Highlights
-
•
The association between daily lifestyle factors and nonalcoholic fatty liver disease (NAFLD) onset is unclear.
-
•
We assessed the association of lifestyle factors with NAFLD onset using health checkup data.
-
•
Eating between meals and eating fast were risk factors for NAFLD onset in men and women, respectively.
-
•
Fast walking was a protective factor against NAFLD onset in women.
-
•
Lifestyle modifications may help to prevent future NAFLD onset.
Keywords: NAFLD, Onset, Lifestyle factors, Eating between meals, Eating Fast, Fast walking
Abstract
Evidence for the influence of lifestyle factors on nonalcoholic fatty liver disease (NAFLD) onset is limited because the association between lifestyle factors and NAFLD has been reported mostly in cross-sectional studies. Our purpose was to elucidate which lifestyle factors are associated with NAFLD onset by performing a longitudinal study. This was a longitudinal study of 1,713 Japanese participants who underwent multiple health checkups from June 2013 to the end of March 2018 and were not diagnosed with NAFLD at the first health checkup at Watari Hospital in Fukushima, Japan. Baseline characteristics, including lifestyle factors, were compared among participants with and without NAFLD. Cox proportional hazards models were used to identify the association between lifestyle factors and NAFLD onset. Among the 1,713 participants, 420 (24.5 %) developed NAFLD during the observation period (median 47 months). There were significant differences in body mass index and hepatobiliary enzyme levels between participants with and without NAFLD. In Cox proportional hazards models, eating between meals (hazard ratio (HR): 2.08, 95 % confidence interval (CI): 1.25–3.45, p < 0.01) and eating fast (HR: 1.59, 95 % CI: 1.26–2.00, p < 0.01) were risk factors for NAFLD onset in men and women, respectively. Moreover, fast walking was a protective factor against NAFLD onset in women (HR: 0.76, 95 % CI: 0.60–0.96, p = 0.02). These findings could help to identify patients at risk and prevent future NAFLD onset.
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease worldwide (Younossi et al., 2016), and its prevalence is expected to continue to increase in the future (Estes et al., 2018). Obesity due to an unhealthy lifestyle is strongly associated with NAFLD (Zelber-Sagi et al., 2011). Therefore, various lifestyle modifications have been proposed as strategies for protection against or the improvement of NAFLD (Younossi et al., 2021) (Kamada et al., 2021).
Bodyweight reduction by caloric restriction is an established treatment for NAFLD (Younossi et al., 2021) (Kamada et al., 2021). Moreover, dietary habits such as fast eating or habitual eating before bedtime have been reported as factors associated with NAFLD(Lee et al., 2016, Nishi et al., 2016), (Cao et al., 2020). However, little is known about the causal relationship between dietary habits and NAFLD.
The efficacy of exercise or physical activity on the resolution or prevention of NAFLD has also been established by numerous studies (Keating et al., 2012) (Choi et al., 2022) (Hashida et al., 2017). However, it is not clear which daily lifestyle factors are associated with NAFLD onset because longitudinal studies simultaneously evaluating exercise or physical activity and eating habits are lacking.
The present study aimed to examine the association of lifestyle factors with NAFLD onset using health checkup data.
2. Materials and methods
2.1. Participants
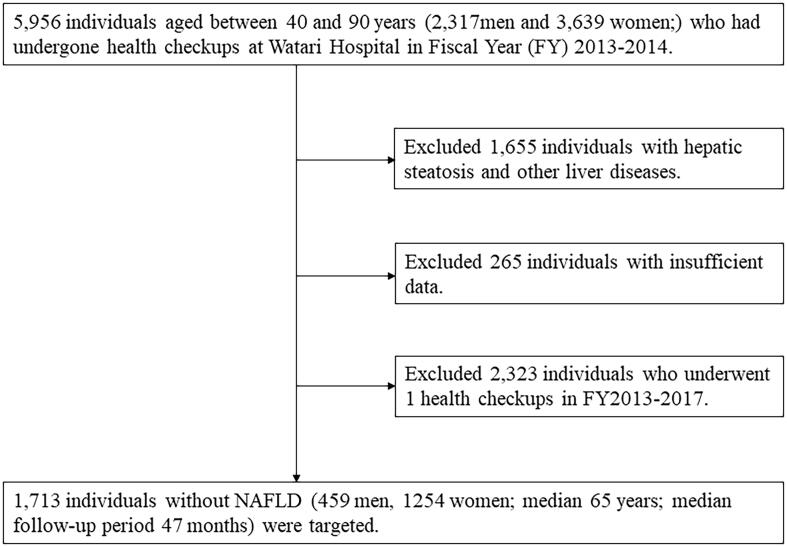
The participants in this study were Japanese men and women who underwent health check-ups more than twice between February 2013 and March 2018 at Watari Hospital Health Center in Fukushima, Japan. NAFLD was diagnosed based on the Japanese NAFLD criteria (Tokushige et al., 2021). Participants with other liver diseases, such as alcoholic liver disease, viral disease, and drug-induced liver disease were excluded. Ultimately, 1,713 participants without NAFLD at the time of the first health checkup during the above period were eligible for analysis (Fig. 1). The study protocol was approved by the ethics committees of Watari Hospital and Fukushima Medical University and conducted in accordance with the ethics guidelines in the Declaration of Helsinki. All participants provided written informed consent prior to study participation.
Fig. 1.
Flow chart of participant selection for this study. This study evaluated Japanese adult participants who underwent multiple health checkups from June 2013 to the end of March 2018 and were not diagnosed with NAFLD at the first health checkup at Watari Hospital in Fukushima, Japan.
2.2. Lifestyle questionnaire
All participants completed the ‘‘Specific health checkups’’ lifestyle questionnaire introduced by the Japanese Ministry of Health, Labour and Welfare (Supplemental table). We collected participants’ ordinary lifestyle data on the following factors: smoking status; alcohol intake; exercise; physical activity; walking speed; and dietary habits, such as eating speed, eating before bedtime, eating between meals and skipping breakfast. Each lifestyle factor was defined as follows: smoking status, current smoker; exercise, a habit of engaging in exercise with light sweating for longer than 30 min per session, two times per week, for over a year; physical activity, walking or engaging in any equivalent amount of physical activity for more than 1 h per day; fast walking, a walking speed faster than the speed of other individuals of the same age and sex; eating fast, a quicker eating speed than the speed of others; eating before bedtime, eating supper 2 h before bedtime more than three times per week; eating between meals, eating snacks or drinking sweet beverages between meals; and skipping breakfast, skipping breakfast more than three times per week. In this study, we evaluated lifestyle data at the time of the first health checkup during the study period; participants were not diagnosed with NAFLD at that time.
2.3. Statistical analysis
Participants with and without NAFLD were compared using the χ2 test and Fisher’s exact test for categorical variables and the Mann-Whitney U test for continuous variables. Adjusted hazard ratios (HRs) and 95 % confidence intervals (95 % CIs) for incident NAFLD were calculated using Cox proportional hazards models. We adjusted for age, smoking (yes or no), exercise (yes or no), physical activity (yes or no), fast walking (yes or no), fast eating (yes or no), eating before bedtime (yes or no), eating between meals (yes or no), and skipping breakfast (yes or no). Then, Kaplan-Meier analysis was performed using the lifestyle factors that were significant in Cox proportional hazard regression models. The statistical significance threshold was set at 5 %. Statistical analyses were performed using SPSS 29.0 for Windows (SPSS, Inc., Chicago, IL).
3. Results
3.1. Baseline characteristics of participants
Among the1,713 participants, 420 (24.5 %) developed NAFLD (men 26.1 %, women 23.9 %) during the median observation period of 47 months (median: 44 months for men, 47 months for women). Table 1 shows the baseline characteristics of the participants. In both sexes, compared to the participants without NAFLD, those with NAFLD had a higher body mass index. Moreover, in both sexes, the levels of alanine aminotransferase, gamma-glutamyl transpeptidase and triglycerides were significantly higher in participants with NAFLD than in participants without NALFD. The level of fasting plasma glucose was higher in the female participants with NAFLD than in those without NAFLD. Regarding lifestyle factors, the prevalence of eating between meals was significantly higher in male participants with NAFLD than in those without NAFLD. Among women, the prevalence of eating fast was significantly higher in participants with NAFLD than in participants without NAFLD.
Table 1.
Baseline characteristics and lifestyle of Japanese adult participants who underwent multiple health checkups from June 2013 to the end of March 2018 and were not diagnosed with nonalcoholic fatty liver disease (NAFLD) at the first health checkup at Watari Hospital in Fukushima, Japan.
| Men (4 5 9) |
Women (1254) |
|||||
|---|---|---|---|---|---|---|
| NAFLD-onset group | Non-onset group | p value | NAFLD-onset group | Non-onset group | p value | |
| Participants | 120 | 339 | 300 | 954 | ||
| Age (years) | 64 (55–70) | 68 (55–74) | 0.01 | 64 (56–70) | 64 (53–71) | 0.71 |
| Body mass index (kg/m2) | 23.2 (21.8–24.7) | 21.9 (20.5–23.6) | <0.01 | 23.0 (21.4–24.6) | 20.9 (19.3–22.7) | <0.01 |
| AST (U/L) | 21 (18–25) | 21 (18–25) | 0.83 | 20 (18–24) | 20 (18–23) | 0.16 |
| ALT (U/L) | 20 (16–28) | 18 (15–22) | <0.01 | 17 (14–21) | 15 (13–18) | <0.01 |
| γ-GTP (U/L) | 24 (18–36) | 22 (16–30) | 0.02 | 17 (13–24) | 15 (12–20) | <0.01 |
| Triglycerides (mg/dL) | 93 (69–118) | 80 (67–107) | <0.01 | 89 (67–115) | 75 (56–97) | <0.01 |
| LDL-C (mg/dL) | 122 (106–139) | 114 (96–137) | 0.12 | 126 (109–142) | 122 (103–142) | 0.08 |
| FPG (mg/dL) | 95 (90–100) | 93 (88–99) | 0.18 | 92 (88–100) | 91 (86–97) | <0.01 |
| Smoking (yes) N (%) | 26 (21.7) | 55 (16.2) | 0.23 | 21 (7.0) | 40 (4.2) | 0.07 |
| Exercise (yes) N (%) | 38 (31.7) | 140 (41.3) | 0.08 | 102 (34.0) | 281 (29.5) | 0.16 |
| Physical activity (yes) N (%) | 52 (43.3) | 151 (44.5) | 0.90 | 106 (35.3) | 357 (37.4) | 0.56 |
| Fast walking (yes) N (%) | 57 (45.7) | 174 (51.3) | 0.54 | 144 (48.0) | 500 (52.4) | 0.21 |
| Eating fast (yes) N (%) | 52 (43.3) | 133 (39.2) | 0.50 | 149 (47.3) | 342 (35.8) | <0.01 |
| Eating before bedtime (yes) N (%) | 24 (20.0) | 77 (22.7) | 0.63 | 51 (17.0) | 130 (13.6) | 0.18 |
| Eating between meals (yes) N (%) | 21 (17.5) | 26 (7.7) | <0.01 | 45 (15.0) | 131 (13.7) | 0.65 |
| Skipping breakfast (yes) N (%) | 15 (12.5) | 24 (7.1) | 0.10 | 20 (6.7) | 54 (5.7) | 0.61 |
Values are expressed as the median (interquartile range) or number (percentage). AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GTP, gamma-glutamyl transpeptidase; LDL-C, low-density lipoprotein cholesterol; FPG, fasting plasma glucose.
Smoking status: Current smoker.
Exercise: A habit of engaging in exercise with light sweating for longer than 30 min per session, two times per week, for over a year.
Physical activity: Walking or engaging in any equivalent amount of physical activity for more than 1 h per day.
Fast walking: A faster walking speed than the speed of other individuals of the same age and sex.
Eating fast: A quicker eating speed than that of other individuals.
Eating before bedtime: Eating supper 2 h before bedtime more than three times per week.
Eating between meals: Eating snacks or drinking sweet beverages between meals.
Skipping breakfast: Skipping breakfast more than three times per week.
3.2. Lifestyle factors associated with NAFLD onset
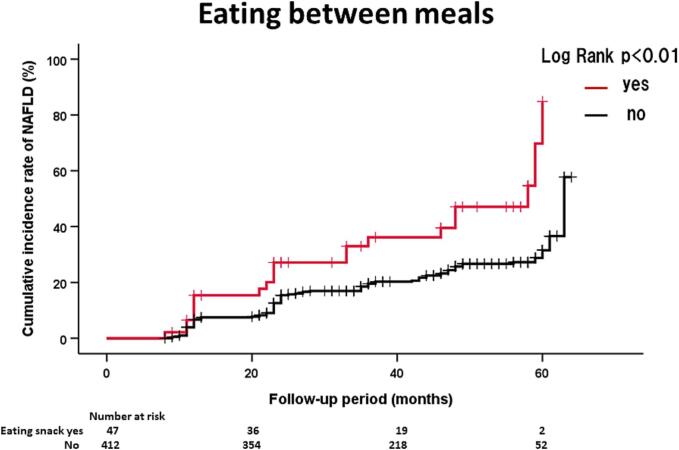
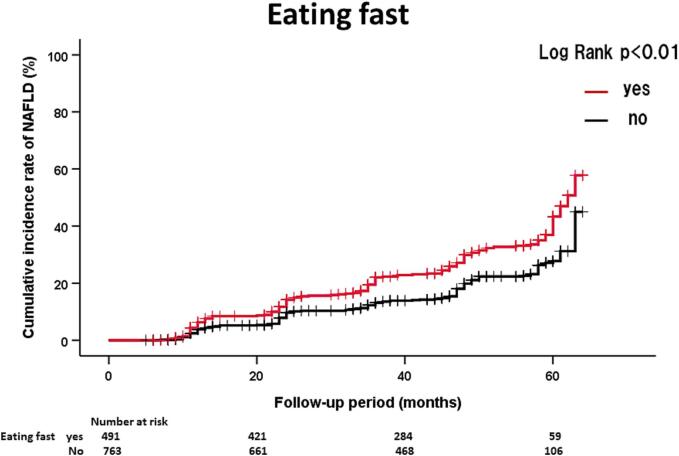
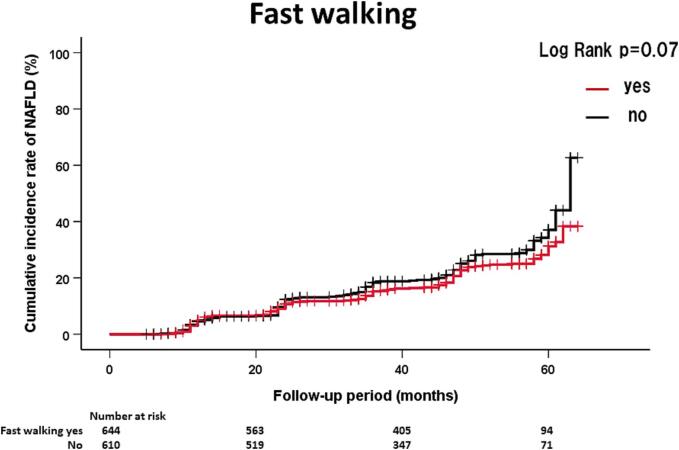
Table 2 shows the HRs and 95 % CIs of significant lifestyle factors influencing NAFLD onset. In men, eating between meals (HR = 2.08, 95 % CI: 1.25–3.45) was significantly associated with NAFLD onset. In women, eating fast (HR = 1.59, 95 % CI: 1.26–2.00) was significantly associated with NAFLD onset. On the other hand, fast walking (HR = 0.76, 95 % CI: 0.60–0.96) was inversely associated with NAFLD onset in women. The above three lifestyle factors were not significant after adjusting for BMI. Fig. 2 shows the details of NAFLD onset in participants with and without the above lifestyle factors. The effects of eating between meals and eating fast on NAFLD onset in men and women, respectively, were also confirmed by the Kaplan-Meier method (Fig. 1).
Table 2.
Cox proportional hazards for nonalcoholic fatty liver disease onset at Watari Hospital in Fukushima, Japan, June 2013 - March 2018.
| Men (4 5 9) |
Women (1,254) |
|||
|---|---|---|---|---|
| Hazard ratio (95 % CI) | p value | Hazard ratio (95 % CI) | p value | |
| Age (1-year increase) | 1.00 (0.98–1.01) | 0.74 | 1.00 (0.99–1.01) | 0.88 |
| Smoking (yes) | 1.11 (0.70–1.11) | 0.66 | 1.30 (0.82–2.09) | 0.27 |
| Exercise (yes) | 0.73 (0.47–1.12) | 0.15 | 1.30 (0.99–1.69) | 0.05 |
| Physical activity (yes) | 1.02 (0.69–1.51) | 0.94 | 0.94 (0.73–1.22) | 0.66 |
| Fast walking (yes) | 0.87 (0.60–1.27) | 0.47 | 0.76 (0.60–0.96) | 0.02 |
| Eating fast (yes) | 1.19 (0.82–1.73) | 0.36 | 1.59 (1.26–2.00) | <0.01 |
| Eating before bedtime (yes) | 0.74 (0.46–1.19) | 0.21 | 1.31 (0.95–1.79) | 0.10 |
| Eating between meals (yes) | 2.08 (1.25–3.45) | <0.01 | 0.99 (0.70–1.38) | 0.93 |
| Skipping breakfast (yes) | 1.30 (0.70–2.43) | 0.41 | 1.27 (0.78–2.06) | 0.34 |
Estimated from Cox proportional hazard models adjusted for all variables.
CI: confidence interval.
Smoking status: Current smoker.
Exercise: A habit of engaging in exercise with light sweating for longer than 30 min per session, two times per week, for over a year.
Physical activity: Walking or engaging in any equivalent amount of physical activity for more than 1 h per day.
Fast walking: A faster walking speed than the speed of other individuals of the same age and sex.
Eating fast: A quicker eating speed than that of other individuals.
Eating before bedtime: Eating supper 2 h before bedtime more than three times per week.
Eating between meals: Eating snacks or drinking sweet beverages between meals.
Skipping breakfast: Skipping breakfast more than three times per week.
Fig. 2.
(A). Kaplan-Meier analysis of NAFLD onset in Japanese men according to eating between meals during 44 months of follow up at Watari Hospital in Fukushima, Japan, June 2013 - March 2018. (B). Kaplan-Meier analysis of NAFLD onset in Japanese women according to eating fast during 47 months of follow up at Watari Hospital in Fukushima, Japan, June 2013 - March 2018. (C). Kaplan-Meier analysis of NAFLD onset in Japanese women according to fast walking during 47 months of follow up at Watari Hospital in Fukushima, Japan, June 2013 - March 2018.
4. Discussion
This study investigated the associations between lifestyle factors and NAFLD onset using a longitudinal approach. We found that participants with suboptimal metabolic factors such as a higher BMI, lipidemia, glycemia and higher levels of hepatobiliary enzymes tended to develop NAFLD. Moreover, eating between meals in men and eating fast and a fast walking speed in women were independently associated with NAFLD onset.
The association between NAFLD and eating fast has been previously reported; however, most of the associations were based on cross-sectional studies (Lee et al., 2016) (Cao et al., 2020) (Takahashi et al., 2020) or evaluations at the time of NAFLD onset (Nishi et al., 2016). In this study, we found that eating fast was associated with future NAFLD onset in women independent of other lifestyle factors, including physical activity. Although the precise reason why fast eating causes NAFLD is unclear, NAFLD-associated factors such as appetite or GLP-1 kinetics are influenced by eating speed, including chewing(Kokkinos et al., 2010) (Sakata et al., 2003).
A previous report showed that a lower frequency of daily food intake was associated with NAFLD (Trovato et al., 2016). However, total energy intake was not evaluated in this study; therefore, subjects with NAFLD may consume more energy than subjects without NAFLD. Another study reported that daily eating frequency was positively associated with total energy intake (Holmbäck et al., 2010). Moreover, a randomized controlled trial showed that a high meal frequency increases intrahepatic triglyceride content (Koopman et al., 2014). These findings may reinforce the association between eating between meals and NAFLD onset in men in this study. The differences in the impacts of two eating habits, eating between meals and eating fast on NAFLD onset in men and women are unknown; however, the significantly higher proportions of participants with both eating habits who develop NAFLD may be a reason for the differences.
Regular physical activity and exercise are recommended treatments for NAFLD (Kamada et al., 2021, Younossi et al., 2021). In this study, only fast walking was associated with NAFLD onset in women; physical activity and exercise were not associated. There are several explanations for this. First, the amounts of exercise and physical activity could not be evaluated in this study using a simple lifestyle questionnaire; thus, the participants’ exercise intensity may not have been sufficient for the prevention of NAFLD onset. Second, participants at a risk for metabolic disease, including NAFLD, may have started exercise or physical activity to prevent NAFLD onset at the time of the questionnaire. In fact, the median levels of blood glucose in the participants with regular exercise habits were higher than those in the participants without exercise habits (92.5 vs. 91.0 mg/dL, p = 0.04). Third, fast walking is categorized as moderate-intensity exercise (Ainsworth et al., 2000). Walking speed was correlated with participation in high-intensity physical activity, a higher exercise frequency and total walking volume (Centers for Disease Control and Prevention, 2000) (Stamatakis et al., 2018). Interestingly, a previous review suggested that resistance exercise was more effective in female NAFLD patients than in male NAFLD patients (Hashida et al., 2017). Moreover, a previous large Japanese cohort study reported that fast walking is a protective factor against new-onset diabetes mellitus (Iwasaki et al., 2021). Accordingly, these findings are appropriate to explain the association between fast walking and NAFLD onset in women. Although we could not confirm the association between fast walking and NAFLD onset by Kaplan-Meier analysis, this result could be attributed to the small sample size for each event.
There are several limitations in this study. First, the lifestyle questionnaire in this study was simple and self-reported; thus, quantities of exercise and food intake were uncertain, and walking pace was subjective. Evaluation of these quantities is needed to clarify the mechanism of the effects of lifestyle factors on NAFLD onset. Second, we evaluated lifestyle factors at baseline in this study. Therefore, lifestyle factors may have changed at the time of NAFLD onset. Analysis of lifestyle changes up to NAFLD onset may help to clarify the significant impact of lifestyle factors on NAFLD onset. However, the lifestyle evaluations used in this study are useful for predicting future NAFLD onset. Third, this study was based on the results of a single center, and the observation period was relatively short. Therefore, our findings need to be validated by large-scale and long-term studies in the future. Fourth, we did not identify lifestyle factors that significantly increased the risk of NAFLD onset after adjusting BMI. This could be due to the strong association between NAFLD and obesity (Semmler et al., 2021) (Fan et al., 2017). On the other hand, genetic predispositions (Salari et al., 2021) and gut microbiota (Gudan et al., 2022) also affect NAFLD; therefore, lifestyle evaluations including various risk factors for NAFLD are essential for developing personalized lifestyle interventions to prevent NAFLD onset. Fifth, we focused only on NAFLD onset in this study. The evaluation of the association of NAFLD with metabolic comorbidities such as diabetes, hypertension, and dyslipidemia is an important task which should be performed in more large-scale cohort analyses.
5. Conclusion
In conclusion, eating between meals and eating fast were risk factors for NAFLD onset in men and women, respectively. On the other hand, fast walking was a protective factor against NAFLD onset in women. Lifestyle modifications considering these three lifestyle factors may help to prevent future NAFLD onset.
Financial disclosure
This research did not receive any grants.
Declaration of Generative AI and AI-assisted technologies in the writing process.
We did not use generative AI and AI-assisted technologies in writing this manuscript.
CRediT authorship contribution statement
Yosuke Takahata: Writing – review & editing, Methodology, Investigation, Conceptualization. Atsushi Takahashi: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Data curation, Conceptualization. Yukio Anzai: Methodology, Investigation, Conceptualization. Naoto Abe: Writing – review & editing, Validation, Conceptualization. Tatsuro Sugaya: Writing – review & editing, Validation, Conceptualization. Masashi Fujita: Writing – review & editing, Validation, Conceptualization. Manabu Hayashi: Writing – review & editing, Validation, Conceptualization. Kazumichi Abe: Writing – review & editing, Validation, Conceptualization. Hiromasa Ohira: Writing – review & editing, Supervision, Methodology, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge all staff of Watari Hospital Health Center. No grants were received to accomplish this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2023.102577.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Ainsworth B.E., Haskell W.L., Whitt M.C., Irwin M.L., Swartz A.M., Strath S.J., O'Brien W.L., Bassett D.R., Jr., Schmitz K.H., et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Cao X., Gu Y., Bian S., Zhang Q., Meng G., Liu L., Wu H., Zhang S., Wang Y., et al. Association between eating speed and newly diagnosed nonalcoholic fatty liver disease among the general population. Nutr Res. 2020;80:78–88. doi: 10.1016/j.nutres.2020.06.012. [DOI] [PubMed] [Google Scholar]
- Choi H.I., Lee M.Y., Kim H., Oh B.K., Lee S.J., Kang J.G., Lee S.H., Kim B.J., Kim B.S., et al. Effect of physical activity on the development and the resolution of nonalcoholic fatty liver in relation to body mass index. BMC Public Health. 2022;22:655. doi: 10.1186/s12889-022-13128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes C., Anstee Q.M., Arias-Loste M.T., Bantel H., Bellentani S., Caballeria J., Colombo M., Craxi A., Crespo J., et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- Fan J.G., Kim S.U., Wong V.W. New trends on obesity and NAFLD in Asia. J Hepatol. 2017;67:862–873. doi: 10.1016/j.jhep.2017.06.003. [DOI] [PubMed] [Google Scholar]
- Gudan, A., Jamioł-Milc, D., Hawryłkowicz, V., Skonieczna-Żydecka, K., Stachowska, E., 2022. The Prevalence of Small Intestinal Bacterial Overgrowth in Patients with Non-Alcoholic Liver Diseases: NAFLD, NASH, Fibrosis, Cirrhosis-A Systematic Review, Meta-Analysis and Meta-Regression. Nutrients 14. [DOI] [PMC free article] [PubMed]
- Hashida R., Kawaguchi T., Bekki M., Omoto M., Matsuse H., Nago T., Takano Y., Ueno T., Koga H., et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66:142–152. doi: 10.1016/j.jhep.2016.08.023. [DOI] [PubMed] [Google Scholar]
- Holmbäck I., Ericson U., Gullberg B., Wirfält E. A high eating frequency is associated with an overall healthy lifestyle in middle-aged men and women and reduced likelihood of general and central obesity in men. Br J Nutr. 2010;104:1065–1073. doi: 10.1017/S0007114510001753. [DOI] [PubMed] [Google Scholar]
- Iwasaki M., Kudo A., Asahi K., Machii N., Iseki K., Satoh H., Moriyama T., Yamagata K., Tsuruya K., et al. Fast walking is a preventive factor against new-onset diabetes mellitus in a large cohort from a Japanese general population. Sci Rep. 2021;11:716. doi: 10.1038/s41598-020-80572-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y., Takahashi H., Shimizu M., Kawaguchi T., Sumida Y., Fujii H., Seko Y., Fukunishi S., Tokushige K., et al. Clinical practice advice on lifestyle modification in the management of nonalcoholic fatty liver disease in Japan: an expert review. J Gastroenterol. 2021;56:1045–1061. doi: 10.1007/s00535-021-01833-9. [DOI] [PubMed] [Google Scholar]
- Keating S.E., Hackett D.A., George J., Johnson N.A. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157–166. doi: 10.1016/j.jhep.2012.02.023. [DOI] [PubMed] [Google Scholar]
- Kokkinos A., le Roux C.W., Alexiadou K., Tentolouris N., Vincent R.P., Kyriaki D., Perrea D., Ghatei M.A., Bloom S.R., et al. Eating slowly increases the postprandial response of the anorexigenic gut hormones, peptide YY and glucagon-like peptide-1. J Clin Endocrinol Metab. 2010;95:333–337. doi: 10.1210/jc.2009-1018. [DOI] [PubMed] [Google Scholar]
- Koopman K.E., Caan M.W., Nederveen A.J., Pels A., Ackermans M.T., Fliers E., la Fleur S.E., Serlie M.J. Hypercaloric diets with increased meal frequency, but not meal size, increase intrahepatic triglycerides: a randomized controlled trial. Hepatology. 2014;60:545–553. doi: 10.1002/hep.27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Ko B.J., Gong Y., Han K., Lee A., Han B.D., Yoon Y.J., Park S., Kim J.H., et al. Self-reported eating speed in relation to non-alcoholic fatty liver disease in adults. Eur J Nutr. 2016;55:327–333. doi: 10.1007/s00394-015-0851-z. [DOI] [PubMed] [Google Scholar]
- Nishi T., Babazono A., Maeda T., Imatoh T., Une H. Effects of eating fast and eating before bedtime on the development of nonalcoholic fatty liver disease. Popul Health Manag. 2016;19:279–283. doi: 10.1089/pop.2015.0088. [DOI] [PubMed] [Google Scholar]
- Sakata T., Yoshimatsu H., Masaki T., Tsuda K. Anti-obesity actions of mastication driven by histamine neurons in rats. Exp Biol Med (Maywood) 2003;228:1106–1110. doi: 10.1177/153537020322801002. [DOI] [PubMed] [Google Scholar]
- Salari N., Darvishi N., Mansouri K., Ghasemi H., Hosseinian-Far M., Darvishi F., Mohammadi M. Association between PNPLA3 rs738409 polymorphism and nonalcoholic fatty liver disease: a systematic review and meta-analysis. BMC Endocr Disord. 2021;21:125. doi: 10.1186/s12902-021-00789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmler G., Datz C., Reiberger T., Trauner M. Diet and exercise in NAFLD/NASH: beyond the obvious. Liver Int. 2021;41:2249–2268. doi: 10.1111/liv.15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis E., Kelly P., Strain T., Murtagh E.M., Ding D., Murphy M.H. Self-rated walking pace and all-cause, cardiovascular disease and cancer mortality: individual participant pooled analysis of 50 225 walkers from 11 population British cohorts. Br J Sports Med. 2018;52:761–778. doi: 10.1136/bjsports-2017-098677. [DOI] [PubMed] [Google Scholar]
- Takahashi F., Hashimoto Y., Kawano R., Kaji A., Sakai R., Kawate Y., Okamura T., Ushigome E., Kitagawa N., et al. Eating fast is associated with nonalcoholic fatty liver disease in men but not in women with type 2 diabetes: a cross-sectional study. Nutrients. 2020;12 doi: 10.3390/nu12082174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokushige K., Ikejima K., Ono M., Eguchi Y., Kamada Y., Itoh Y., Akuta N., Yoneda M., Iwasa M., et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis 2020. J Gastroenterol. 2021;56:951–963. doi: 10.1007/s00535-021-01796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trovato F.M., Martines G.F., Brischetto D., Catalano D., Musumeci G., Trovato G.M. Fatty liver disease and lifestyle in youngsters: diet, food intake frequency, exercise, sleep shortage and fashion. Liver Int. 2016;36:427–433. doi: 10.1111/liv.12957. [DOI] [PubMed] [Google Scholar]
- Younossi Z.M., Koenig A.B., Abdelatif D., Fazel Y., Henry L., Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- Younossi Z.M., Corey K.E., Lim J.K. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2021;160:912–998. doi: 10.1053/j.gastro.2020.11.051. [DOI] [PubMed] [Google Scholar]
- Zelber-Sagi S., Ratziu V., Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377–3389. doi: 10.3748/wjg.v17.i29.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.