Abstract
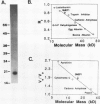
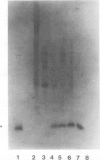
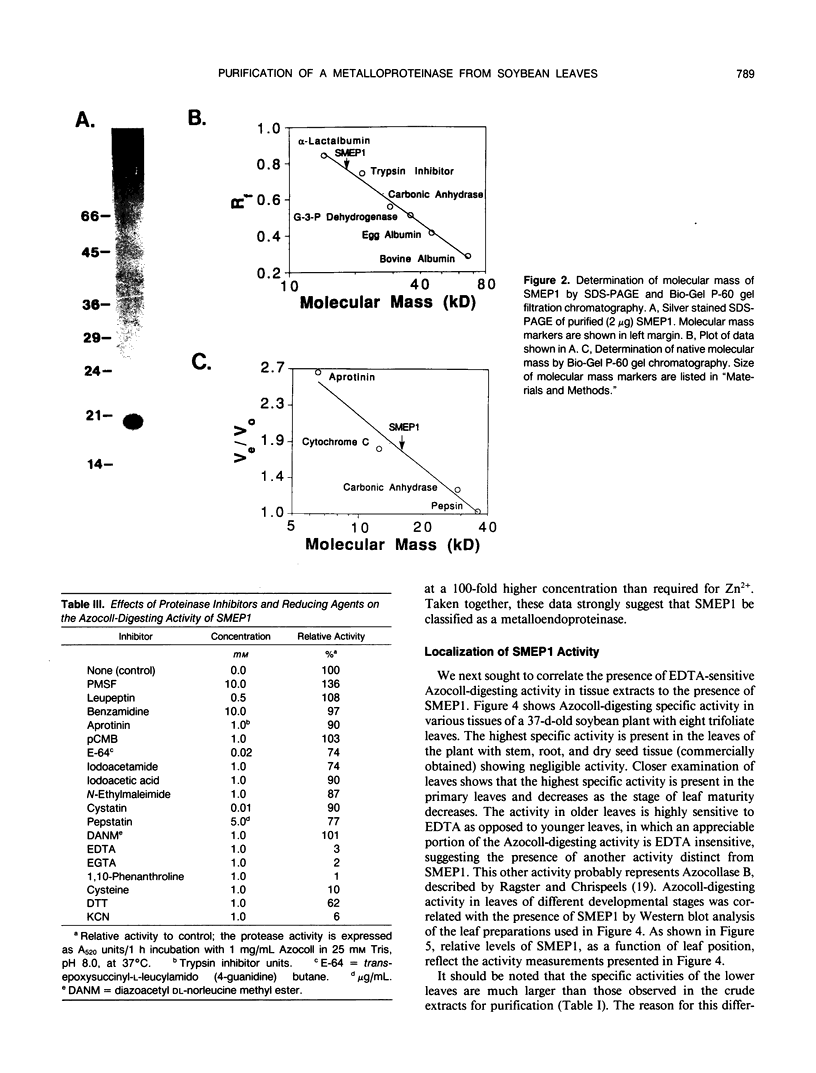
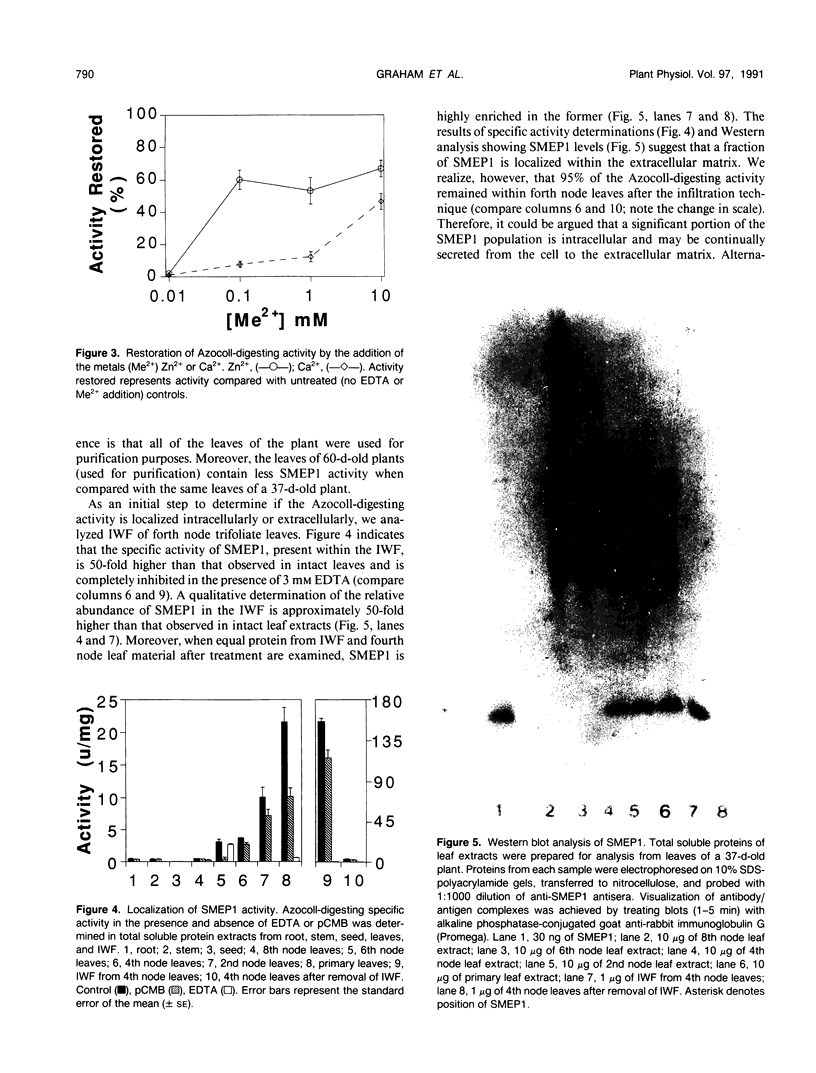
A metalloendoproteinase from leaves of soybean (Glycine max) has been purified 1160-fold to electrophoretic homogeneity. The native protein is monomeric with a molecular mass of 15 kilodaltons as estimated by gel filtration and 19 kilodaltons as estimated by denaturing gel electrophoresis. The enzyme has a pH optima of 8.0 to 9.0 using Azocoll as substrate. The proteolytic activity is susceptible to metal chelating agents and the inactivated enzyme can be restored to 69% of original activity by the addition of ZnCl2. Western analysis shows that a fraction of the soybean metalloendoproteinase is present within the extracellular space of older leaves. Soybean metalloendoproteinase 1 is the Azocollase A activity first described by Ragster and Chrispeels (Plant Physiol 64: 857-862; 1979).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Bond H. M., Bowles D. J. Characterization of soybean endopeptidase activity using exogenous and endogenous substrates. Plant Physiol. 1983 Jun;72(2):345–350. doi: 10.1104/pp.72.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles D. J. Defense-related proteins in higher plants. Annu Rev Biochem. 1990;59:873–907. doi: 10.1146/annurev.bi.59.070190.004301. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Huffaker R. C. Proteolytic activity during senescence of plants. New Phytol. 1990;116:199–231. doi: 10.1111/j.1469-8137.1990.tb04710.x. [DOI] [PubMed] [Google Scholar]
- Koehler S. M., Ho T. H. Hormonal regulation, processing, and secretion of cysteine proteinases in barley aleurone layers. Plant Cell. 1990 Aug;2(8):769–783. doi: 10.1105/tpc.2.8.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Mauch F., Staehelin L. A. Functional Implications of the Subcellular Localization of Ethylene-Induced Chitinase and [beta]-1,3-Glucanase in Bean Leaves. Plant Cell. 1989 Apr;1(4):447–457. doi: 10.1105/tpc.1.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi W., Minamikawa T. Synthesis and Posttranslational Activation of Sulfhydryl-Endopeptidase in Cotyledons of Germinating Vigna mungo Seeds. Plant Physiol. 1989 Jan;89(1):274–279. doi: 10.1104/pp.89.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Ragster L. V., Chrispeels M. J. Azocoll-digesting Proteinases in Soybean Leaves: Characteristics and Changes during Leaf Maturation and Senescence. Plant Physiol. 1979 Nov;64(5):857–862. doi: 10.1104/pp.64.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Nelson D. E., Kuhn D., Hasegawa P. M., Bressan R. A. Molecular Cloning of Osmotin and Regulation of Its Expression by ABA and Adaptation to Low Water Potential. Plant Physiol. 1989 Jul;90(3):1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Active-site zinc ligands and activated H2O of zinc enzymes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):220–224. doi: 10.1073/pnas.87.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z. H., Varner J. E. Tissue-Specific Expression of Cell Wall Proteins in Developing Soybean Tissues. Plant Cell. 1991 Jan;3(1):23–37. doi: 10.1105/tpc.3.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wilden W., Segers J. H., Chrispeels M. J. Cell Walls of Phaseolus vulgaris Leaves Contain the Azocoll-Digesting Proteinase. Plant Physiol. 1983 Nov;73(3):576–578. doi: 10.1104/pp.73.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]