Abstract
Sodium–glucose cotransporter 2 (SGLT2) inhibitors reduce major adverse cardiovascular events (MACE) in type 2 diabetic (T2DM) patients. Pharmacological selectivity of these agents to SGLT2 over SGLT1 is highly variant, with unknown clinical relevance. Genetically reduced SGLT1—but not SGLT2—activity correlates with lower risk of heart failure and mortality, therefore additional non-selective SGLT1 inhibition might be beneficial. In this prespecified meta-analysis, we included 6 randomized, placebo-controlled cardiovascular outcome trials of SGLT2 inhibitors assessing MACE in 57,553 patients with T2DM. Mixed-effects meta-regression revealed that pharmacological selectivity of SGLT2 inhibitors (either as continuous or dichotomized variable) had no significant impact on most outcomes. However, lower SGLT2 selectivity correlated with significantly lower risk of stroke (pseudo-R2 = 78%; p = 0.011). Indeed, dual SGLT1/2 inhibitors significantly reduced the risk of stroke (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.64–0.94), unlike selective agents (p for interaction = 0.018). The risk of diabetic ketoacidosis and genital infections was higher in both pharmacological groups versus placebo. However, hypotension occurred more often with non-selective SGLT2 inhibitors (odds ratio [OR], 1.87; 95% CI, 1.20–2.92) compared with selective agents (p for interaction = 0.044). In conclusion, dual SGLT1/2 inhibition reduces stroke in high-risk T2DM patients but has limited additional effect on other clinical outcomes.
Subject terms: Cardiology, Endocrinology, Diabetes
Introduction
Sodium–glucose cotransporter 2 (SGLT2) inhibitors were originally designed to aid glucose control in patients with diabetes mellitus by blocking SGLT2 in the proximal convoluted tubule of the kidneys, resulting in glucosuria. However, their antidiabetic effect turned out to be modest1, rather, these medications consistently reduced the relative risk of hospitalization for heart failure (HF) in these type 2 diabetic patients across all cardiovascular outcome trials, but some clinical endpoints were heterogeneous2–7. Later it became clear that SGLT2 inhibitors exert salutary cardiovascular effects independently of the presence of diabetes, nonetheless, the mechanism of action is currently incompletely understood8–10.
Of note, SGLT2 inhibitors show substantial variance in pharmacological selectivity to SGLT2 over SGLT11,11,12. Whereas sotagliflozin is considered to be a dual SGLT1/2 inhibitor, canagliflozin also shows clinically relevant renal, gastrointestinal, and myocardial SGLT1 inhibitory effect at therapeutic plasma concentrations13–19. On the other hand, the SGLT2 inhibitors empagliflozin, dapagliflozin, and ertugliflozin are highly selective to SGLT2. These pharmacodynamic differences might be clinically relevant given the fact that SGLT1 is responsible for the majority of glucose absorption from the gastrointestinal system and contributes to postprandial glucose excursions20,21, which phenomenon is well-known risk factor for adverse cardiovascular events22. Furthermore, humans with alterations in the gene encoding SGLT1 resulting in production of functionally limited transporter (resembling pharmacological SGLT1 inhibition) are protected from the development of HF, and all-cause death is significantly lower as compared with non-affected subjects23. On the contrary, those with functionally limited SGLT2 (resembling pharmacological SGLT2 inhibition) derive no meaningful benefit pertinent to these outcomes24. Additionally, preclinical studies have linked SGLT1 to the pathophysiology of HF25–28, as well as myocardial29, cerebral30,31, and renal ischemic injury32. Therefore, additional pharmacological inhibition of SGLT1 might have significant clinical implications on top of SGLT2 blockade in high-risk type 2 diabetic patients, which have not been established yet.
In this meta-analysis, our goal was to quantify the contribution of pharmacological SGLT2 selectivity to clinical efficacy and safety outcomes in type 2 diabetic patients treated with SGLT2 inhibitors, according to large-scale cardiovascular outcomes trials.
Materials and methods
Protocol and registration
The present meta-analysis was conducted and reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline33. The protocol was prespecified and published on PROSPERO (registration no.: CRD42021273914).
Eligibility criteria
Population: We selected randomized, placebo-controlled cardiovascular outcome trials assessing major adverse cardiovascular events (MACE: composite of cardiovascular death, nonfatal myocardial infarction [MI], and nonfatal stroke) with SGLT2 inhibitors in adult patients with type 2 diabetes mellitus. Trials enrolling patients without diabetes were excluded. We considered peer-reviewed, English-language publications without date restriction.
Intervention: The intervention constituted treatment with an SGLT2 inhibitor (empagliflozin, canagliflozin, dapagliflozin, ertugliflozin, or sotagliflozin).
Comparator: Placebo was considered as the comparator in all cases.
Outcomes: The main outcomes included MACE (composite of cardiovascular death, nonfatal MI, and nonfatal stroke); cardiovascular death; fatal and nonfatal stroke; fatal and nonfatal MI; hospitalization for HF; all-cause mortality; and renal composite endpoint. All trials reported total fatal and nonfatal stroke (regardless of subtype), except for the DECLARE-TIMI 58 trial, which reported ischemic stroke events only (included also in the composite of MACE in that trial)4. The primary and secondary outcomes, and definition of the renal composite endpoint are contained within Table 1.
Table 1.
Participants and characteristics of included clinical trials.
| EMPA-REG OUTCOME 20152 | CANVAS Program 20173 | DECLARE-TIMI 58 20184 | CREDENCE 20195 | VERTIS CV 20206 | SCORED 20207 | |
|---|---|---|---|---|---|---|
| Study design | Interventional, randomized, parallel-assigned, double blind, placebo-controlled (placebo vs. 10 mg or 25 mg empagliflozin) | Interventional, randomized, parallel-assigned, quadruple blind, placebo-controlled (placebo vs. 100 mg or 300 mg canagliflozin) | Interventional, randomized, parallel-assigned, double blind, placebo-controlled (placebo vs. 10 mg dapagliflozin) | Interventional, randomized, parallel-assigned, double blind, placebo-controlled (placebo vs. 100 mg canagliflozin) | Interventional, randomized, parallel-assigned, double blind, placebo-controlled (placebo vs. 5 mg or 15 mg ertugliflozin) | Interventional, randomized, parallel-assigned, quadruple blind, placebo-controlled (placebo vs. 200 mg or 400 mg sotagliflozin) |
| Pharmacological selectivity of tested medication (SGLT2:SGLT1), ratio | Empagliflozin: ~ 2700 | Canagliflozin: ~ 260 | Dapagliflozin: ~ 1200 | Canagliflozin: ~ 260 | Ertugliflozin: ~ 2200 | Sotagliflozin: ~ 20 |
| No. of participants | 7020 | 10,142 | 17,160 | 4401 | 8246 | 10,584 |
| Median follow-up, years | 3.1 | 2.4 | 4.2 | 2.6 | 3.0 | 1.3 |
| Age—mean ± SD, years | 63 ± 9 | 63 ± 8 | 64 ± 7 | 63 ± 9 | 64 ± 8 | 69 ± 8 |
| Male sex—no. (%) | 5016 (71.5%) | 6509 (64.2%) | 10,738 (62.6%) | 2907 (66.1%) | 5769 (70.0%) | 5830 (55%) |
| Race: White—no. (%) | 5081 (72.4%) | 7944 (78.3%) | 13,653 (79.6%) | 2931 (66.6%) | 7240 (87.8%) | 8749 (82.7%) |
| Baseline HbA1C—mean ± SD, % | 8.1 ± 0.8 | 8.2 ± 0.9 | 8.3 ± 1.2 | 8.3 ± 1.3 | 8.2 ± 1.0 | 8.4 ± 1.3 |
| Baseline eGFR—mean ± SD, mL/min/1.73 m2 | 74 ± 22 | 77 ± 21 | 85 ± 16 | 56 ± 18 | 76 ± 21 | 44 ± 11 |
| Key inclusion criteria | Type 2 diabetes mellitus; > = 18 y–o; HbA1C = 7.0‒10.0% if on background therapy; HbA1C = 7.0‒9.0% if no background therapy; high CV risk | Type 2 diabetes mellitus; > = 30 y-o; HbA1C = 7.0‒10.5%; high CV risk | Type 2 diabetes mellitus; > = 40 y-o; HbA1C 6.5‒12.0%; high CV risk | Type 2 diabetes mellitus; > = 30 y-o; HbA1C = 6.5‒12.0%; eGFR = 30‒90 mL/min/1.73 m2 AND albuminuria; on ACEI or ARB | Type 2 diabetes mellitus; > = 40 y-o; HbA1C = 7.0‒10.5%; high CV risk | Type 2 diabetes mellitus; > = 18 y-o; HbA1C > = 7%; eGFR = 25‒60 mL/min/1.73 m2; high CV risk |
| Key exclusion criteria | eGFR < 30 mL/min/1.73 m2 | eGFR < 30 mL/min/1.73 m2 | CrCl < 60 mL/min | eGFR < 30 mL/min/1.73 m2 | eGFR < 30 mL/min/1.73 m2 | eGFR < 25 mL/min/1.73 m2 |
| Primary outcomes | 3-P MACE (excluding silent MI) | 3-P MACE | Co-primary: 3-P MACE (with nonfatal ischemic stroke); CVD AND HHF | Renal composite #1 (ESRD AND doubling of serum creatinine level AND renal death AND CVD) | 3P-MACE | CVD AND HHF AND urgent visits for HF (original co-primary: 3P-MACE; CVD AND HHF) |
| Key secondary outcomes | 4-P MACE (3-P MACE AND hospitalization for UA); renal composite (doubling of serum creatinine level with eGFR < 45 mL/min/1.73 m2 AND dialysis AND renal death) | All-cause mortality; CVD; progression of albuminuria; CVD AND HHF; renal composite (> = 40% decrease in eGFR AND dialysis AND renal death) | Renal composite #1 (> = 40% decrease in eGFR AND ESRD AND renal death AND CVD); all-cause mortality; renal composite #2 (> = 40% decrease in eGFR AND ESRD AND renal death) | CVD AND HHF; 3-P MACE; HHF; renal composite #2 (ESRD AND doubling of serum creatinine level AND renal death); CVD; all-cause mortality; MACE AND HHF AND hospitalization for UA | CVD AND HHF; CVD; renal composite (renal death AND dialysis/transplant AND doubling of serum creatinine level) | Total no. of HHF AND urgent visits for HF; CVD; all-cause mortality; 3-P MACE AND HHF; CVD AND HHF AND urgent visits for HF AND no. of HF events during hospitalization; renal composite (50% decline in eGFR AND dialysis AND renal transplantation AND eGFR < 15 mL/min/1.73 m2); 3P-MACE |
| Link to trial registry | NCT01131676 | NCT01032629; NCT01989754 | NCT01730534 | NCT02065791 | NCT01986881 | NCT03315143 |
When trials defined more than one renal composite endpoint, for the meta-analysis we used the one that was devoid of cardiovascular death (CVD) ensuring more homogeneity across studies.
3P-MACE three-point major adverse cardiovascular events comprising cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor blocker, CrCl creatinine clearance, CV cardiovascular, CVD cardiovascular death, eGFR estimated glomerular filtration rate, ESRD end-stage renal disease, HbA1C glycated hemoglobin, HHF hospitalization for heart failure, MI myocardial infarction, SD standard deviation, UA unstable angina.
Literature search, study selection, data collection, and quality assessment
The search terms are provided as Supplementary material. Two collaborators independently assessed the publications in line with the predefined selection criteria as outlined above. Disagreement was resolved by the senior author. For each involved trial, data were extracted on trial design, baseline characteristics of study populations, and outcomes. The Cochrane Risk of Bias Tool34 was used to evaluate individual study quality.
Statistical analysis
The hazard ratios (HRs) and their 95% confidence intervals (CIs) were extracted for the binary prespecified outcomes and pooled using the Sidik–Jonkman35 random-effects model. Statistical heterogeneity (referred to as heterogeneity) was assessed using the Cochran Q homogeneity test, Higgins and Thompson I2, and Tau2. As per the Cochrane Handbook for Systematic Reviews of Interventions36, we considered the following for heterogeneity: I2 = 0% to 40% might not be important; I2 = 30–60% may represent moderate heterogeneity; I2 = 50–90% may represent substantial heterogeneity; I2 = 75–100% is considerable heterogeneity.
We performed mixed-effects meta-regression (based on the Sidik–Jonkman method) to assess the influence of pharmacological receptor selectivity (SGLT2:SGLT1 selectivity ratio as a continuous variable1,11,12) of each individual medication on the given outcome (yielding pseudo-R2 values, which describes the proportion of heterogeneity explained by this factor). Additionally, analyses were carried out by dichotomizing studies based on whether the studied medication has clinically relevant SGLT1 inhibitory effect (i.e. agents with low pharmacological SGLT2 selectivity: canagliflozin, sotagliflozin) or has no clinically meaningful SGLT1 inhibitory property (i.e. agents with high pharmacological SGLT2 selectivity: empagliflozin, dapagliflozin, ertugliflozin). In each case, mixed-effects meta-regression (based on the Sidik–Jonkman method) was used to assess difference between the pooled estimates of low vs. high SGLT2 selectivity groups, pertinent to each outcome.
Evaluation of reported adverse events (including all severe adverse events) was performed by extracting odd ratios (ORs) and their 95% CI (SGLT2 inhibitor treatment versus placebo) from the included trial. Then, the influence of pharmacological selectivity on these safety outcomes, either as dichotomous or continuous variable, was computed as described above.
All statistical analyses were carried out in Stata 17.0 (StataCorp LLC, College Station, TX, USA). A two-tailed p < 0.05 was considered statistically significant.
Sensitivity analysis
Sensitivity analysis was carried out post hoc. Fatal and nonfatal stroke as an outcome was analyzed in individual study subgroups with baseline estimated glomerular filtration rate (eGFR) lower than 60 mL/min/1.73 m2 according to a previous meta-analysis37. The effect of selectivity of SGLT2 inhibitors on these subgroups was investigated as outlined above.
Results
Eligible studies, patient characteristics, and risk of bias
We identified an overall of 6 placebo-controlled randomized cardiovascular outcome trials (EMPA-REG OUTCOME2, CANVAS Program3, DECLARE-TIMI 584, CREDENCE5, VERTIS CV6, and SCORED7) of three selective SGLT2 inhibitors (empagliflozin2, dapagliflozin4, and ertugliflozin6) and two SGLT2 inhibitors with clinically relevant SGLT1-inhibitory property (canagliflozin3,5 and sotagliflozin7), including a total of 57,553 patients with type 2 diabetes mellitus and high cardiovascular risk (Table 1). The mean (± standard deviation) age of all trial participants was 65 ± 8 years; 36,769 (63.9%) were men, and 20,784 (36.1%) were women; and 45,598 (79.2%) were White (Table 1). Across the 6 trials, the median follow-up ranged from 1.3 to 4.2 years, average HbA1C ranged from 8.1 to 8.3%, baseline average eGFR ranged from 44 to 85 mL/min/1.73 m2 (Table 1).
Supplementary materials contain the search terms, whereas Supplementary Fig. 1 depicts the selection process. All included trials showed low overall risk of bias according to the Cochrane Risk of Bias Tool34 (Supplementary Table 1); the SCORED trial7 ended prematurely due to loss of funding, therefore the primary endpoint was changed during the trial and investigator-reported events were used for endpoint analyses.
Outcomes
Efficacy
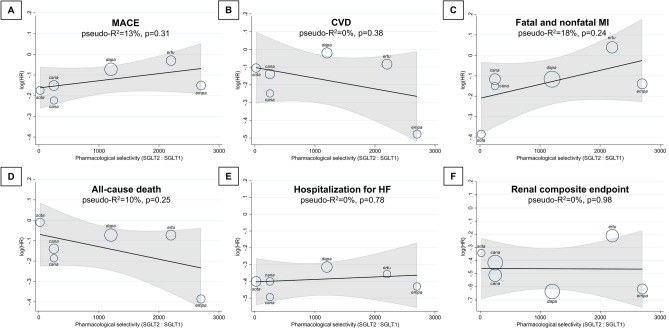
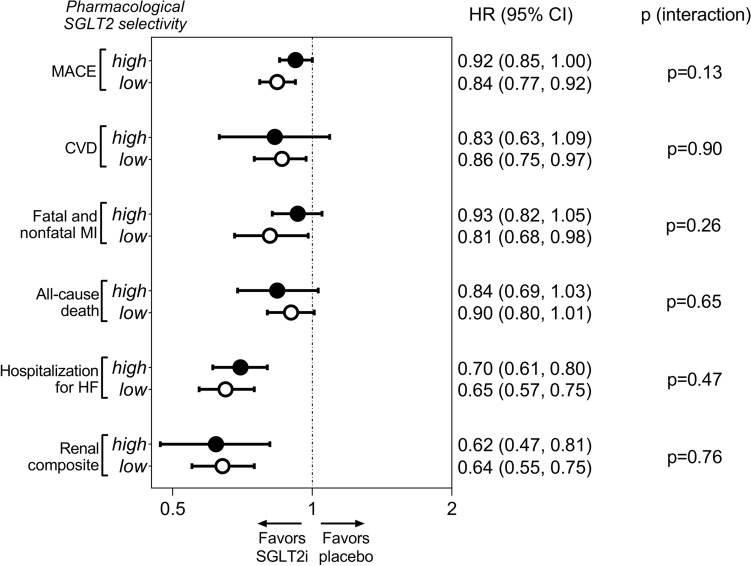
Overall, SGLT2 inhibitors significantly reduced the relative risk of MACE compared with placebo (HR, 0.88; 95% CI, 0.83–0.95; p < 0.001) without relevant heterogeneity (I2 = 31%; p = 0.45) (Supplementary Fig. 2). The SGLT2:SGLT1 selectivity ratio of individual study medications as a continuous variable had no notable effect on MACE (pseudo-R2 = 13%; p = 0.31) (Fig. 1). When trials were grouped according to the pharmacological selectivity of the trial medications, low selectivity agents significantly reduced MACE versus placebo (HR, 0.84; 95% CI, 0.77–0.92), whereas in case of highly selective agents, the confidence interval crossed the line of unity (HR, 0.92; 95% CI, 0.85–1.00) (Fig. 2). However, there was no significant difference between these two pharmacological groups (p = 0.13) (Fig. 2).
Figure 1.
Effect of pharmacological selectivity of SGLT2 inhibitors on risk of MACE (A), CVD (B), fatal and nonfatal MI (C), all-cause death (D), hospitalization for HF (E), and renal composite endpoint (F) using the SGLT2:SGLT1 pharmacological selectivity ratio as continuous explanatory variable in a mixed-effects meta-regression analysis. CVD cardiovascular death, HF heart failure, HR hazard ratio, MACE major adverse cardiovascular events, MI myocardial infarction, SGLT1/2 sodium–glucose cotransporter 1/2.
Figure 2.
Comparison of the effect of high versus low pharmacological selectivity of SGLT2 inhibitors on clinical outcomes. CI confidence interval, CVD cardiovascular death, HF heart failure, HR hazard ratio, MACE major adverse cardiovascular events, MI myocardial infarction, SGLT1/2 sodium–glucose cotransporter 1/2, SGLT2i sodium–glucose cotransporter 2 inhibitor.
Altogether, SGLT2 inhibitor treatment reduced the risk of cardiovascular death compared with placebo (HR, 0.84; 95% CI, 0.74–0.97; p = 0.014) with substantial heterogeneity (I2 = 62%; p = 0.043) (Supplementary Fig. 2), which was not explained by differences in selectivity of SGLT2 inhibitors to SGLT2 over SGLT1 (pseudo-R2 = 0%; p = 0.38) (Fig. 1). Accordingly, while only non-selective SGLT2 inhibitors reduced significantly the risk of cardiovascular death (HR, 0.86; 95% CI, 0.75–0.97) as compared with highly selective agents (HR, 0.83; 95% CI, 0.63–1.09) (Supplementary Fig. 2), there was no significant interaction (p = 0.90) (Fig. 2).
Fatal and nonfatal MI was slightly, but significantly reduced by SGLT2 inhibitors overall (HR, 0.88; 95% CI, 0.78–0.99; p = 0.031), with moderate heterogeneity (I2 = 47%; p = 0.26) (Supplementary Fig. 2). The ratio of pharmacological selectivity of SGLT2 inhibitors to SGLT2 over SGLT1 did not significantly correlate with clinical outcomes (pseudo-R2 = 18%; p = 0.24) (Fig. 1). Only non-selective SGLT2 inhibitors reduced significantly the risk of fatal and nonfatal MI compared with placebo (HR, 0.81; 95% CI, 0.68–0.98), whereas highly selective agents did not (HR, 0.93; 95% CI, 0.82–1.05), but there was no significant difference between the two groups (p = 0.26) (Fig. 2).
Across all 6 trials, the risk of all-cause death was significantly reduced by SGLT2 inhibitors (HR, 0.87; 95% CI, 0.78–0.97; p = 0.013), while heterogeneity was significant (I2 = 63%; p = 0.049) (Supplementary Fig. 3). Pharmacological selectivity of SGLT2 inhibitors did not affect all-cause death (pseudo-R2 = 10%; p = 0.25) (Fig. 1). Indeed, neither non-selective (HR, 0.90; 95% CI, 0.80–1.01) nor highly selective (HR, 0.84; 95% CI, 0.69–1.03) SGLT2 inhibitors reduced all-cause death as compared with placebo (p = 0.65) (Fig. 2).
In all trials, SGLT2 inhibitors significantly reduced the risk of hospitalization for HF with large effect size (HR, 0.68; 95% CI, 0.62–0.75; p < 0.001) and no heterogeneity (I2 = 5%; p = 0.92) (Supplementary Fig. 3). The SGLT2:SGLT1 pharmacological selectivity ratio did not correlate with this outcome (pseudo-R2 = 0%; p = 0.78) (Fig. 1). Agents with high (HR, 0.70; 95% CI, 0.61–0.80) and low (HR, 0.65; 95% CI, 0.57–0.75) SGLT2 selectivity reduced risk of HF hospitalization to a similar extent, with no significant difference (p = 0.47) (Fig. 2).
Next, SGLT2 inhibitors significantly reduced the risk of the composite renal endpoint (HR, 0.63; 95% CI, 0.54–0.73; p < 0.001), referring to a large effect with moderate heterogeneity (I2 = 46%; p = 0.16) (Supplementary Fig. 3). The extent of SGLT2 selectivity did not significantly affect this outcome (pseudo-R2 = 0%; p = 0.98) (Fig. 1). In fact, both highly selective (HR, 0.62; 95% CI, 0.47–0.81) and non-selective (HR, 0.64; 95% CI, 0.55–0.75) SGLT2 inhibitors significantly reduced the risk of the renal endpoint to a similar magnitude (p = 0.76) (Fig. 2).
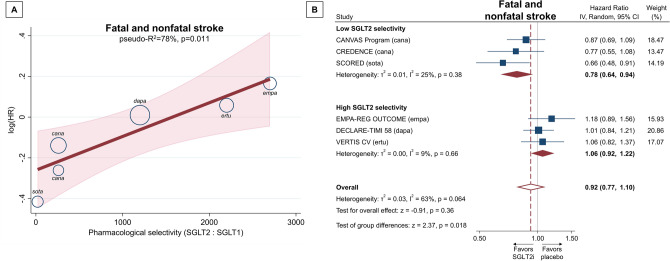
Altogether, SGLT2 inhibitors did not alter the risk of fatal and nonfatal stroke in high-risk type 2 diabetic patients (HR, 0.92; 95% CI, 0.77–1.10; p = 0.36), but there was a substantial heterogeneity (I2 = 63%; p = 0.064) (Fig. 3B). The SGLT2:SGLT1 pharmacological selectivity ratio explained a considerable amount of heterogeneity in the risk of stroke (pseudo-R2 = 78%; p = 0.011) (Fig. 3A). Accordingly, less selectivity towards SGLT2 (i.e. more pronounced SGLT1 inhibitory effect) favored lower risk of stroke (Fig. 3A). In fact, only non-selective SGLT2 inhibitors reduced the risk of fatal and nonfatal stroke (HR, 0.78; 95% CI, 0.64–0.94) as compared with placebo, whereas those with high selectivity did not (HR, 1.06; 95% CI, 0.92–1.22) (Fig. 3B), with a significant interaction between the two pharmacological groups (p = 0.018). In a head-to-head comparison, this refers to a ~ 26% relative risk reduction in stroke with non-selective versus selective SGLT2 inhibitors (HR, 0.74; 95% CI, 0.58–0.93).
Figure 3.
Effect of pharmacological selectivity of SGLT2 inhibitors on risk of fatal and nonfatal stroke either as continuous (SGLT2:SGLT1 pharmacological selectivity ratio) (A) or as binary (high vs. low SGLT2 selectivity) (B) explanatory variable. CI confidence interval, HR hazard ratio, IV inverse variance, SGLT1/2 sodium–glucose cotransporter 1/2, SGLT2i sodium–glucose cotransporter 2 inhibitor.
Safety
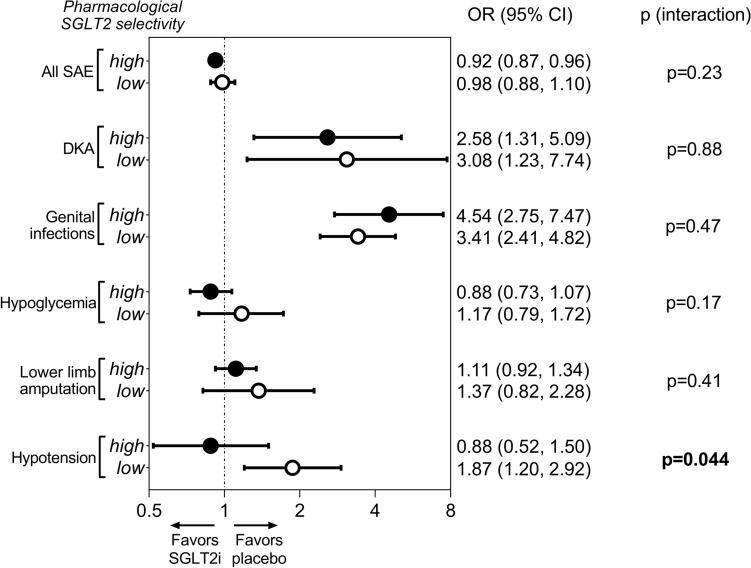
Both highly selective and non-selective SGLT2 inhibitors appeared to be safe compared with placebo. In fact, agents with high SGLT2 selectivity reduced the risk of severe adverse events (OR, 0.92; 95% CI, 0.87–0.96) whereas non-selective agents did not (OR, 0.98; 95% CI, 0.88–1.10), but there was no significant between-group difference (p = 0.23) (Fig. 4 and Supplementary Fig. 4). As compared with placebo, non-selective and highly selective SGLT2 inhibitors were associated with significantly higher risk of diabetic ketoacidosis (OR, 3.08; 95% CI, 1.23–7.74 vs. OR, 2.58; 95% CI, 1.31–5.09; p = 0.88) and genital infections (OR, 3.41; 95% CI, 2.41–4.82 vs. OR, 4.54; 95% CI, 2.75–7.47; p = 0.47), respectively, to a similar extent (Fig. 4 and Supplementary Figs. 5, 6). On the contrary, the risk of hypoglycemia (OR, 1.17; 95% CI, 0.79–1.72 vs. OR, 0.88; 95% CI, 0.73–1.07; p = 0.17) and lower limb amputation (OR, 1.37; 95% CI, 0.82–2.28 vs. OR, 1.11; 95% CI, 0.92–1.34; p = 0.41), respectively, was not significantly altered by these agents compared with placebo (Fig. 4 and Supplementary Figs. 7, 8). However, non-selective SGLT2 inhibitors significantly increased the risk of hypotension compared with placebo (HR, 1.87; 95% CI, 1.20–2.92), whereas highly selective agents did not (HR, 0.88; 95% CI, 0.52–1.50), with a significant between-group difference (p = 0.044) (Fig. 4 and Supplementary Fig. 9). In fact, less selectivity towards SGLT2 (i.e. more pronounced SGLT1 inhibitory effect) was associated with higher risk of hypotension (pseudo-R2 = 65%; p = 0.015) (Supplementary Fig. 9). The risk of hypotension was ~ 2-times higher with non-selective inhibitors as compared with highly selective SGLT2 inhibitors (OR, 2.13; 95% CI, 1.06–4.24).
Figure 4.
Comparison of the effect of high versus low pharmacological selectivity of SGLT2 inhibitors on safety outcomes. CI confidence interval, DKA diabetic ketoacidosis, HR hazard ratio, SAE severe adverse events, SGLT2 sodium–glucose cotransporter 2, SGLT2i sodium–glucose cotransporter 2 inhibitor.
Sensitivity analysis
For fatal and nonfatal stroke, we analyzed data of patients with baseline eGFR lower than 60 mL/min/1.73 m2 pooling data from a previous meta-analysis37. Data from the VERTIS CV trial6 were unavailable for this analysis. In patients with eGFR lower than 60 mL/min/1.73 m2, SGLT2 inhibitors tended to reduce the risk of stroke (HR, 0.75; 95% CI, 0.55–1.02; p = 0.066) with some heterogeneity (I2 = 58%; p = 0.14) (Supplementary Fig. 10). Compared to agents with high SGLT2 selectivity, low SGLT2 selectivity was associated with a significantly lower risk of stroke (HR, 0.63; 95% CI, 0.48–0.81) in patients with eGFR lower than 60 mL/min/1.73 m2, marking a significant difference between the two pharmacological groups (p = 0.047) (Supplementary Fig. 10).
Discussion
In this meta-analysis, we investigated the impact of pharmacological selectivity of SGLT2 inhibitors on cardiovascular outcomes in patients with type 2 diabetes mellitus, according to large-scale cardiovascular outcome trials. We found that a more pronounced SGLT1 inhibitory property had little additional effect on most outcomes, however, it was significantly associated with lower risk of stroke as compared with high pharmacological SGLT2 selectivity. In terms of adverse events, the risk of hypotension appears to be higher with non-selective SGLT2 inhibitors.
The substantial differences between the extent of pharmacological selectivity of SGLT2 inhibitors to SGLT2 over SGLT1 might be clinically relevant since individuals with partially reduced transport activity of SGLT1 (corresponding to pharmacological SGLT1 inhibition), but not that of SGLT2, derive significant cardiovascular and survival benefits as compared with non-affected controls23,24. A recent meta-analysis of dedicated HF studies found that lower SGLT2 selectivity was significantly more favorable in terms of the composite of hospitalization for HF or cardiovascular death38. In line with these clinical data, a number of preclinical studies have linked SGLT1 to pathological processes in the heart25–29, brain30,31, and kidney32. Yet, in high-risk type 2 diabetic patients, the effect of the pharmacological selectivity of SGLT2 inhibitors on clinical outcomes has been ill-defined.
Here we report that pharmacological selectivity of SGLT2 inhibitors does not significantly correlate with risk of MACE, cardiovascular death, fatal and nonfatal MI, all-cause mortality, hospitalization for HF, or the renal composite outcome in patients with type 2 diabetes mellitus and high cardiovascular risk. Therefore, it seems that additional SGLT1 inhibition on top of SGLT2 blockade might not affect these outcomes in the studied patient groups. On the contrary, pharmacological SGLT2:SGLT1 selectivity ratio significantly correlated with stroke outcomes, with lower SGLT2 selectivity (i.e. more pronounced inhibitory effect on SGLT1) corresponding with reduced risk. In addition, this difference remained significant even in patients with a baseline eGFR lower than 60 mL/min/1.73 m2. Therefore, SGLT2 inhibitors with pronounced SGLT1 inhibitory effect might reduce the risk of stroke, which effect seems to be less affected by baseline eGFR.
Previous meta-analyses of large cardiovascular outcome trials39,40 found that SGLT2 inhibitors have only modest effect on atherosclerotic major adverse cardiovascular outcomes in patients with type 2 diabetes mellitus, and this is confined to those with established atherosclerotic cardiovascular disease (ASCVD). Specifically, the risk of MACE and (fatal and nonfatal) MI, respectively, were shown to be significantly reduced by SGLT2 inhibitors only in type 2 diabetic patients with established ASCVD, but not in those without ASCVD39,40. However, SGLT2 inhibitors had neutral effect on stroke risk in patients with and without ASCVD39. Therefore, our present results might complement previous meta-analyses by adding that selectivity of SGLT2 inhibitors is a significant predictor of stroke outcomes, in fact, combined SGLT1/2 inhibition might constitute a novel pharmacological approach to reduce adverse stroke outcomes in type 2 diabetic patients who are inherently at greater risk.
Ischemic stroke was the predominant subtype in these outcome trials, and SGLT2 inhibitors overall reduce new onset atrial fibrillation or flutter (AF/AFL)37, a major risk factor for ischemic stroke. On individual trial level, only dapagliflozin (DECLARE-TIMI 58 trial) reduced the risk of AF/AFL, which was independent of patient’s previous history of AF, ASCVD, or HF4,41. However, dapagliflozin had neutral effect on risk of ischemic stroke4. Therefore, it is unlikely that SGLT2 inhibitor treatment alters stroke risk by reducing new onset AF/AFL occurrence. Other SGLT1-related mechanisms have recently been suggested by Pitt and colleagues20, including favorable alteration of gut microbiome due to intestinal inhibition of SGLT1-mediated glucose absorption, and increased native glucagon-like peptide-1 levels, both having direct and indirect antithrombotic effects, and at the same time, postprandial serum glucose excursions are blunted20. Interestingly, small animal studies have linked SGLT1 upregulation during ischemic stroke to neuronal damage possibly through enhanced glucose uptake, its knockdown reduced lesion volume30 and brain injury31. Currently, it is unclear whether pharmacological inhibition of cerebral SGLT1 itself has any clinical relevance and whether it plays any role in prevention of stroke.
Regarding safety outcomes, both highly selective and non-selective SGLT2 inhibitors increased the risk of genital infections and diabetic ketoacidosis to a similar extent in patients with type 2 diabetes, as compared with placebo. On the contrary, neither pharmacological subgroup heightened significantly the risk of hypoglycemia or lower limb amputation. However, non-selective SGLT2 inhibitors were associated with a twofold increased risk of hypotension compared with highly selective agents. This might again reflect on distinct mechanistic effects of selective versus non-selective SGLT2 inhibitors.
In summary, we found that lower pharmacological selectivity of SGLT2 inhibitors with a more pronounced inhibitory effect on SGLT1 is associated with reduced risk of fatal and nonfatal stroke in high-risk type 2 diabetic patients, according to large-scale cardiovascular outcome trials. Combined SGLT1/2 inhibition could be a novel pharmacological approach to prevent stroke in these patients. Future confirmatory studies are warranted to elucidate the clinical significance of these hypothesis-generating findings.
Limitations
Our meta-analysis has inherent limitations, rendering the findings hypothesis-generating only. The included trials were not powered to assess individual endpoints of the composite outcome. The number of studies included is relatively small, however, these trials enrolled a relatively high number of patients. Differences in eligibility criteria and baseline characteristics may have affected the calculations, but the included cardiovascular outcome trials similarly enrolled type 2 diabetic patients with high cardiovascular risk. Definitions of outcomes were slightly different across trials. The definition of the renal composite varied significantly, limiting the interpretation of this endpoint. The DECLARE-TIMI 584 trial reported fatal and nonfatal stroke of ischemic origin only, however the incidence of hemorrhagic stroke was only 0.09% in the overall trial population, suggesting that the composite endpoint would not meaningfully change if hemorrhagic stroke was included. The EMPA-REG OUTCOME2 trial excluded silent MI from the endpoints of ‘MACE’ and ‘fatal and nonfatal MI’, respectively. Finally, the SCORED trial7 was terminated prematurely due to loss of funding, therefore the primary endpoint was changed during the trial and investigator-reported events were used for endpoint analyses.
Supplementary Information
Author contributions
A.A.S. conceived and designed the study, acquired, analyzed and interpreted the data, and drafted the article. A.O., M.R., and B.A.B. acquired and interpreted the data, and drafted the article. B.M. conceived and designed the study, interpreted the data, and revised the article for important intellectual content. T.R. conceived and designed the study, analyzed and interpreted the data, and revised the article for important intellectual content.
Funding
Open access funding provided by Semmelweis University. This work was supported by the National Research, Development and Innovation Fund of Hungary, financed under the NVKP_16 funding scheme [grant no. NVKP_16-1–2016-0017, ’National Heart Program’]; by the Thematic Excellence Programme [grant no. 2020-4.1.1.-TKP2020] of the Ministry of Innovation and Technology of Hungary, within the framework of the Therapeutic Development and Bioimaging thematic programmes of Semmelweis University; by a grant from the National Research, Development and Innovation Office (NKFIH) of Hungary [grant no. K134939 to T.R.]; and by the New National Excellence Program of the Ministry of Innovation and Technology [grant no. ÚNKP-21-3-II-SE-45 to A.A.S.]. TKP2021-EGA-23 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-EGA funding scheme. Project no. RRF-2.3.1-21-2022-00003 (‘National Cardiovascular Laboratory’) has been implemented with the support provided by the European Union. The study funders were not involved in the design of the study; the collection, analysis, and interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Data availability
Original data generated and analyzed during this study are included in this manuscript.
Competing interests
B.M. received speaker fees from AstraZeneca, Boehringer Ingelheim, and Novartis (not related to this study). The other authors declare that they have no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Béla Merkely and Tamás Radovits.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-52331-w.
References
- 1.Zelniker TA, Braunwald E. Clinical Benefit of cardiorenal effects of sodium–glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75:435–447. doi: 10.1016/j.jacc.2019.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Zinman B, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 3.Neal B, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 4.Wiviott SD, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 5.Perkovic V, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 6.Cannon CP, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N. Engl. J. Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N. Engl. J. Med. 2021;384:129–139. doi: 10.1056/NEJMoa2030186. [DOI] [PubMed] [Google Scholar]
- 8.Packer M. Critical examination of mechanisms underlying the reduction in heart failure events with SGLT2 inhibitors: Identification of a molecular link between their actions to stimulate erythrocytosis and to alleviate cellular stress. Cardiovasc. Res. 2021;117:74–84. doi: 10.1093/cvr/cvaa064. [DOI] [PubMed] [Google Scholar]
- 9.Zelniker TA, Braunwald E. Mechanisms of cardiorenal effects of sodium–glucose cotransporter 2 inhibitors: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2020;75:422–434. doi: 10.1016/j.jacc.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Cowie MR, Fisher M. SGLT2 inhibitors: Mechanisms of cardiovascular benefit beyond glycaemic control. Nat. Rev. Cardiol. 2020;17:761–772. doi: 10.1038/s41569-020-0406-8. [DOI] [PubMed] [Google Scholar]
- 11.Dominguez Rieg JA, Rieg T. What does sodium–glucose co-transporter 1 inhibition add: Prospects for dual inhibition. Diabetes Obes. Metab. 2019;21(Suppl 2):43–52. doi: 10.1111/dom.13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018;61:2079–2086. doi: 10.1007/s00125-018-4654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium–glucose cotransporter inhibitors: Effects on renal and intestinal glucose transport: From bench to bedside. Diabetes Care. 2015;38:2344–2353. doi: 10.2337/dc15-0642. [DOI] [PubMed] [Google Scholar]
- 14.Sokolov V, et al. Differentiating the sodium-glucose cotransporter 1 inhibition capacity of canagliflozin vs. dapagliflozin and empagliflozin using quantitative systems pharmacology modeling. CPT Pharmacom. Syst. Pharmacol. 2020;9:222–229. doi: 10.1002/psp4.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohgaki R, et al. Interaction of the sodium/glucose cotransporter (SGLT) 2 inhibitor canagliflozin with SGLT1 and SGLT2. J. Pharmacol. Exp. Ther. 2016;358:94–102. doi: 10.1124/jpet.116.232025. [DOI] [PubMed] [Google Scholar]
- 16.Sha S, et al. Pharmacodynamic differences between canagliflozin and dapagliflozin: Results of a randomized, double-blind, crossover study. Diabetes Obes. Metab. 2015;17:188–197. doi: 10.1111/dom.12418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polidori D, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: Results of a randomized, placebo-controlled study. Diabetes Care. 2013;36:2154–2161. doi: 10.2337/dc12-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein P, et al. Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces post-meal glucose excursion in patients with type 2 diabetes by a non-renal mechanism: Results of a randomized trial. Metabolism. 2014;63:1296–1303. doi: 10.1016/j.metabol.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Kondo H, et al. Effects of canagliflozin on human myocardial redox signalling: Clinical implications. Eur. Heart J. 2021 doi: 10.1093/eurheartj/ehab420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitt B, Steg G, Leiter LA, Bhatt DL. The role of combined SGLT1/SGLT2 inhibition in reducing the incidence of stroke and myocardial infarction in patients with type 2 diabetes mellitus. Cardiovasc. Drugs Ther. 2021 doi: 10.1007/s10557-021-07291-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Posch MG, et al. Metabolic, intestinal, and cardiovascular effects of sotagliflozin compared with empagliflozin in patients with type 2 diabetes: A randomized, double-blind study. Diabetes Care. 2022;45:2118–2126. doi: 10.2337/dc21-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: The epidemiological evidence. Diabetologia. 2001;44:2107–2114. doi: 10.1007/s001250100020. [DOI] [PubMed] [Google Scholar]
- 23.Seidelmann SB, et al. Genetic variants in SGLT1, glucose tolerance, and cardiometabolic risk. J. Am. Coll. Cardiol. 2018;72:1763–1773. doi: 10.1016/j.jacc.2018.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katzmann JL, et al. Genetic variation in sodium–glucose cotransporter 2 and heart failure. Clin. Pharmacol. Ther. 2021 doi: 10.1002/cpt.2153. [DOI] [PubMed] [Google Scholar]
- 25.Banerjee SK, et al. SGLT1, a novel cardiac glucose transporter, mediates increased glucose uptake in PRKAG2 cardiomyopathy. J. Mol. Cell. Cardiol. 2010;49:683–692. doi: 10.1016/j.yjmcc.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramratnam M, et al. Transgenic knockdown of cardiac sodium/glucose cotransporter 1 (SGLT1) attenuates PRKAG2 cardiomyopathy, whereas transgenic overexpression of cardiac SGLT1 causes pathologic hypertrophy and dysfunction in mice. J. Am. Heart Assoc. 2014 doi: 10.1161/JAHA.114.000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushita N, et al. Chronic pressure overload induces cardiac hypertrophy and fibrosis via increases in SGLT1 and IL-18 gene expression in mice. Int. Heart J. 2018;59:1123–1133. doi: 10.1536/ihj.17-565. [DOI] [PubMed] [Google Scholar]
- 28.Lin H, Guan L, Meng L, Uzui H, Guo H. SGLT1 knockdown attenuates cardiac fibroblast activation in diabetic cardiac fibrosis. Front. Pharmacol. 2021 doi: 10.3389/fphar.2021.700366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, et al. Cardiac sodium-dependent glucose cotransporter 1 is a novel mediator of ischaemia/reperfusion injury. Cardiovasc. Res. 2019;115:1646–1658. doi: 10.1093/cvr/cvz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki Y, Ogihara S, Harada S, Tokuyama S. Activation of cerebral sodium–glucose transporter type 1 function mediated by post-ischemic hyperglycemia exacerbates the development of cerebral ischemia. Neuroscience. 2015;310:674–685. doi: 10.1016/j.neuroscience.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Sebastiani A, et al. RS1 (Rsc1A1) deficiency limits cerebral SGLT1 expression and delays brain damage after experimental traumatic brain injury. J. Neurochem. 2018;147:190–203. doi: 10.1111/jnc.14551. [DOI] [PubMed] [Google Scholar]
- 32.Nespoux J, et al. Gene deletion of the Na(+)-glucose cotransporter SGLT1 ameliorates kidney recovery in a murine model of acute kidney injury induced by ischemia-reperfusion. Am. J. Physiol. Renal Physiol. 2019;316:F1201–F1210. doi: 10.1152/ajprenal.00111.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higgins JP, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.IntHout J, Ioannidis JP, Borm GF. The Hartung–Knapp–Sidik–Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian–Laird method. BMC Med. Res. Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins, J. P. T. et al.Cochrane Handbook for Systematic Reviews of Interventions (eds.) (Cochrane, 2020).
- 37.Zhou Z, et al. Effect of SGLT2 inhibitors on stroke and atrial fibrillation in diabetic kidney disease: Results from the CREDENCE Trial and meta-analysis. Stroke. 2021;52:1545–1556. doi: 10.1161/STROKEAHA.120.031623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tager T, et al. Influence of receptor selectivity on benefits from SGLT2 inhibitors in patients with heart failure: A systematic review and head-to-head comparative efficacy network meta-analysis. Clin. Res. Cardiol. 2021 doi: 10.1007/s00392-021-01913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGuire DK, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: A meta-analysis. JAMA Cardiol. 2021;6:148–158. doi: 10.1001/jamacardio.2020.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zelniker TA, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: A systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393:31–39. doi: 10.1016/S0140-6736(18)32590-X. [DOI] [PubMed] [Google Scholar]
- 41.Zelniker TA, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: Insights from the DECLARE-TIMI 58 trial. Circulation. 2020;141:1227–1234. doi: 10.1161/CIRCULATIONAHA.119.044183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original data generated and analyzed during this study are included in this manuscript.