Abstract
Murine models of invasive candidiasis were used to study the in vivo importance of gamma interferon (IFN-γ) and interleukin-4 (IL-4) in host defense against Candida albicans and to characterize the tissue inflammatory reactions, with special reference to macrophages (Mφ). Knockout (KO) IFN-γ-deficient (GKO) and IL-4-deficient (IL-4 KO) and C57BL/6 parental mouse strains were challenged intraperitoneally with 108 C. albicans blastoconidia. Survival of GKO mice was significantly lower (16.7%) than that of C57BL/6 control (55.5%) and IL-4 KO (61.1%) animals, but was not correlated with the extent of organ colonization. Immunohistological analysis with a panel of myeloid and lymphoid markers revealed multiple renal abscesses, myocarditis, hepatitis, meningoencephalitis, and pneumonia in each strain, with a dominant presence of Mφ. In the absence of IFN-γ, C. albicans induced striking changes in the phenotype of alveolar Mφ and extensive perivascular lymphoid infiltrates in the lung. Impairment in nitric oxide production by peritoneal Mφ was shown only in GKO mice, and they produced Candida-specific immunoglobulin G (IgG), IgM, IgA, and IgG subclasses in lower titers. Our in vivo studies with KO mice elucidate a critical role for IFN-γ, but not IL-4, in host defense against C. albicans.
Candida albicans is a common commensal organism in humans, and its importance as an opportunistic pathogen, particularly in immunocompromised patients, has continued to increase over the last two decades. According to the National Nosocomial Infections Surveillance System, the ratio of C. albicans isolates among nosocomial fungal infections increased from 52% to 63% in the 1980s (4). Phagocytic cell defects generally predispose to disseminated candidiasis; candidemia was calculated to result in 38% excess mortality and extend hospitalization by approximately 30 days (40). Besides the efforts to develop more effective and safer antifungal agents, a new therapeutic approach to augment the antifungal capacity of the host’s immune system should be investigated.
The mechanisms of host defense and pathogenesis of candidiasis are not completely understood. Optimal phagocytosis of C. albicans requires opsonization; however, unopsonized yeast can be internalized by macrophages (Mφ) through the mannose receptor (21). Efficient killing of C. albicans by mononuclear phagocytes requires respiratory burst-associated toxic compounds (22), and recent data suggest that nitric oxide (NO) may also be involved in anticandidal functions of Mφ (5). Experimental evidence suggests that mononuclear phagocytes could play an important role in eradication of this pathogen, and their anticandidal activity can be augmented in vitro with granulocyte-Mφ and Mφ colony-stimulating factors and cytokines (no significant change could be measured in the level of specific immunoglobulin A [IgA] in serum or among the levels of interleukin-3 [IL-3] and gamma interferon [IFN-γ]) in both human and murine systems (23, 25, 28, 39).
The in vivo benefit of cytokine treatment in disseminated candidiasis has not been established, and data from different murine models are controversial. Administration of IFN-γ has been reported to be associated with improved survival of mice after lethal challenge with C. albicans, which correlated with the anticandidal activity of peritoneal Mφ (28); another study showed a reduction in tissue fungal burden in IFN-γ-treated mice (19). However, in a different murine model, in vivo administration of IFN-γ resulted in increased susceptibility and organ colonization of four infected inbred strains (13). In vivo administration of IL-12, which has been reported to prime naive T cells for high IFN-γ expression and skew cytokine production toward a Th1-type response (38), did not modify the course of systemic candidiasis (32). In contrast, Th2-type cytokines IL-4 and IL-10 have been reported to exacerbate infection, and neutralization of IL-4 by specific antibody or soluble IL-4 receptor resulted in an enhanced production of Th1 cytokines, associated with increased resistance to systemic murine candidiasis (26, 30, 37). The controversial results of in vivo cytokine treatment may be the consequence of genetic differences among the infected strains and also the variation in protocols; the kinetics of cytokine production are influenced by several host and pathogen factors, and the effect of exogenous cytokine might depend on the condition of the infected host and stage of infection.
Cytokine and receptor gene disruption strategies make it possible to examine the role of cytokines in host response to different pathogens directly. Recent studies showed an increased susceptibility of IFN-γ–receptor knockout (KO) mice to Mycobacterium bovis or Mycobacterium tuberculosis, but not to Schistosoma mansoni (1, 7, 8). Another study reported that disruption of the IFN-γ receptor gene was associated with higher susceptibility to Leishmania major and that IL-4 deficiency resulted in increased resistance, but only in certain inbred strains (17).
Our study was undertaken to investigate the in vivo role of IFN-γ and IL-4 in disseminated C. albicans infection and characterize the tissue inflammatory cells by immunohistochemistry and by functional assays ex vivo. We demonstrate that IFN-γ, but not IL-4, is essential for survival in invasive candidiasis and show the dominant participation of Mφ in the inflammatory lesions of different tissues in KO as well as wild-type mice. In the absence of IFN-γ, a striking local immune regulatory alteration was observed in the lungs.
MATERIALS AND METHODS
Mice.
IFN-γ-deficient KO (GKO) mice were generated on the C57BL/6 background by D. Dalton et al. (8), and IL-4 KO mice were generated on the (129Sv × C57BL/6) background by M. Kopf et al. (16). They were backcrossed for 14 generations onto C57BL/6 mice at the Sir William Dunn School of Pathology, Oxford, United Kingdom.
Experimental infection and semiquantitative organ culture.
C. albicans (ATCC 18804) was cultured as described previously (21). Specific-pathogen-free inbred C57BL/6, IL-4 KO, and GKO mice of both sexes, 6 to 8 weeks old, were challenged intraperitoneally (i.p.) with 108 C. albicans blastoconidia in two separate experiments. The half-lethal dose of pathogen in a month had been determined previously by titration in C57BL/6 mice. At 7, 14, 21, and 28 days after the C. albicans challenge, four to five mice from each strain were sacrificed by CO2 asphyxiation. Quantitation of viable C. albicans within the various organs of infected mice was made by colony counting. The brain, lungs, liver, spleen, and kidneys were removed aseptically, weighed, and homogenized in 0.1% Triton X-100 (Sigma Chemical Co., St. Louis, Mo.). Samples were plated onto Sabouraud dextrose agar (Difco Laboratories, Detroit, Mich.) in duplicate serial dilutions and incubated for 24 to 48 h at 37°C. Data were recorded as the mean log10 CFU per gram of organ. Animals were observed over 28 days.
Antibodies for immunohistology.
The following rat monoclonal antibodies (MAbs) were prepared in our laboratory and used at optimal concentrations for immunohistology: FA/11, which is specific for macrosialin and is a pan-Mφ murine homolog of CD68 (27, 33); and 7/4, which defines a polymorphic differentiation antigen on mouse neutrophils (15) and on immunologically activated murine Mφ (37a). Other antibodies used were B220 (PharMingen, San Diego, Calif.); CD19 (Serotec Ltd., Oxford, United Kingdom); and MAbs recognizing T-cell markers, which included CD3 (KT3.1.1), CD4 (YTS 191.1.1.2), and CD8 (YTS 169.4.2.1), a gift from S. P. Cobbold (Sir William Dunn School of Pathology). A hybridoma producing a MAb against major histocompatibility complex II (MHC II) (TIB120) was obtained from the American Type Culture Collection (Rockville, Md.).
Immunohistology.
Organs were excised, immersed in Tissue-Tek OCT compound (BDH-Merck, Poole, Dorset, United Kingdom), and rapidly frozen in isopentane-dry ice. Frozen sections were cut at a depth of 5 μm onto glass slides and stored at −20°C. Shortly before staining, sections were thawed at room temperature for 30 min and fixed for 10 min in 2% paraformaldehyde in HEPES-buffered isotonic saline on ice. Fixed sections were washed in 0.1% Triton X-100 in phosphate-buffered saline (PBS) and then incubated with 10 mM glucose–1 mM NaN3–0.4 U of glucose oxidase per ml (Sigma) in PBS for 15 min at 37°C to block endogenous peroxidase activity. Slides were treated with 5% normal rabbit serum for 30 min and then with the primary MAb (FA/11, 7/4, TIB120, B220, CD3, CD4, or CD8) or PBS as a control for 60 min at room temperature. Affinity-purified biotinylated rabbit anti-rat IgG (Vector Laboratories, Peterborough, United Kingdom) was used as a secondary antibody at 1% for 30 min, followed by avidin-biotin-peroxidase complex (ABC elite standard; Vector Laboratories). The presence of antigens was revealed by incubation with 0.5 mg of diaminobenzidine (Polysciences, Inc., Northampton, United Kingdom) per ml–0.024% H2O2 in 10 mM imidazole in PBS (pH 7.4). Sections were counterstained in Cresyl fast violet acetate and mounted in DPX (BDH-Merck).
For detection of fungi in tissues, sections were fixed in 10% neutral buffered formalin for 10 min and then stained with Gomori’s methenamine silver (Sigma). In brief, sections were treated with 10% periodic acid solution for 5 min to oxidize the polysaccharides to aldehydes and then with 0.11% silver methenamine in 4% borax solution for 20 min at 62°C. The aldehyde group at alkaline pH reduced silver ion to metallic silver. Sections were rinsed in 2% gold chloride to form a more stable gold complex. Finally, excess silver was removed by washing in 20% sodium thiosulfate. Fungi appeared brown to black; sections were counterstained with light green (BDH-Merck).
Ex vivo nitrite assay.
Peritoneal cells were harvested by peritoneal lavage 5 days after i.p. challenge with a lower dose of C. albicans (105 CFU/mouse). The dose of pathogen was reduced to avoid the ex vivo infection and killing of Mφ by extracellular Candida in the peritoneal fluid. Peritoneal Mφ (5 × 105/well) were cultured in 96-well plates (Falcon) in Optimem 1 (GIBCO BRL, Paisley, United Kingdom) supplemented with 2 mM l-glutamine (Sigma) and 50 IU of penicillin per ml–50 μg of streptomycin (Sigma) per ml. Culture supernatants were collected after 48 h of culture, and the nitrite concentration was assayed by the Griess reaction adapted for microplates (14). Briefly, equal volumes of 2% sulfanilamide (Sigma) in 10% phosphoric acid and 0.2% naphthylethylene diamine dihydrochloride (Sigma) were mixed to prepare the Griess reagent. Reagent (100 μl) was added to equal volumes of test supernatants, and then these mixtures were incubated for 30 min in the dark. The A550 of the formed chromophore was measured by means of a plate reader. The nitrite content of the samples was calculated by using sodium nitrite as a standard and was used as a relative measure of NO synthesis. The viability of the adherent cells was >92% by Trypan blue assay. The proportion of peritoneal Mφ was >90%, as assayed by FA/11 antibody staining.
Candida-specific antibody detection.
A standard enzyme-linked immunosorbent assay (ELISA) was done to quantify specific antibodies in mice sera. Briefly, polystyrene microtiter plates (Falcon, Becton Dickinson, Paramus, N.J.) were coated overnight at 4°C with 107 heat-killed candida per well in 0.1 M bicarbonate buffer (pH 9.6). Wells were then saturated with 1% bovine serum albumin (Sigma) in PBS for 1 h at 37°C. Appropriate serial dilutions of the samples were incubated in the plates for 1 h at 37°C. After extensive washing, bound antibodies were revealed by the addition of alkaline phosphatase-conjugated rabbit anti-mouse IgG1, IgG2a, IgG2b, IgG3 (Zymed Laboratories, San Francisco, Calif.), goat anti-mouse IgG, IgM, IgA (Sigma), or peroxidase-conjugated rat anti-mouse IgE (Serotec) for 1 h at 37°C. The colorimetric change was measured by means of a plate reader with 405- and 492-nm filters. The antibody titers were expressed as the reciprocal of the dilution giving an absorbance of 0.1 above that of the control (no serum added).
Statistical analysis.
The significance of differences between the mean survival of infected animals, organ colony counts, candida-specific antibody titers in different strains, and nitrite production in different strains was determined by Student’s t test. A difference was considered statistically significant at P < 0.05.
RESULTS
Mortality of different infected strains.
Survival of C57BL/6, IL-4 KO, and GKO strains was followed over 28 days, after i.p. challenge with 108 C. albicans (Fig. 1). No significant differences could be observed in the first 2 weeks among the different infected strains; the mortality of each group reached 22% by the end of the second week. During the third week, the survival of infected GKO mice decreased rapidly, and by day 28, their mortality rate was significantly higher (83.3%) than that of the infected IL-4 KO (38.9%) and C57BL/6 (44.5%) mice. The rates of survival of the IL-4 KO and C57BL/6 groups were not significantly different at the P < 0.05 level during the first month of the Candida infection.
FIG. 1.
Survival of GKO, IL-4 KO, and C57BL/6 control mice challenged i.p. with 108 C. albicans blastoconidia. Survival of GKO mice was significantly lower than that of IL-4 KO and C57BL/6 control animals from day 14 (P < 0.05 at days 21 and 28 for both). Each group contained 40 animals initially. The figure represents pooled data of two separate sets of experiments, which gave results in close agreement.
Semiquantitative organ cultures.
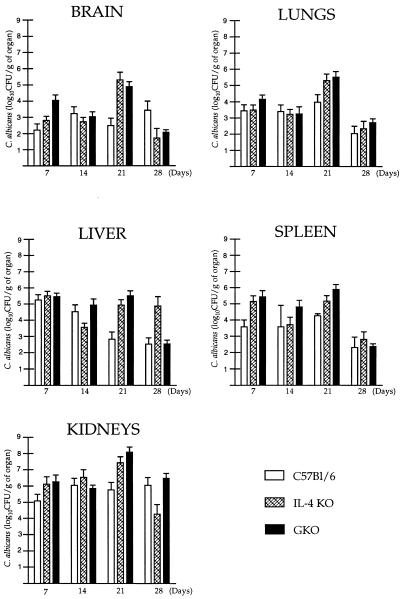
Semiquantitative organ cultures of the brain, lungs, spleen, liver, and kidneys were analyzed 7, 14, 21, and 28 days after i.p. C. albicans infection of mice (Fig. 2); data from at least five animals were pooled at each time. The highest numbers of CFU were found in the kidneys of both KO and control mice at each time point, except at day 28 in IL-4 KO mice. The lowest numbers of CFU were observed in the brain and lungs in each strain during the first 2 weeks of candidiasis. Comparing the different strains, the overall tissue fungal burden was moderately higher in most of the organs of GKO mice, but the differences were not significant at days 7, 14, and 28 (P < 0.05). At day 21, the numbers of CFU in each organ were significantly higher in both GKO and IL-4 KO mice than in the parental strain (P < 0.05). The results of two separate experiments showing significant differences in tissue fungal burden at day 21 were in close agreement; pooled data of these two sets of experiments are presented. No correlation, however, was found between the fungal burden of organs and the mortality of the different groups.
FIG. 2.
CFU of C. albicans from various organs of GKO, IL-4 KO, and C57BL/6 control mice after i.p. challenge with 108 Candida blastoconidia. Each bar represents the mean ± standard error. Experiments were performed in duplicate with five or more mice.
Histology.
Characteristics of the kidney, heart, liver, brain, spleen, and lungs of C57BL/6, GKO, and IL-4 KO mice were studied by immunohistology 3, 14, and 28 days after i.p. C. albicans injection (Table 1). The pathogen was detected in different tissues by Gomori’s silver methenamine staining.
TABLE 1.
Kinetics and cell contents of tissue inflammatory reactions in murine candidiasisa
| Candidiasis effect | Result in tissue at postinfection day:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kidney
|
Heart
|
Liver
|
Brain
|
|||||||||
| 3 | 14 | 28 | 3 | 14 | 28 | 3 | 14 | 28 | 3 | 14 | 28 | |
| Lesions | ++ | + | +/− | ++ | + | +/− | +/− | ++ | + | − | ++ | +/− |
| Macrophages | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | − | ++ | ++ |
| T cells | +/− | + | +/− | +/− | + | +/− | +/− | + | +/− | − | + | +/− |
| B cells | +/− | + | +/− | +/− | + | +/− | +/− | +/− | +/− | − | − | − |
| Neutrophils | +/− | − | − | +/− | − | − | + | +/− | − | − | − | − |
The extension or multiplicity of lesions and the ratio of different inflammatory cells were scored between − (none) and ++ (dominant). Note that Mφ were the dominant cell type at each time of infection, the ratio of lymphocytes was increased at 14 days, and small numbers of neutrophil granulocytes could only be observed at an early stage. Most of the T cells were CD4+, and Mφ became MHC II+ at a later stage of the infection.
In the kidney, extensive necrotic abscesses containing filamentous and yeast forms of the fungus were present in each strain from day 3. Evidence of local, capsular invasion of C. albicans could also be detected besides lymphohematogenous spread (Fig. 3). The vast majority of the inflammatory cells were FA/11+ Mφ and stained weakly with MAb 7/4; few of them were MHC II+. By day 14, lymphoid infiltrates with CD3+, CD4+, and CD8+ T cells and B220+ B cells appeared in the lesions beside FA/11+ giant Mφ, and the proportion of MHC II+ cells increased. Lesions were smaller, but still complex, localized both to the cortex and medulla at day 28, and contained fewer inflammatory cells; there were MHC II+ cells, mostly FA/11+ Mφ.
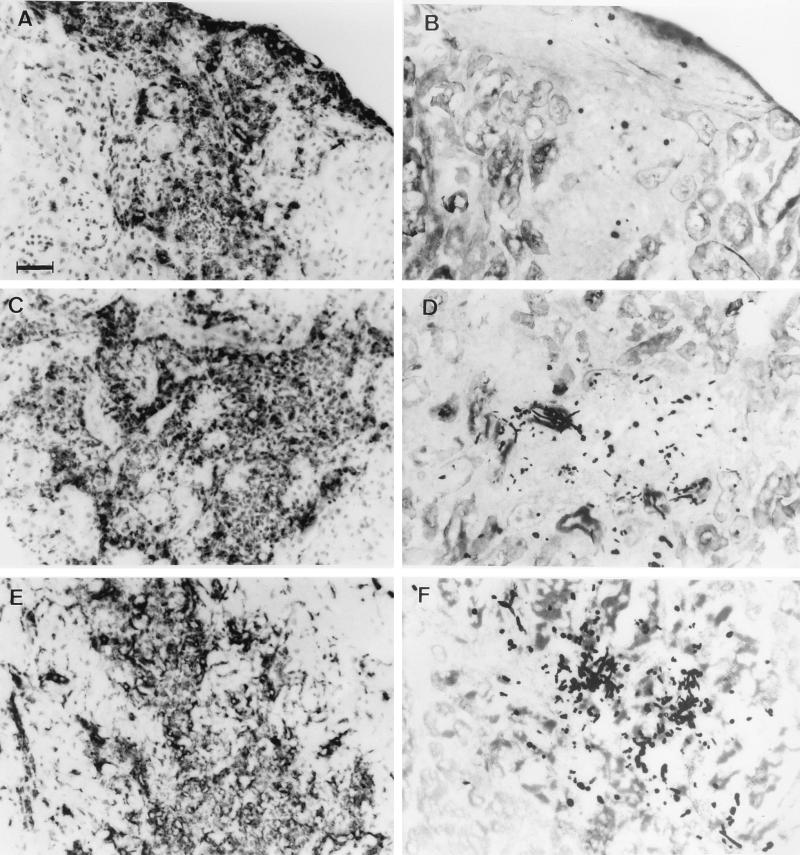
FIG. 3.
Histological appearance of lesions in the kidneys (A to D) and heart (E and F) at day 3 of murine invasive candidiasis. Inflammatory lesions in both the kidneys and heart consisted of mostly FA/11+ macrophages (A, C, and E). Yeast and mycelial forms of fungus were shown in the lesions by Gomori’s silver methenamine staining and were both extra- and intracellular (B, D, and F). The histopathologies of the kidneys and heart in GKO, IL-4 KO, and C57BL/6 control mice were similar. Representative photographs were taken of sections from IL-4 KO (A and B) and GKO mice (C to F). Bar, 50 μm.
In the myocardium, similar to the kidney, extensive necrotic abscesses were observed at day 3 with filamentous and yeast forms of fungus both extracellularly and inside Mφ (Fig. 3). Again the vast majority of inflammatory cells were FA/11+ Mφ and stained only weakly with 7/4 MAb, and few were MHC II+. At day 14, no focal lesions but diffuse mononuclear cell reactions were detected in the myocardium of each strain; the inflammatory cells were MHC II+, mostly FA/11+ Mφ, and there were fewer lymphoid cells. Diffuse mononuclear infiltrates were still observed at day 28.
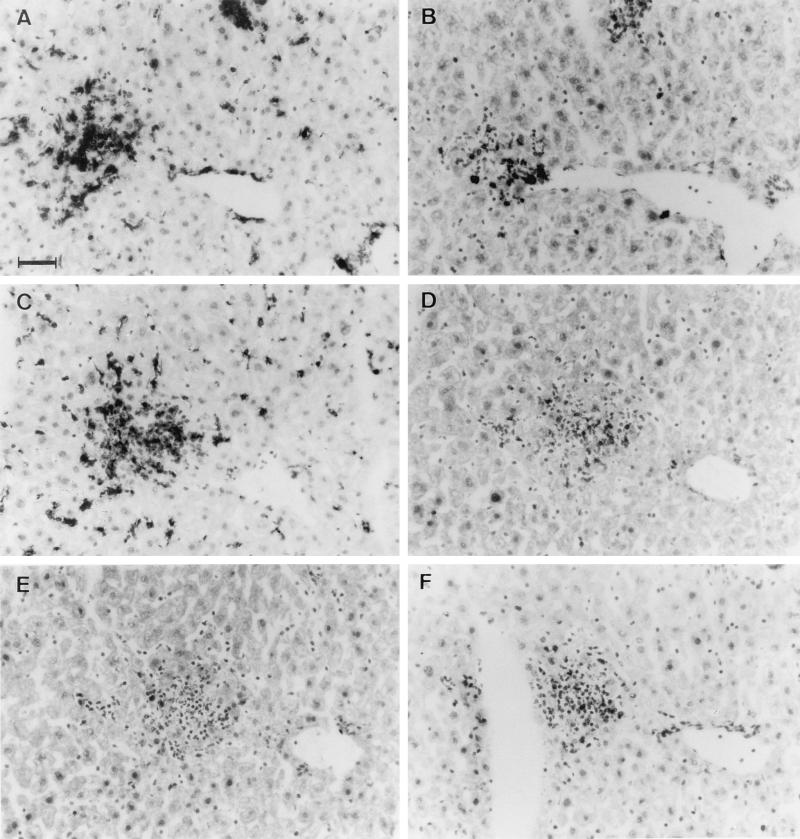
In the liver, no necrotic lesions could be observed at day 3. At day 14, multifocal inflammatory reactions appeared mostly perivascularly. The inflammatory cells were MHC II+ (Fig. 4A), the vast majority of which were FA/11+ Mφ (Fig. 4C) and were weakly stained with MAb 7/4+. There were fewer T cells—mostly CD4+ (Fig. 4D) and fewer CD8+ (4F)—and very few B220+ B cells (Fig. 4E), and debris of C. albicans was associated with the inflammatory lesions. At day 28 in the survivors, multifocal hepatitis could still be observed, with mononuclear infiltrates.
FIG. 4.
Histopathology of the liver in invasive candidiasis at day 14. Perivascular infiltrates contained MHC II+ cells (A) and included CD3+ (B), CD4+ (D), and CD8+ (F) T cells and FA/11+ Mφ (C); only a few B220+ B cells (E) were associated with the lesions. The histopathologies of the liver in GKO, IL-4 KO, and C57BL/6 control mice were similar; representative photographs of liver in C57BL/6 mice are shown. Bar, 50 μm.
In the brain (not shown), multifocal inflammatory reactions could be observed from day 14, which were associated with the blood vessels, and the cerebrospinal fluid-brain barrier. The mononuclear cells were MHC II+, mostly FA/11+ microglia or Mφ, and there were fewer T cells, mostly CD4+ cells. Debris of fungus could also be seen inside the blood vessels. At day 28 in the survivors, fewer lesions were present with MHC II+, FA/11+ microglia or Mφ.
In the spleen (data not shown), there were no necrotic lesions resembling those in the kidneys and heart at any time studied; the structure and cell content of white and red pulp were similar in infected KO and control mice and showed diffuse lymphoid hyperplasia with no disorganization of organ microarchitecture.
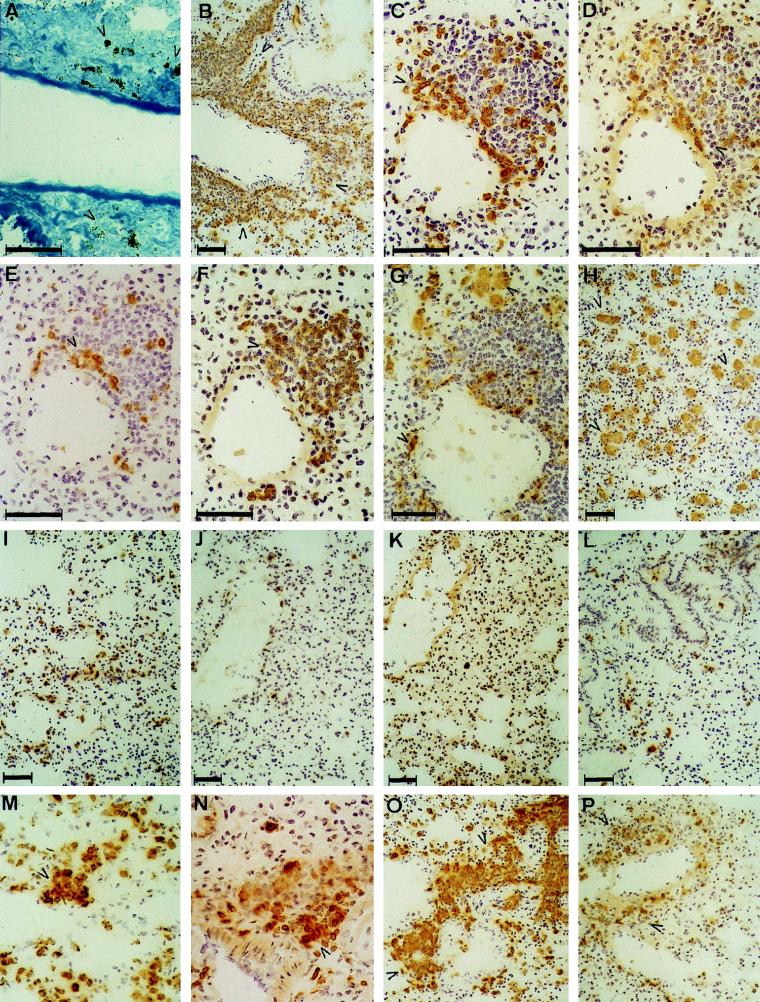
The histopathologies of the kidneys, heart, liver, brain, and spleen were not significantly different in the control and immunocompromised mice at any time. However, the lungs of GKO mice presented a striking, distinct phenotype by day 3 of candidiasis, having extensive perivascular lymphoid infiltrates with B220+ and CD19+ B cells (arrowheads) (Fig. 5F) and CD3+ (Fig. 5C), CD4+ (Fig. 5D), and CD8+ (Fig. 5D) T cells. Mφ (FA/11+) (arrowheads in Fig. 5G and H) appeared in two distinct phenotypes: disseminated, large alveolar Mφ and elongated cells, peripherally associated with the perivascular lymphoid infiltrates and beneath the endothelium. Neutrophil granulocytes (7/4+) could not be observed among the inflammatory cells in the infiltrates. By day 14, the extension of the infiltrates was reduced, became more focal, and contained MHC II+ cells (arrowheads) (Fig. 5O): FA/11+ Mφ (not shown) and mainly CD3+ (Fig. 5P), CD4+ T cells, and fewer B220+ B cells (not shown). The size of alveolar Mφ decreased by that time. Diffuse pneumonia, but no perivascular infiltrates, could be seen in the survivors at day 28. Extensive necrotic lesions with filamentous or yeast forms of C. albicans, similar to those in the kidneys and heart, could not be observed in the lungs of GKO mice by Gomori’s staining at any time, which revealed only debris of fungal origin (Fig. 5A). In marked contrast to the results presented above, only diffuse mononuclear infiltrates could be observed from day 14 in the lungs of C57BL/6 and IL-4 KO mice (Fig. 5I to L), without the perivascular infiltrates characteristic of lesions in GKO mice. In order to determine the onset of perivascular infiltrate formation, GKO and control mice were also examined at 1 and 2 days after i.p. challenge. At day 1, the histopathologies of lungs in GKO and control mice were similar: Mφ and CD3+ CD4+ T cells appeared perivascularly and formed more prominent aggregates by day 2 in GKO mice (Fig. 5M and N).
FIG. 5.
Histopathology of the lungs in invasive murine candidiasis at day 3 in GKO (A to H) and C57BL/6 mice (I to L). Inflammatory lesions in GKO mice at day 2 (M and N) and day 14 (O and P) are also shown. Perivascular infiltrates appeared only in GKO mice and contained MHC II+ (TIB120+) mononuclear cells (B), CD3+ T cells (C), mostly CD4+ (D) and fewer CD8+ (E) cells and B220+ B cells (F), which were also CD19+ (not shown). Mφ FA/11+ (G) were not a dominant component of the infiltrates, but were present in two different phenotypes in the lungs: elongated, perivascular cells and engorged, diffusely distributed alveolar Mφ (H). Perivascular infiltrates contained only debris of C. albicans, as shown by Gomori’s staining (A). Histopathology of the lungs in IL-4 KO and C57BL/6 mice was markedly different from that of GKO mice; a similar inflammatory reaction did not develop in these strains even at later stages of infection. Lung sections obtained at day 3 from C57BL/6 mice were stained with TIB120 (I), FA/11 (J), B220 (K), and anti-CD3 (L) MAbs and showed only minimal inflammatory responses. At day 2, perivascular MHC II+ cell aggregates (M) appeared in GKO mice, most of which were CD3+ T cells (N), and by day 14, the extension of perivascular infiltrates was reduced compared with that of day 3, containing MHC II+ cells (O), which were partly CD3+ T cells (P). Bars, 50 μm.
Nitrite production by peritoneal Mφ.
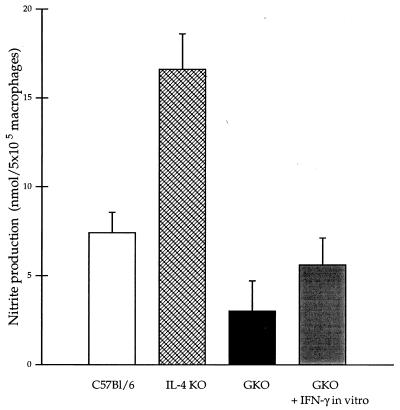
The nitrite concentrations in the culture supernatant of peritoneal Mφ from noninfected mice were comparably low in each strain. Increased nitrite production was detected by Mφ isolated from infected animals after in vivo challenge. The increase was significantly lower in GKO mice (16.1-fold) and higher in IL-4 KO mice (55.3-fold) than in the C57BL/6 control mice (24.6-fold) compared to the baseline (Fig. 6). Impaired NO production by Mφ from GKO mice could be restored in vitro by addition of IFN-γ (100 U/ml) to the culture suspension (Fig. 6).
FIG. 6.
Nitrite concentration in 48-h culture supernatants of peritoneal Mφ isolated from C57BL/6, IL-4 KO and GKO infected mice at day 5 of infection. The nitrite production of peritoneal macrophages from IL-4 KO mice was significantly higher than that of the control. The impaired function of Mφ from GKO animals could be restored by ex vivo IFN-γ treatment for 48 h (100 U/ml). The height of each bar represents the mean ± standard error of three experiments performed in duplicate.
C. albicans-specific serum antibody responses.
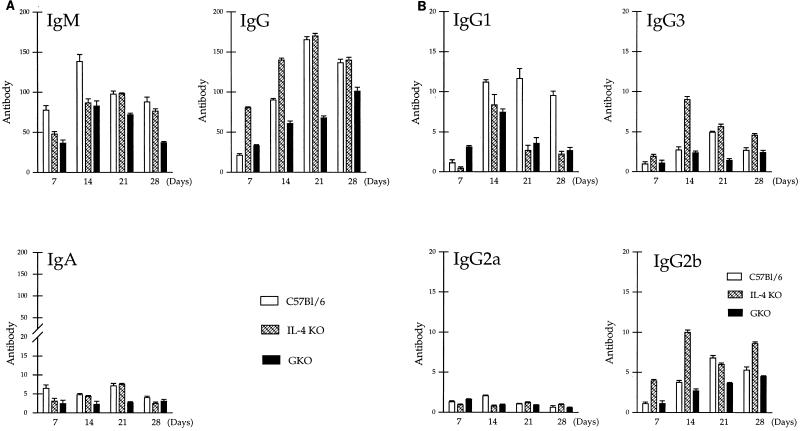
In control C57BL/6 mice, an increase in Candida-specific IgM titer was observed from day 7, with a peak at day 14 after the challenge; the specific IgG titer reached its maximum 1 week later at day 21 (Fig. 7A), whereas among different IgG subclasses, the IgG1 response was most prominent in C57BL/6 mice, followed by the IgG2b and IgG3 responses, and only a moderate increase could be detected in the level of IgG2a in serum during candidiasis (Fig. 7B [IgG1>IgG2b≥IgG3>IgG2a]).
FIG. 7.
(A) C. albicans-specific IgM, IgG, and IgA titers in sera of mice infected i.p. with 108 Candida blastoconidia. Pooled sera of at least four mice/group were analyzed for specific antibodies by standard ELISA. The height of each bar represents the mean ± standard error of three experiments performed in duplicate. (B) C. albicans-specific IgG1, IgG3, IgG2a, and IgG2b titers in sera of mice infected i.p. with 108 Candida blastoconidia. Pooled serum samples of at least four mice per group were analyzed for specific antibodies by standard ELISA. The height of each bar represents the mean ± standard error of three experiments performed in duplicate.
In GKO mice, the increase in Candida-specific IgM, IgG, and IgG subclass levels was lower than that measured in control mice from day 14 (Fig. 7A and B). The patterns of the IgM, IgG, and IgG subclass responses were similar to those of the control group (IgG1>IgG2b≥IgG3>IgG2a). The level of specific IgA in serum in GKO mice was low and remained unchanged during the infection.
The Candida-specific titers of IgM, IgG, and IgA in the sera of IL-4 KO mice did not differ significantly from those of controls (P < 0.05). Titers of specific IgG1 were significantly lower, whereas production of IgG3 and IgG2b was significantly higher in IL-4 KO mice than in controls (P < 0.05) (Fig. 7B [IgG2b≥IgG3>IgG1>IgG2a]).
DISCUSSION
This study is the first to report disseminated candidiasis in GKO and IL-4 KO mice and to analyze the kinetics and cell content of the different tissue inflammatory reactions. In situ analysis establishes that Mφ play a key role in the host response to Candida. Our results indicate that IFN-γ, but not IL-4, is essential for survival in invasive candidiasis. In contrast to previous reports, which suggested that neutralization of IL-4 or IL-4 receptor was associated with an increased resistance to C. albicans (26, 30), in our model, the susceptibility of IL-4 KO mice was similar to that of control mice. This difference could be explained by unrelated genetic factors, which influence the immune response and susceptibility of other inbred strains to the growing pathogen. Previous studies reported that C57BL/6 mice are relatively resistant to C. albicans infection, with a predominance of Th1-type anticandidal response, while BALB/c and especially DBA/2 strains are more susceptible to this fungal pathogen and produce Th2 cytokines in higher titer (30, 31, 36); we hypothesize that anti-IL-4 treatment can improve the resistance only of certain strains, which exhibit a predominantly Th2-type immune response to the pathogen, skewing the immune response towards a Th1 type.
In our model, higher mortality of GKO mice could be observed only from day 14 of candidiasis, although the infection became disseminated within 24 h of the i.p. C. albicans challenge. Pyelonephritis and myocarditis developed at an early stage (day 3), while hepatitis and meningoencephalitis could be observed only later in the infection (day 14) in each strain. The tissue fungal burden was marginally higher in GKO mice, and it peaked at day 21 in each strain associating with increased mortality in GKO mice alone, which suggests that Candida overgrowth was not the only factor responsible for higher mortality. The increased mortality of GKO mice after 14 days of candidiasis could be explained by a combination of different factors, including an altered inflammatory reaction and immune responses to C. albicans.
Immunohistology indicated that Mφ were the dominant cell type in the inflammatory reaction of several organs, both in KO and control mice. The kinetics and phenotype of tissue inflammatory lesions were organ rather than mouse strain specific, except in the lungs. Lesions appeared first in the kidney and heart and were more extensive and necrotic. Kidneys are often reported to be one of the main targets in different candidiasis models, although their phagocytic system can eliminate C. albicans as effectively as those of the spleen and liver (2). The proteoglycan- and glycoprotein-rich basement membrane of glomeruli contains ligands for the CR2/CR3 receptor on the Candida surface, and the acidic condition in renal tubules may favor fungal growth (6). The rapid development of necrotic lesions in the heart might be explained by limited local innate immune defenses. The tissue inflammatory reactions in both the liver and brain were most prominent at later stages of infection (day 14), but were less extensive and necrotic, which could be explained by the efficiency of the phagocytic system in the liver and by anatomical segregation in the brain. The dominance of Mφ rather than neutrophils in the tissue infiltrates from the early stages of infection and in immunocompetent as well as KO mice differs from our unpublished observations with M. bovis BCG infection, in which Mφ are poorly recruited to lesions in GKO mice, which also contain abundant neutrophils. The appearance of lymphoid, especially CD4+, T cells at later stages (day 14) was similar in most organs of immunocompetent and KO mice, except for the lungs, in which the host cellular response in the absence of IFN-γ was strikingly different in invasive candidiasis.
In the lungs, activated Mφ (but few neutrophil granulocytes) and CD4+ T cells appeared initially (day 1) at perivascular sites in all strains, but lymphoid infiltrates developed only in GKO mice (day 3). These infiltrates were dominated by B cells and were associated peripherally with FA/11+ elongated, dendritic cells. The phenotype of alveolar Mφ was also distinctive and unusual in GKO mice; from an early stage of candidiasis, they were engorged and scattered throughout the lungs. In the present study, the FA/11 MAb could detect not only Mφ, but also dendritic cells, which are constitutively MHC II+, as well as Mφ that had been induced to express MHC II, and we have not distinguished between the different potential antigen-presenting cells.
The mechanism of the development of perivascular infiltrates in the lungs of GKO mice could be due to relative excess of Th2-type cytokines. Our model illustrates the role of IFN-γ in regulating the local inflammatory response, and we suggest that it may act by (i) influencing the dose and persistence of antigens, (ii) down-regulating directly or indirectly the local production of certain chemokines and cytokines, and/or (iii) terminating the proliferation or survival of activated lymphoid cells. Formation of extensive lymphoid infiltrates might be the consequence of persistent recruitment, in situ proliferation, and impaired apoptosis of lymphoid cells. The role of the different Mφ populations in this process is also undefined: the dendritic cells at the periphery of the infiltrates might provide an important inductive function, but these require further investigations.
In our model, C. albicans was disseminated to the lungs via the bloodstream, adhered to the endothelial cells, and penetrated through them. Germinated C. albicans has been reported to stimulate endothelial cells to produce proinflammatory cytokines in vitro, such as IL-6, IL-8, and monocyte chemoattractant protein (MCP-1), and to induce adhesion molecules (ICAM-1 and VCAM-1) (12), resulting in the recruitment of phagocytes and initiation of a local inflammatory reaction. Mφ and, possibly, dendritic cells are able to ingest Candida even in the absence of opsonins (21, 29) and then produce proinflammatory cytokines, such as IL-1β, IL-6, Mφ inflammatory proteins (MIP-1β and MIP-2), and granulocyte-Mφ colony-stimulating factor (35, 41). In transgenic mice, which overexpress IL-6 in airway epithelial cells, the phenotype of peribronchial inflammatory reactions resembles that we have observed in GKO mice (10), indicating that overproduction of IL-6 might contribute to the development of the B-cell-rich lymphoid infiltrates. IL-12 produced by Mφ, as well as dendritic cells, acts both as a proinflammatory cytokine and an immunomodulator, priming naive T cells for high IFN-γ production and therefore bridging the innate and adaptive immune responses (38). The importance of innate immune mechanisms (phagocytes and/or NK cells) in host defense to C. albicans (3, 20) has also been demonstrated in candidiasis models with SCID mice.
NO production by peritoneal Mφ, collected after i.p. injection with C. albicans, reflected the function and activation of Mφ in the different strains. In GKO mice, NO production was impaired, but could be restored by in vitro IFN-γ treatment, excluding an intrinsic deficiency of Mφ, while in IL-4 KO mice, the increased NO production can be explained by the absence of inhibition by IL-4 and/or an increased stimulatory effect by IFN-γ. The decreased NO production by GKO peritoneal Mφ correlated with enhanced susceptibility to C. albicans, but enhanced NO in IL-4 KO Mφ did not increase in vivo resistance to infection, compared with that in the wild-type mice.
In our disseminated candidiasis model, the levels of MHC II expression on Mφ were similar in GKO and other strains, in contrast to a previous study, which established that IFN-γ plays a crucial role in MHC II expression on activated Mφ in murine M. bovis BCG infection (8). Our finding indicates the existence of an IFN-γ-independent mechanism which regulates MHC II expression, possibly via IL-4 and IL-13 (11).
The pattern of Candida-specific Ig subclass production was altered in IL-4 KO mice. The specific IgG1 response was reduced, and IgG2b and IgG3 were increased, which can be explained by the absence of a stimulatory effect of IL-4 on expression of IgG1 and inhibition of IgG2a and IgG3 production (18, 24, 34). IFN-γ also influences isotype switching; it can inhibit the expression of IgG1, IgG2b, and IgG3 and augment the production of IgG2 (9, 34). However, in our model, the production of Candida-specific IgG1, IgG2b, and IgG3 was not upregulated in GKO mice compared with that in the control strain.
In conclusion, the findings reported here illustrate the in vivo importance of IFN-γ for survival in disseminated candidiasis and indicate the role of Mφ in the tissue inflammatory reactions in both immunocompetent and immunodeficient animals. We have also demonstrated localized immune regulatory disturbances in organs such as lungs, which might contribute to higher susceptibility to this increasingly important pathogen.
ACKNOWLEDGMENTS
This work was supported by grants from the Medical Research Council to S. Gordon (06BI) and the National Science Foundation of Hungary (OTKA T 017 100) and European Commission (PECO no. CIPD CT 9400303) to L. Maródi. The first author is a participant in the UK-Hungary Health Scientist Exchange Program jointly funded by the British Council, Hungarian Ministry of Education, and Soros Foundation.
We thank Rosangela da Silva (Sir William Dunn School of Pathology, Oxford) for helpful discussion and Elizabeth Darley, Lance Tomlinson, Stephen Clark, and Jeremy Sanderson (Sir William Dunn School of Pathology, Oxford) for technical assistance.
REFERENCES
- 1.Akhiani A A, Lycke N, Nilsson L-Å, Olling S, Ouchterlony Ö. Lack of interferon-γ receptor does not influence the outcome of infection in murine Schistosomiasis mansoni. Scand J Immunol. 1996;43:257–262. doi: 10.1046/j.1365-3083.1996.d01-33.x. [DOI] [PubMed] [Google Scholar]
- 2.Baghian A, Lee K W. Elimination of Candida albicans from kidneys of mice during short-term systemic infections. Kidney Int. 1991;40:400–405. doi: 10.1038/ki.1991.225. [DOI] [PubMed] [Google Scholar]
- 3.Balish E, Jensen J, Warner T, Brekke J, Leonard B. Mucosal and disseminated candidiasis in gnotobiotic SCID mice. J Med Vet Mycol. 1993;31:143–154. doi: 10.1080/02681219380000161. [DOI] [PubMed] [Google Scholar]
- 4.Beck-Sagué C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 5.Blasi E, Pitzurra L, Puliti M, Chimienti A R, Mazzolla R, Barluzzi R, Bistoni F. Differential susceptibility of yeast and hyphal forms of Candida albicans to macrophage-derived nitrogen-containing compounds. Infect Immun. 1995;63:1806–1809. doi: 10.1128/iai.63.5.1806-1809.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calderone, R., R. Diamond, J.-M. Seneet, J. Warmington, S. Filler, and J. E. Edwards. 1994. Host cell-fungal cell interactions. J. Med. Vet. Mycol. 32(Suppl. 1):151–168. [DOI] [PubMed]
- 7.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russel D G, Orme I M. Disseminated tuberculosis in interferon γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 9.Damore M A, Omori S A, Wall R. IFN-γ induces the κ intron enhancer via an IFN-stimulated response element. J Immunol. 1996;156:2451–2457. [PubMed] [Google Scholar]
- 10.DiCosmo B F, Geba G P, Picarella D, Elias J A, Rankin J A, Stripp B R, Whitsett J A, Flavell R A. Airway epithelial cell expression of interleukin-6 in transgenic mice. Uncoupling of airway inflammation and bronchial hyperreactivity. J Clin Invest. 1994;94:2028–2035. doi: 10.1172/JCI117556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle A G, Herbein G, Montaner L J, Minty A J, Caput D, Ferrara P, Gordon S. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-γ. Eur J Immunol. 1994;24:1441–1445. doi: 10.1002/eji.1830240630. [DOI] [PubMed] [Google Scholar]
- 12.Filler S G, Pfunder A S, Spellberg B J, Spellberg J P, Edwards J E., Jr Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun. 1996;64:2609–2617. doi: 10.1128/iai.64.7.2609-2617.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garner R E, Kuruganti U, Czarniecki C W, Chiu H H, Domer J E. In vivo immune responses to Candida albicans modified by treatment with recombinant murine gamma interferon. Infect Immun. 1989;57:1800–1808. doi: 10.1128/iai.57.6.1800-1808.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite and [15N]nitrite in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch S, Gordon S. Polymorphic expression of a neutrophil differentiation antigen revealed by monoclonal antibody 7/4. Immunogenetics. 1983;18:229–239. doi: 10.1007/BF00952962. [DOI] [PubMed] [Google Scholar]
- 16.Kopf M, Le Gros G, Bachmann M, Lamers M C, Bluethmann H, Kohler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–247. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 17.Kopf M, Brombacher F, Köhler G, Kienzle G, Widmann K-H, Lefrang K, Ledermann B, Solbach W. IL-4-deficient Balb/c mice resist infection with Leishmania major. J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kühn R, Rajewsky K, Müller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 19.Kulberg B J, van’t Wout J W, Hoogstraten C, van Furth R. Recombinant interferon-γ enhances resistance to acute disseminated Candida albicans infection in mice. J Infect Dis. 1993;168:436–443. doi: 10.1093/infdis/168.2.436. [DOI] [PubMed] [Google Scholar]
- 20.Mahanty S, Greenfield R A, Joyce W A, Kincade P W. Inoculation candidiasis in a murine model of severe combined immunodeficiency syndrome. Infect Immun. 1988;56:3162–3166. doi: 10.1128/iai.56.12.3162-3166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maródi L, Korchak H M, Johnston R B., Jr Mechanism of host defense against Candida species. I. Phagocytosis by monocytes and monocyte-derived macrophages. J Immunol. 1991;146:2783–2789. [PubMed] [Google Scholar]
- 22.Maródi L, Forehand J R, Johnston R B., Jr Mechanisms of host defense against Candida species. II. Biochemical basis for the killing of Candida by mononuclear phagocytes. J Immunol. 1991;146:2790–2794. [PubMed] [Google Scholar]
- 23.Maródi L, Schreiber S, Anderson D C, Macdermott R P, Korchak H M, Johnston R B., Jr Enhancement of macrophage candidacidal activity by interferon-γ. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J Clin Invest. 1993;91:2596–2601. doi: 10.1172/JCI116498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mongini P K A, Highet P F, Inman J K. Human B cell activation. Effect of T cell cytokines on the physicochemical binding requirements for achieving cell cycle progression via the membrane IgM signaling pathway. J Immunol. 1995;155:3385–3400. [PubMed] [Google Scholar]
- 25.Perfect J R, Granger D L, Durack D T. Effects of antifungal agents and γ-interferon on macrophage cytotoxicity for fungi and tumor cells. J Infect Dis. 1987;156:316–323. doi: 10.1093/infdis/156.2.316. [DOI] [PubMed] [Google Scholar]
- 26.Pucetti P, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Enssle K-H, Romani L, Bistoni F. Cure of murine candidiasis by recombinant soluble interleukin-4 receptor. J Infect Dis. 1994;169:1325–1331. doi: 10.1093/infdis/169.6.1325. [DOI] [PubMed] [Google Scholar]
- 27.Rabinowitz S S, Gordon S. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J Exp Med. 1991;174:827–836. doi: 10.1084/jem.174.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Redmond H P, Shou J, Gallagher H J, Kelly C J, Daly J M. Macrophage-dependent candidacidal mechanisms in the murine system. Comparison of murine Kupffer cell and peritoneal macrophage candidacidal mechanisms. J Immunol. 1993;150:3427–3433. [PubMed] [Google Scholar]
- 29.Reis-E-Sousa C, Stahl P D, Austyn J M. Phagocytosis of antigens by Langerhans cells in vitro. J Exp Med. 1993;178:509–519. doi: 10.1084/jem.178.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romani L, Mencacci A, Grohmann U, Mocci S, Mosci P, Pucetti P, Bistoni F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J Exp Med. 1992;176:19–25. doi: 10.1084/jem.176.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romani L, Mencacci A, Cenci E, Spaccapelo R, Schiaffella E, Tonetti L, Puccetti P, Bistoni F. Natural killer cells do not play a dominant role in CD4+ subset differentiation in Candida albicans-infected mice. Infect Immun. 1993;61:3769–3774. doi: 10.1128/iai.61.9.3769-3774.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romani L, Mencacci A, Tonetti L, Spaccapelo R, Cenci E, Pucetti P, Wolf S F, Bistoni F. IL-12 is both required and prognostic in vivo for T helper type differentiation in murine candidiasis. J Immunol. 1994;152:5167–5175. [PubMed] [Google Scholar]
- 33.Smith M J, Koch G L E. Differential expression of murine macrophage surface glycoprotein antigens in intracellular membranes. J Cell Sci. 1987;87:113–119. doi: 10.1242/jcs.87.1.113. [DOI] [PubMed] [Google Scholar]
- 34.Snapper C M, Paul W E. Interferon-γ and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 35.Taub D D, Conlon K, Lloyd A R, Oppenheim J J, Klein D J. Preferential migration of activated CD4+ and CD8+ cells in response to MIP-1α and MIP-1β. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 36.Tavares D, Ferreira P, Vilanova M, Videira A, Arala-Chaves M. Immunoprotection against systemic candidiasis in mice. Int Immunol. 1995;7:785–796. doi: 10.1093/intimm/7.5.785. [DOI] [PubMed] [Google Scholar]
- 37.Tonetti L, Spaccapello R, Cenci E, Mencacci A, Pucetti P, Coffmann R L, Bistoni F, Romani L. Interleukin-4 and -10 exacerbate candidiasis in mice. Eur J Immunol. 1995;25:1559–1565. doi: 10.1002/eji.1830250614. [DOI] [PubMed] [Google Scholar]
- 37a.Tree, P., and S. Gordon. Unpublished observations.
- 38.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukocyte Biol. 1996;59:505–511. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Friedman H, Kjeu J Y. Enhancement of human monocyte function against Candida albicans by the colony-stimulating factors (CSF): IL-3, granulocyte-macrophage-CSF, and macrophage-CSF. J Immunol. 1989;143:671–677. [PubMed] [Google Scholar]
- 40.Wenzel R P. Nosocomial candidemia: risk factors and attributable mortality. Clin Infect Dis. 1995;20:1531–1534. doi: 10.1093/clinids/20.6.1531. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto Y, Klein T W, Friedman H. Involvement of mannose receptor in cytokine interleukin-1β (IL-1β), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1β (MIP-β), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages. Infect Immun. 1997;65:1077–1082. doi: 10.1128/iai.65.3.1077-1082.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]