Abstract
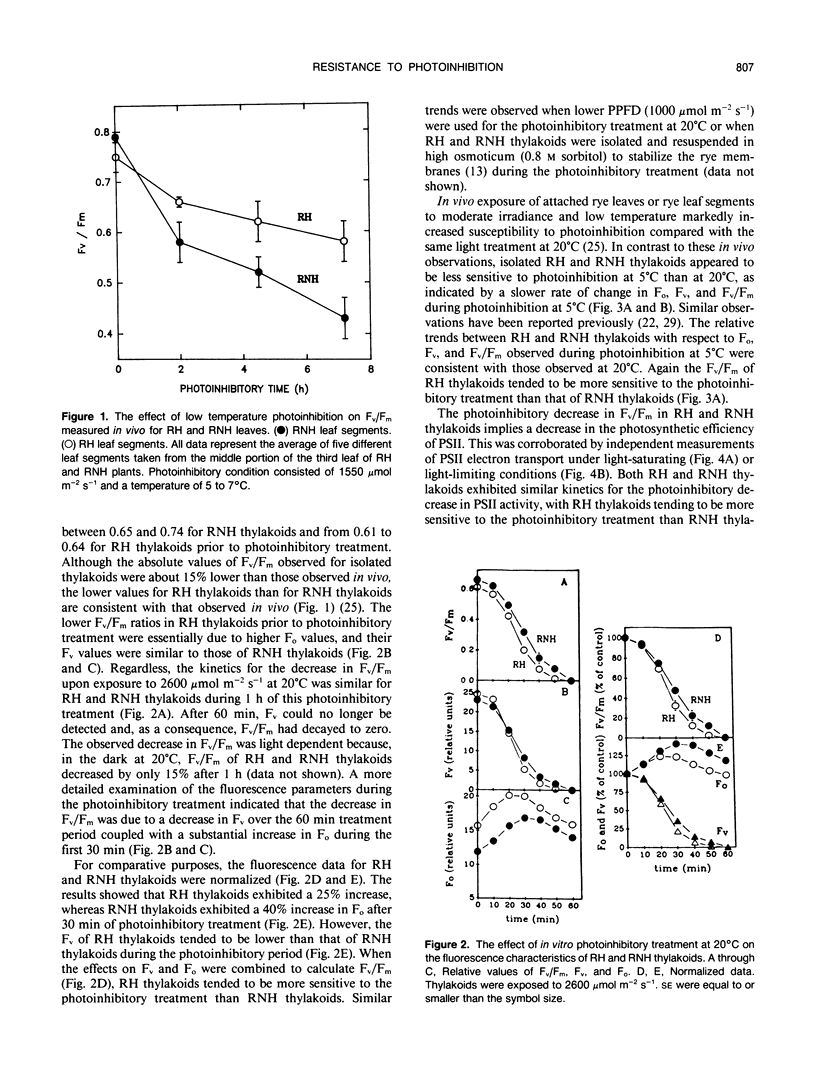
In vivo measurements of chlorophyll a fluorescence indicate that cold-hardened winter rye (Secale cereale L. cv Musketeer) develops a resistance to low temperature-induced photoinhibition compared with nonhardened rye. After 7.2 hours at 5°C and 1550 micromoles per square meter per second, the ratio of variable fluorescence/maximum fluorescence was depressed by only 23% in cold-hardened rye compared with 46% in nonhardened rye. We have tested the hypothesis that the principal site of this resistance to photoinhibition resides at the level of rye thylakoid membranes. Thylakoids were isolated from cold-hardened and nonhardened rye and exposed to high irradiance (1000-2600 micromoles per square meter per second) at either 5 or 20°C. The photoinhibitory response measured by room temperature fluorescence induction, photosystem II electron transport, photoacoustic spectroscopy, or [14C]atrazine binding indicates that the differential resistance to low temperature-induced photoinhibition in vivo is not observed in isolated thylakoids. Similar results were obtained whether isolated rye thylakoids were photoinhibited or thylakoids were isolated from rye leaves preexposed to a photoinhibitory treatment. Thus, we conclude that increased resistance to low temperature-induced photoinhibition is not a property of thylakoid membranes but is associated with a higher level of cellular organization.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boese S. R., Huner N. P. Effect of growth temperature and temperature shifts on spinach leaf morphology and photosynthesis. Plant Physiol. 1990 Dec;94(4):1830–1836. doi: 10.1104/pp.94.4.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan F. E., Becker D. W., Cheniae G. M. Studies on the Photoactivation of the Water-Oxidizing Enzyme: II. Characterization of Weak Light Photoinhibition of PSII and Its Light-Induced Recovery. Plant Physiol. 1986 Sep;82(1):261–269. doi: 10.1104/pp.82.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier R., Larue B., Leblanc R. M. Photoacoustic spectroscopy of Anacystis nidulans. I. Effect of sample thickness on the photoacoustic signal. Arch Biochem Biophys. 1983 Apr 15;222(2):403–410. doi: 10.1016/0003-9861(83)90537-4. [DOI] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huner N. P., Krol M., Williams J. P., Maissan E., Low P. S., Roberts D., Thompson J. E. Low Temperature Development Induces a Specific Decrease in trans-Delta-Hexadecenoic Acid Content which Influences LHCII Organization. Plant Physiol. 1987 May;84(1):12–18. doi: 10.1104/pp.84.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huner N. P., Williams J. P., Maissan E. E., Myscich E. G., Krol M., Laroche A., Singh J. Low Temperature-Induced Decrease in trans-Delta-Hexadecenoic Acid Content Is Correlated with Freezing Tolerance in Cereals. Plant Physiol. 1989 Jan;89(1):144–150. doi: 10.1104/pp.89.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupa Z., Huner N. P., Williams J. P., Maissan E., James D. R. Development at Cold-Hardening Temperatures : The Structure and Composition of Purified Rye Light Harvesting Complex II. Plant Physiol. 1987 May;84(1):19–24. doi: 10.1104/pp.84.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle D. J., Ohad I., Arntzen C. J. Membrane protein damage and repair: Selective loss of a quinone-protein function in chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser-Ross N., Malkin S., Cahen D. Photoacoustic detection of photosynthetic activities in isolated broken chloroplasts. Biochim Biophys Acta. 1980 Dec 3;593(2):330–341. doi: 10.1016/0005-2728(80)90070-5. [DOI] [PubMed] [Google Scholar]
- Tischer W., Strotmann H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron transport. Biochim Biophys Acta. 1977 Apr 11;460(1):113–125. doi: 10.1016/0005-2728(77)90157-8. [DOI] [PubMed] [Google Scholar]


