., et al. REM sleep deprivation impairs learning and memory by decreasing brain O-GlcNAc cycling in mouse. Neurotherapeutics. 2021;18:2504–2517. doi: 10.1007/s13311-021-01094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Richards A.M., Nicholls M.G., Espiner E.A., Ikram H., Cullens M., Hinton D. Diurnal patterns of blood pressure, heart rate and vasoactive hormones in normal man. Clin. Exp. Hypertens. A. 1986;8:153–166. doi: 10.3109/10641968609074769. [DOI] [PubMed] [Google Scholar]
- 181.Isobe S., Ohashi N., Ishigaki S., Tsuji T., Sakao Y., Kato A., et al. Augmented circadian rhythm of the intrarenal renin–angiotensin systems in anti-thymocyte serum nephritis rats. Hypertens. Res. 2016;39:312–320. doi: 10.1038/hr.2015.151. [DOI] [PubMed] [Google Scholar]
- 182.Tuomela T., Viinikka L., Perheentupa J. Epidermal growth factor in mice: changes during circadian and female reproductive cycles. Acta Endocrinol. (Copenh) 1990;123:643–648. doi: 10.1530/acta.0.1230643. [DOI] [PubMed] [Google Scholar]
- 183.Haus E., Haus E., Dumitriu L., Nicolau G.Y., Bologa S., Sackett-Lundeen L. Circadian rhythms of basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), insulin-like growth factor binding protein-3 (IGFBP-3), cortisol, and melatonin in women with breast cancer. Chronobiol. Int. 2001;18:709–727. doi: 10.1081/cbi-100106083. [DOI] [PubMed] [Google Scholar]
- 184.Vaghefi S.S.E., Mousavi F., Khaksari M., Asadikaram G., Soltani Z. Sex-related changes in circadian rhythm of inflammatory and oxidative stress Markers in CKD. Iran. J. Kidney Dis. 2021;15:13. [PubMed] [Google Scholar]
- 185.Yang H., Yang L.-T., Liu J., Tang S., Zhao X., Wang Q., et al. Circadian protein CLK suppresses transforming growth factor-β expression in peripheral B cells of nurses with day-night shift rotation. Am. J. Transl. Res. 2018;10:4331–4337. [PMC free article] [PubMed] [Google Scholar]
- 186.Zimmet P., Alberti K.G.M.M., Stern N., Bilu C., El-Osta A., Einat H., et al. The circadian syndrome: is the metabolic syndrome and much more. J. Intern. Med. 2019;286:181–191. doi: 10.1111/joim.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kohsaka A., Laposky A.D., Ramsey K.M., Estrada C., Joshu C., Kobayashi Y., et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6:414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 188.Eckel-Mahan K.L., Patel V.R., de Mateo S., Orozco-Solis R., Ceglia N.J., Sahar S., et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155:1464–1478. doi: 10.1016/j.cell.2013.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhou L., Zhang Z., Nice E., Huang C., Zhang W., Tang Y. Circadian rhythms and cancers: the intrinsic links and therapeutic potentials. J. Hematol. Oncol. 2022;15:21. doi: 10.1186/s13045-022-01238-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rutters F., Nefs G. Sleep and circadian rhythm disturbances in diabetes: a narrative review. Diabetes Metab. Syndr. Obes. 2022;15:3627–3637. doi: 10.2147/DMSO.S354026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shen Y., Lv Q., Xie W., Gong S., Zhuang S., Liu J., et al. Circadian disruption and sleep disorders in neurodegeneration. Transl. Neurodegener. 2023;12:8. doi: 10.1186/s40035-023-00340-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Horta M.A.C., Thieme N., Gao Y., Burnum-Johnson K.E., Nicora C.D., Gritsenko M.A., et al. Broad substrate-specific phosphorylation events are associated with the initial stage of plant cell wall recognition in Neurospora crassa. Front. Microbiol. 2019;10:2317. doi: 10.3389/fmicb.2019.02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Krahmer J., Hindle M., Perby L.K., Mogensen H.K., Nielsen T.H., Halliday K.J., et al. The circadian clock gene circuit controls protein and phosphoprotein rhythms in arabidopsis thaliana. Mol. Cell. Proteomics. 2022;21 doi: 10.1016/j.mcpro.2021.100172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mauvoisin D., Atger F., Dayon L., Núñez Galindo A., Wang J., Martin E., et al. Circadian and feeding rhythms orchestrate the diurnal liver acetylome. Cell Rep. 2017;20:1729–1743. doi: 10.1016/j.celrep.2017.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Masri S., Patel V.R., Eckel-Mahan K.L., Peleg S., Forne I., Ladurner A.G., et al. Circadian acetylome reveals regulation of mitochondrial metabolic pathways. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3339–3344. doi: 10.1073/pnas.1217632110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Szabó Á., Papin C., Cornu D., Chélot E., Lipinszki Z., Udvardy A., et al. Ubiquitylation dynamics of the clock cell proteome and TIMELESS during a circadian cycle. Cell Rep. 2018;23:2273–2282. doi: 10.1016/j.celrep.2018.04.064. [DOI] [PubMed] [Google Scholar]
- 197.Mauvoisin D., Wang J., Jouffe C., Martin E., Atger F., Waridel P., et al. Circadian clock-dependent and -independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proc. Natl. Acad. Sci. U. S. A. 2014;111:167–172. doi: 10.1073/pnas.1314066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hughes M.E., DiTacchio L., Hayes K.R., Vollmers C., Pulivarthy S., Baggs J.E., et al. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pizarro A., Hayer K., Lahens N.F., Hogenesch J.B. CircaDB: a database of mammalian circadian gene expression profiles. Nucleic Acids Res. 2013;41:D1009–1013. doi: 10.1093/nar/gks1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pembroke W.G., Babbs A., Davies K.E., Ponting C.P., Oliver P.L. Temporal transcriptomics suggest that twin-peaking genes reset the clock. Elife. 2015;4 doi: 10.7554/eLife.10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Li S., Shui K., Zhang Y., Lv Y., Deng W., Ullah S., et al. CGDB: a database of circadian genes in eukaryotes. Nucleic Acids Res. 2017;45:D397–D403. doi: 10.1093/nar/gkw1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Buel S.M., Debopadhaya S., De los Santos H., Edwards K.M., David A.M., Dao U.H., et al. The PAICE suite reveals circadian posttranscriptional timing of noncoding RNAs and spliceosome components in Mus musculus macrophages. G3 (Bethesda) 2022;12:jkac176. doi: 10.1093/g3journal/jkac176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Janich P., Arpat A.B., Castelo-Szekely V., Lopes M., Gatfield D. Ribosome profiling reveals the rhythmic liver translatome and circadian clock regulation by upstream open reading frames. Genome Res. 2015;25:1848–1859. doi: 10.1101/gr.195404.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Du N.-H., Arpat A.B., De Matos M., Gatfield D. MicroRNAs shape circadian hepatic gene expression on a transcriptome-wide scale. Elife. 2014;3 doi: 10.7554/eLife.02510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hughes M.E., Hong H.-K., Chong J.L., Indacochea A.A., Lee S.S., Han M., et al. Brain-specific rescue of Clock reveals system-driven transcriptional rhythms in peripheral tissue. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kuintzle R.C., Chow E.S., Westby T.N., Gvakharia B.O., Giebultowicz J.M., Hendrix D.A. Circadian deep sequencing reveals stress-response genes that adopt robust rhythmic expression during aging. Nat. Commun. 2017;8 doi: 10.1038/ncomms14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Castellana S., Biagini T., Petrizzelli F., Cabibbo A., Mazzoccoli G., Mazza T. RhythmicDB: a database of predicted Multi-Frequency rhythmic transcripts. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.882044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Negoro H., Kanematsu A., Doi M., Suadicani S.O., Matsuo M., Imamura M., et al. Involvement of urinary bladder Connexin43 and the circadian clock in coordination of diurnal micturition rhythm. Nat. Commun. 2012;3:809. doi: 10.1038/ncomms1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Haspel J.A., Chettimada S., Shaik R.S., Chu J.-H., Raby B.A., Cernadas M., et al. Circadian rhythm reprogramming during lung inflammation. Nat. Commun. 2014;5:4753. doi: 10.1038/ncomms5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jouffe C., Cretenet G., Symul L., Martin E., Atger F., Naef F., et al. The circadian clock coordinates ribosome biogenesis. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang R., Lahens N.F., Ballance H.I., Hughes M.E., Hogenesch J.B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl. Acad. Sci. U. S. A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kim E.Y., Jeong E.H., Park S., Jeong H.-J., Edery I., Cho J.W. A role for O -GlcNAcylation in setting circadian clock speed. Genes Dev. 2012;26:490–502. doi: 10.1101/gad.182378.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Terajima H., Yoshitane H., Ozaki H., Suzuki Y., Shimba S., Kuroda S., et al. ADARB1 catalyzes circadian A-to-I editing and regulates RNA rhythm. Nat. Genet. 2017;49:146–151. doi: 10.1038/ng.3731. [DOI] [PubMed] [Google Scholar]
- 214.Masri S., Rigor P., Cervantes M., Ceglia N., Sebastian C., Xiao C., et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell. 2014;158:659–672. doi: 10.1016/j.cell.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yang G., Chen L., Grant G.R., Paschos G., Song W.-L., Musiek E.S., et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci. Transl. Med. 2016;8:324ra16. doi: 10.1126/scitranslmed.aad3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Atger F., Gobet C., Marquis J., Martin E., Wang J., Weger B., et al. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E6579–E6588. doi: 10.1073/pnas.1515308112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cretenet G., Le Clech M., Gachon F. Circadian clock-coordinated 12 Hr period rhythmic activation of the IRE1α pathway controls lipid metabolism in mouse liver. Cell Metab. 2010;11:47–57. doi: 10.1016/j.cmet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 218.Shao M., Shan B., Liu Y., Deng Y., Yan C., Wu Y., et al. Hepatic IRE1α regulates fasting-induced metabolic adaptive programs through the XBP1s–PPARα axis signalling. Nat. Commun. 2014;5:3528. doi: 10.1038/ncomms4528. [DOI] [PubMed] [Google Scholar]
- 219.Hong S.-H., Kang M., Lee K.-S., Yu K. High fat diet-induced TGF-β/Gbb signaling provokes insulin resistance through the tribbles expression. Sci. Rep. 2016;6 doi: 10.1038/srep30265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Cao Y., Gao X., Zhang W., Zhang G., Nguyen A.K., Liu X., et al. Dietary fiber enhances TGF- signaling and growth inhibition in the gut. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;301:G156–G164. doi: 10.1152/ajpgi.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bosch-Queralt M., Cantuti-Castelvetri L., Damkou A., Schifferer M., Schlepckow K., Alexopoulos I., et al. Diet-dependent regulation of TGFβ impairs reparative innate immune responses after demyelination. Nat. Metab. 2021;3:211–227. doi: 10.1038/s42255-021-00341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hermetet F., Buffière A., Aznague A., Pais De Barros J.-P., Bastie J.-N., Delva L., et al. High-fat diet disturbs lipid raft/TGF-β signaling-mediated maintenance of hematopoietic stem cells in mouse bone marrow. Nat. Commun. 2019;10:523. doi: 10.1038/s41467-018-08228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rahman S.A., Castanon-Cervantes O., Scheer F.A.J.L., Shea S.A., Czeisler C.A., Davidson A.J., et al. Endogenous circadian regulation of pro-inflammatory cytokines and chemokines in the presence of bacterial lipopolysaccharide in humans. Brain Behav. Immun. 2015;47:4–13. doi: 10.1016/j.bbi.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.D’Esposito V., Di Tolla M.F., Lecce M., Cavalli F., Libutti M., Misso S., et al. Lifestyle and dietary habits affect plasma levels of specific cytokines in healthy subjects. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.913176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dauletbaev N., Herscovitch K., Das M., Chen H., Bernier J., Matouk E., et al. Down-regulation of IL-8 by high-dose vitamin D is specific to hyperinflammatory macrophages and involves mechanisms beyond up-regulation of DUSP1: vitamin D suppresses IL-8 in hyperinflammatory MDM. Br. J. Pharmacol. 2015;172:4757–4771. doi: 10.1111/bph.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Allen L.A., Schmidt J.R., Thompson C.T., Carlson B.E., Beard D.A., Lombard J.H. High salt diet impairs cerebral blood flow regulation via salt-induced angiotensin II suppression. Microcirculation. 2019;26 doi: 10.1111/micc.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wolfrum C., Asilmaz E., Luca E., Friedman J.M., Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 228.Lee J.H., Sancar A. Regulation of apoptosis by the circadian clock through NF-κB signaling. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12036–12041. doi: 10.1073/pnas.1108125108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Thapaliya S., Wree A., Povero D., Inzaugarat M.E., Berk M., Dixon L., et al. Caspase 3 Inactivation protects against hepatic cell death and ameliorates fibrogenesis in a diet-induced NASH model. Dig. Dis. Sci. 2014;59:1197–1206. doi: 10.1007/s10620-014-3167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lakshmanan M.R., Nepokroeff C.M., Porter J.W. Control of the synthesis of fatty-acid Synthetase in rat liver by insulin, Glucagon, and Adenosine 3′:5′ cyclic Monophosphate. Proc. Natl. Acad. Sci. U. S. A. 1972;69:3516–3519. doi: 10.1073/pnas.69.12.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Volpe J.J., Vagelos P.R. Regulation of mammalian fatty-acid Synthetase. The roles of Carbohydrate and insulin. Proc. Natl. Acad. Sci. U. S. A. 1974;71:889–893. doi: 10.1073/pnas.71.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Saito T., Hirano M., Ide T., Ichiki T., Koibuchi N., Sunagawa K., et al. Pivotal role of Rho-associated kinase 2 in generating the intrinsic circadian rhythm of vascular contractility. Circulation. 2013;127:104–114. doi: 10.1161/CIRCULATIONAHA.112.135608. [DOI] [PubMed] [Google Scholar]
- 233.Soliman H., Nyamandi V., Garcia-Patino M., Varela J.N., Bankar G., Lin G., et al. Partial deletion of ROCK2 protects mice from high-fat diet-induced cardiac insulin resistance and contractile dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H70–H81. doi: 10.1152/ajpheart.00664.2014. [DOI] [PubMed] [Google Scholar]
- 234.Early J.O., Menon D., Wyse C.A., Cervantes-Silva M.P., Zaslona Z., Carroll R.G., et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E8460–E8468. doi: 10.1073/pnas.1800431115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Hansen K.F., Sakamoto K., Obrietan K. MicroRNAs: a potential interface between the circadian clock and human health. Genome Med. 2011;3:10. doi: 10.1186/gm224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Kura B., Parikh M., Slezak J., Pierce G.N. The influence of diet on MicroRNAs that impact cardiovascular disease. Molecules. 2019;24:1509. doi: 10.3390/molecules24081509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kang T.-H., Leem S.-H. Modulation of ATR-mediated DNA damage checkpoint response by cryptochrome 1. Nucleic Acids Res. 2014;42:4427–4434. doi: 10.1093/nar/gku094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ünsal-Kaçmaz K., Mullen T.E., Kaufmann W.K., Sancar A. Coupling of human circadian and cell cycles by the Timeless protein. Mol. Cell. Biol. 2005;25:3109–3116. doi: 10.1128/MCB.25.8.3109-3116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Bu Y., Yoshida A., Chitnis N., Altman B.J., Tameire F., Oran A., et al. A PERK–miR-211 axis suppresses circadian regulators and protein synthesis to promote cancer cell survival. Nat. Cell Biol. 2018;20:104–115. doi: 10.1038/s41556-017-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Deldicque L., Cani P.D., Philp A., Raymackers J.-M., Meakin P.J., Ashford M.L.J., et al. The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am. J. Physiol. Endocrinol. Metab. 2010;299:E695–E705. doi: 10.1152/ajpendo.00038.2010. [DOI] [PubMed] [Google Scholar]
- 241.Tumanovska L.V., Swanson R.J., Serebrovska Z.O., Portnichenko G.V., Goncharov S.V., Kysilov B.A., et al. Cholesterol enriched diet suppresses ATF6 and PERK and upregulates the IRE1 pathways of the unfolded protein response in spontaneously hypertensive rats: Relevance to pathophysiology of atherosclerosis in the setting of hypertension. Pathophysiology. 2019;26:219–226. doi: 10.1016/j.pathophys.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 242.Manzella N., Bracci M., Strafella E., Staffolani S., Ciarapica V., Copertaro A., et al. Circadian modulation of 8-oxoguanine DNA damage repair. Sci. Rep. 2015;5 doi: 10.1038/srep13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Komakula S.S.B., Tumova J., Kumaraswamy D., Burchat N., Vartanian V., Ye H., et al. The DNA repair protein OGG1 protects against obesity by altering mitochondrial energetics in white adipose tissue. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-33151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sampath H., Vartanian V., Rollins M.R., Sakumi K., Nakabeppu Y., Lloyd R.S. 8-Oxoguanine DNA glycosylase (OGG1) deficiency increases susceptibility to obesity and metabolic dysfunction. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.O-Sullivan I., Zhang W., Wasserman D.H., Liew C.W., Liu J., Paik J., et al. FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization. Nat. Commun. 2015;6:7079. doi: 10.1038/ncomms8079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Carrano A.C., Liu Z., Dillin A., Hunter T. A conserved ubiquitination pathway determines longevity in response to diet restriction. Nature. 2009;460:396–399. doi: 10.1038/nature08130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pang L., Dunterman M., Xuan W., Gonzalez A., Lin Y., Hsu W.-H., et al. Circadian regulator CLOCK promotes tumor angiogenesis in glioblastoma. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Dimova E.Y., Jakupovic M., Kubaichuk K., Mennerich D., Chi T.F., Tamanini F., et al. The circadian clock protein CRY1 is a negative regulator of HIF-1α. iScience. 2019;13:284–304. doi: 10.1016/j.isci.2019.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Suyama K., Silagi E.S., Choi H., Sakabe K., Mochida J., Shapiro I.M., et al. Circadian factors BMAL1 and RORα control HIF-1α transcriptional activity in nucleus pulposus cells: implications in maintenance of intervertebral disc health. Oncotarget. 2016;7:23056–23071. doi: 10.18632/oncotarget.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Vaughan M.E., Wallace M., Handzlik M.K., Chan A.B., Metallo C.M., Lamia K.A. Cryptochromes Suppress HIF1α in muscles. iScience. 2020;23 doi: 10.1016/j.isci.2020.101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Peek C.B., Levine D.C., Cedernaes J., Taguchi A., Kobayashi Y., Tsai S.J., et al. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 2017;25:86–92. doi: 10.1016/j.cmet.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gaspar J.M., Mendes N.F., Corrêa-da-Silva F., Lima-Junior J.C.D., Gaspar R.C., Ropelle E.R., et al. Downregulation of HIF complex in the hypothalamus exacerbates diet-induced obesity. Brain Behav. Immun. 2018;73:550–561. doi: 10.1016/j.bbi.2018.06.020. [DOI] [PubMed] [Google Scholar]