Abstract
Bordetella pertussis induces in vitro apoptosis of murine alveolar macrophages by a mechanism that is dependent on expression of bacterial adenylate cyclase-hemolysin. Using a murine respiratory model, we found in this study that intranasal infection with a parental B. pertussis strain, but not with an isogenic variant deficient in the expression of all toxins and adhesins, induced a marked neutrophil accumulation in the bronchoalveolar lavage fluid and an early decrease in macrophage numbers. These phenomena paralleled a time-dependent rise in the proportion of apoptotic nuclei, as detected by flow cytometry, and of macrophages which had engulfed apoptotic bodies. Apoptotic death of bronchopulmonary cells was observed exclusively following intranasal infection with bacteria reisolated from lungs of infected animals and not with B. pertussis collected after in vitro subculture. Using the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling technique coupled to fluorescence microscopy and morphological analysis, we established that the apoptotic cells in bronchoalveolar lavage fluids were neutrophils and macrophages. Histological analysis of the lung tissues from B. pertussis-infected mice showed increased numbers of apoptotic cells in the alveolar compartments. Cellular accumulation in bronchoalveolar lavage fluids and apoptosis of alveolar macrophages were significantly attenuated in mice infected with a mutant deficient in the expression of adenylate cyclase-hemolysin, indicating a role of this enzyme in these processes.
Most Bordetella species, such as Bordetella pertussis, the agent of whooping cough, induce respiratory infection and colonize the upper respiratory tracts of their hosts. B. pertussis differs from other bacterial pathogens in that it produces numerous adhesins and toxins with similar functions involved in the pathogenesis of the disease. Regulation of expression of these proteins is influenced by environmental conditions. Although the functions of some of these proteins have been analyzed in studies using mutants of B. pertussis, the purified proteins themselves, cell cultures, and murine respiratory models, the pathogenesis of whooping cough remains poorly understood (9, 17).
Once in the respiratory tract, B. pertussis expresses and secretes many proteins implicated in its adhesion to the host ciliated cells. These adhesins include filamentous hemagglutinin, pertactin, fimbriae, tracheal colonization factor, and pertussis toxin (PT) (9, 17). It is hypothesized that these adhesins act either sequentially or synergistically during the course of the disease. After adhesion and multiplication, B. pertussis secretes numerous toxins, such as tracheal cytotoxin, PT, and adenylate cyclase-hemolysin (AC-Hly), which are responsible for the tissue lesions and destruction of the host cells (9, 17). Thus, the tracheal cytotoxin, a muramyl peptide, is involved in the initiation of the disease and causes ciliostasis, while PT, an ADP-ribosylating toxin, promotes inflammation and leukocytosis in vivo. Finally, AC-Hly, a protein belonging to the repeat-in-toxin family, is able to enter phagocytic cells, to synthesize high amounts of cyclic AMP, and to disrupt cellular functions (3, 18, 25). Along with filamentous hemagglutinin, AC-Hly was recently shown to be necessary for B. pertussis to inhibit monocyte-dependent T-cell proliferation in vitro (2). However, the exact roles of the two toxins AC-Hly and PT during the disease have not been fully elucidated.
Using a murine respiratory model, we demonstrated previously that intranasal (i.n.) infection with B. pertussis results in lung lesions, characterized by bronchopneumonia and alveolitis, as well as in neutrophil, macrophage, and lymphocyte accumulation in bronchoalveolar lavage (BAL) fluid. Infection with mutants deficient in PT or AC-Hly failed to promote cell influx and tissue injury, suggesting that both toxins are involved in these phenomena (10). Compelling evidence has established that bacterial pathogens may overcome natural defenses and cause disease by killing the immune and phagocytic cells by apoptosis (1, 24, 26). This phenomenon, characterized by DNA fragmentation, chromatin condensation, cell shrinkage, and formation of apoptotic bodies, which are engulfed by phagocytic cells (8), has been implicated in a number of diseases, such as viral, parasitic, and bacterial infections (1, 24, 26, 27). Indeed, apoptosis of defense cells may either allow the bacteria to escape host microbicidal abilities, thus prolonging pathogen survival (24) or contribute by itself to the initiation of the inflammatory process, as recently proposed (26, 27).
Most of the findings showing apoptosis of host cells during bacterial infection have been obtained in vitro; only a few studies have focused on the emergence of this phenomenon in vivo. Accordingly, Shigella flexneri and Listeria monocytogenes were shown to promote apoptotic death in vivo of macrophages, B cells, and T cells and of hepatocytes and lymphocytes, respectively (15, 19, 28). Using different mutants, we established previously that B. pertussis induces in vitro apoptosis of alveolar macrophages (13) by a mechanism depending exclusively on AC-Hly production (11).
This study was designed to verify whether B. pertussis infection also promotes apoptotic death of bronchopulmonary cells in vivo and identify the cell type(s) involved in this process. Using flow cytometry and the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) technique coupled to fluorescence microscopy and morphological analysis, we demonstrated the presence of apoptotic neutrophils and macrophages in the BAL fluid of mice infected with bacteria cultured under specific growth conditions. The use of mutants deficient in the expression of all toxins and adhesins or of AC-Hly indicates that the latter protein is involved in B. pertussis-induced lung inflammation and in apoptotic death of alveolar macrophages.
MATERIALS AND METHODS
B. pertussis strains and culture conditions.
The strains used in this study were the parental strain B. pertussis 18323 (16), B. pertussis 18323H− (13), a variant deficient in the expression of all adhesins and toxins, and B. pertussis 18HS19 (12), a mutant deficient in the expression of AC-Hly. B. pertussis strains were freshly isolated from lungs of infected mice, from days 5 to 7 after infection, as described previously (7). After growth on Bordet-Gengou agar medium supplemented with 1% glycerol and 15% defibrinated sheep blood (BG) at 36°C for 72 h to visualize hemolysis, bacteria were plated for an additional 24 h before each experiment and then grown in Stainer-Scholte liquid medium (21) for 20 h at 36°C until the optical density measured at 650 nm reached 1.0. Bacterial suspensions prepared under these culture conditions are defined as freshly isolated B. pertussis. In selected experiments, B. pertussis was administered to mice after three subcultures in vitro, conditions defined as in vitro cultures.
Adenylate cyclase assay.
Adenylate cyclase activity was measured on bacterial suspensions as described previously (14). One unit corresponds to 1 nmol of cAMP formed per min at 30°C and pH 8.0.
i.n. infections.
Bacteria, grown as described above, were resuspended in Stainer-Scholte liquid medium. Fifty-microliter doses of bacterial suspensions containing 1.5 × 106 bacteria were instilled via the i.n. route to groups of 3- to 4-week-old female BALB/c mice (CERJ, St. Berthevin, France) under light ether anesthesia. Animals were sacrificed at 2, 7, 14, and 21 days after infection for BAL cell counts, for determination of alveolar macrophages which had ingested apoptotic cells or bodies, and for identification of apoptotic cells in BAL fluids and lung tissue sections as described below.
For studying bacterial colonization in the lungs, infected mice were sacrificed by cervical dislocation at 1 h (designated day 0) and 3, 6, 13, and 21 days after exposure. The lungs were removed and homogenized in saline with tissue grinders. Dilutions of lung homogenates were plated on BG, and CFU were counted after 3 to 4 days of incubation at 36°C.
Cellular analysis of BAL fluids.
To examine the cellular contents of BAL fluids, infected mice were anesthetized with 1.8 mg of ethyl urethane (Sigma Chemical Co., St. Louis, Mo.) per kg of body weight, the tracheae were cannulated, and BAL fluids were collected by eight successive washes with 0.5-ml aliquots of phosphate-buffered saline (PBS; pH 7.2). The total cell numbers were determined with a Coulter ZM counter (Coultronics, Margency, France). Aliquots of cell suspensions were diluted to a final concentration of 8 × 104 cells/ml, and cytospin (Hettich Universal, Tuttingen, Germany) preparations were made. Slides were stained with Diff-Quick dye (Merz-Dade, Baxter Dade, Duedingen, Switzerland) and examined at a magnification of ×100 by oil immersion light microscopy. The percentages of neutrophils, macrophages, and lymphocytes were determined after counting 300 cells in randomly selected fields. Results are expressed as number of each cell population/milliliter of BAL fluid. The slides were further examined in a blind fashion to establish the proportions of macrophages which had engulfed intact apoptotic cells or their apoptotic bodies, determined by counting approximately 500 alveolar macrophages in randomly selected fields.
In vitro and in vivo assessment of apoptosis.
Apoptotic cells were detected in the BAL fluid from infected mice by using an Apopdetek kit (Enzo Diagnostics, Farmingdale, N.Y.), which is based on the TUNEL technique (6). Briefly, dUTP-conjugated biotin and terminal transferase were applied on cytospin preparations previously fixed during 10 min in acetone and air dried according to the manufacturer’s instructions. Slides were then incubated with fluorescein isothiocyanate-conjugated streptavidin and counterstained with Evans blue dye. The percentages of total apoptotic cells were determined after counting 500 to 1,000 cells/slide in randomly selected fields, using an oil immersion fluorescence microscope at a magnification of ×100. In parallel, the proportions of apoptotic neutrophils and alveolar macrophages over the total number of each cell type were determined. Apoptosis was also evaluated by flow cytometry using a previously described technique (5). Briefly, 3 × 105 BAL cells were washed in PBS containing 2% fetal calf serum (Boehringer Mannheim, Mannheim, Germany) by centrifugation at 400 × g for 10 min at 4°C. Cell pellets were fixed with 1% paraformaldehyde, permeabilized with a 0.2% Tween 20 solution in PBS, and incubated for 30 min in PBS containing 2% fetal calf serum, 10 μg of propidium iodide (Sigma) per ml, 5 mM EDTA, and 5 μg of RNase A (Boehringer Mannheim) per ml. Samples were then applied to a FACScan flow cytometer (Becton Dickinson, Le Pont de Claix, France). A total of 10,000 events were analyzed with LYSIS II software (Becton Dickinson). Results are expressed as percentage of apoptotic nuclei (low-propidium iodide-stained nuclei).
In separate experiments, mice were anesthesized as described above and exsanguinated via the abdominal aorta. The lungs were inflated by injecting into the trachea 0.7 ml of a solution of optimum cutter temperature compound (OCT; BDH, Poole, England) in PBS (by volume). The lobes were dissected and mounted over cork disks, covered by OCT, and snap-frozen in isopentane (Prolabo, Paris, France). The frozen blocks were kept at −80°C until required. Sections (5 μm) alongside the main intrapulmonary bronchus were cut in a cryostat kept at −21°C and collected on glass slides previously coated with 3-aminopropyltriethoxysilane (Sigma). They were then fixed in acetone for 10 min, air dried, wrapped in a plastic film, and kept at −20°C until use. In situ apoptosis was determined by the TUNEL technique as described above except that slides were incubated with streptavidin-conjugated alkaline phosphatase followed by substrate solution consisting of 0.48 mM naphthol AS-XM phosphate, 2% dimethylformamide, 100 mM Tris (pH 8.2), 1.3 mM levamisole, and 194 mM fast blue salt BB (all from Sigma), with nuclear red counterstaining.
Statistical analysis.
Results are expressed as means ± standard error of the means (SEM) of the indicated number of experiments. One-way analysis of variance was used to determine difference among the groups. If a significant variance was found, an unpaired Student t test was used to assess comparability between means. P values of ≤0.05 were considered significant.
RESULTS
Changes in cellular composition of BAL fluid induced by B. pertussis respiratory infection.
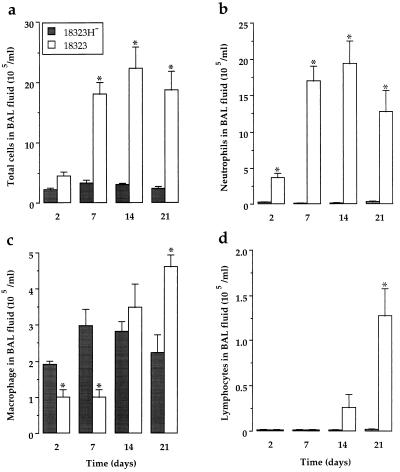
The i.n. infection with freshly isolated parental strain B. pertussis 18323 induced a time-dependent increase in total cell numbers in the BAL fluid of BALB/c mice (Fig. 1a). This recruitment, which was observed as early as 2 days after infection and lasted for 3 weeks, was mostly accounted for by a rise in neutrophil counts (Fig. 1b). A significant decrease in alveolar macrophage numbers was also observed 2 and 7 days after parental strain 18323 infection (Fig. 1c). These changes were followed by a late (14 to 21 days) and moderate increment in macrophage and lymphocyte counts (Fig. 1c and d). Infection with adhesin- and toxin-deficient variant 18323H− failed to modify the cellular composition of BAL fluid at any time point (Fig. 1). The intensity of cellular recruitment depends on bacterial growth conditions. Indeed, infection with in vitro cultures of B. pertussis was followed after 7 days by an increase in total cell and in neutrophil counts which was lower than that observed with freshly isolated bacteria. At this time point, numbers of total cells and of neutrophils in BAL fluids from mice infected with in vitro cultures were 7.7 ± 2.2 and 4.7 ± 0.2 (n = 3), respectively, compared to 18.0 ± 2.0 and 17.0 ± 2.0 (n = 9, P < 0.05), after infection with freshly isolated parental strain 18323.
FIG. 1.
Cellular distribution in BAL fluids from B. pertussis-infected mice. BAL fluids were collected from mice infected via the i.n. route with 1.5 × 106 CFU of parental strain 18323 or variant 18323H− and killed at different time points after the infection. Numbers of total cells (a), neutrophils (b), macrophages (c), and lymphocytes (d) were determined after cytocentrifugation and staining with Diff-Quick dye. Results are expressed as means ± SEM of six to nine experiments at each time point. ∗, P < 0.05 compared to variant 18323H−-infected mice.
Sustained colonization of B. pertussis within the lung after i.n. delivery in mice.
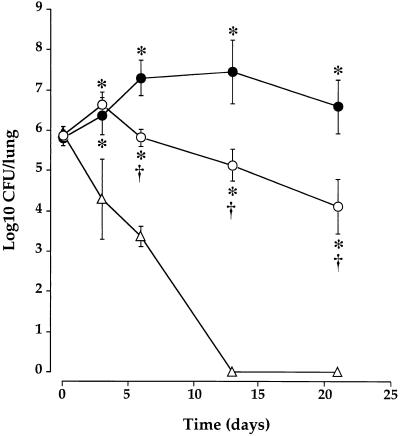
The difference observed in the intensity of cellular recruitment is correlated with the behavior of the strain after infection of the respiratory tract. As shown in Fig. 2 and as generally observed after infection (10, 12), B. pertussis adheres to, multiplies in, and colonizes mouse lungs, whereas a variant deficient in the expression of toxins and adhesins fails to multiply and is cleared much faster after infection (10). However, as shown in Fig. 2, when mice were infected with freshly isolated bacteria, we observed a longer bacterial persistence in lungs compared to strains subcultured in vitro (10). Furthermore, the freshly cultured mutant deficient in AC-Hly expression, 18HS19, multiplies until day 3 in lungs of infected mice, whereas it failed to do so when subcultured in vitro (12). However, this mutant did not colonize lungs for as long as the parental strain and is cleared faster, confirming previous data (12).
FIG. 2.
B. pertussis colonization of the lungs of mice. Mice were challenged i.n. with 1.5 × 106 CFU of freshly isolated parental strain 18323 (•), variant 18323H− (▵), and mutant strain 18HS19 (○). The plots show means ± SEM of 3 to 10 experiments at each time point. ∗, P < 0.05 compared to variant 18323H−-infected mice. †, P < 0.05 compared to parental strain 18323-infected mice.
Kinetics of apoptosis in BAL cells collected from B. pertussis-infected mice.
A time-dependent rise in the proportion of apoptotic nuclei was detected by flow cytometry in BAL cells collected from mice infected with freshly isolated B. pertussis 18323 (Table 1). This increase was observed as early as 2 days after the infection, peaked at 14 days, and decreased slowly thereafter. No significant changes were observed in BAL cells from freshly isolated variant 18323H−-infected mice (Table 1). The kinetics of cellular apoptosis paralleled that of leukocyte accumulation in BAL fluids (Table 1; Fig. 1a and b).
TABLE 1.
Kinetics of apoptosis in BAL fluid and lung tissue of B. pertussis-infected mice
| Days after infection | Mean ± SEM (n = 3–12)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| BAL fluid
|
Lung tissue, apoptotic cellsc
|
||||||||
| Apoptotic nuclei (%)a
|
Phagocytosis (%)b
|
||||||||
| 18323H− | 18323 | 18HS19 | 18323H− | 18323 | 18HS19 | 18323H− | 18323 | 18HS19 | |
| 2 | 4.3 ± 0.9 | 7.8 ± 0.5 | 3.3 ± 0.3 | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.1 ± 0.1 | 29.3 ± 5.8 | 86.3 ± 39.4 | NDd |
| 7 | 1.8 ± 0.1 | 13.3 ± 2.1e | 6.3 ± 0.7e,f | 0.3 ± 0.1 | 1.3 ± 0.3e | 1.2 ± 0.4e | 79.2 ± 11.1 | 290.7 ± 84.4e | 74.7 ± 18.5f |
| 14 | 2.5 ± 0.6 | 17.1 ± 3.8e | 1.4 ± 0.1e | 0.4 ± 0.1 | 5.3 ± 1.2e | 0.3 ± 0.1f | 44.0 ± 21.4 | 343.7 ± 108.7e | 23.3 ± 5.6f |
| 21 | 2.4 ± 0.4 | 10.7 ± 1.7e | ND | 0.3 ± 0.1 | 6.0 ± 1.8e | ND | 38.3 ± 19.9 | 52.7 ± 24.9 | ND |
Detected by flow cytometry.
Proportion of alveolar macrophages ingesting apoptotic cells or their bodies (light microscopy).
Number of apoptotic cells in 20 fields of the alveolar compartments (TUNEL technique).
ND, not determined.
P < 0.05 compared to 18323H−-infected mice.
P < 0.05 compared to 18323-infected mice.
The i.n. administration of in vitro cultures of parental strain 18323 failed to induce changes in apoptotic cell numbers in the BAL fluid, since only 3.2% ± 0.3% (n = 3) of bronchopulmonary cells were apoptotic.
The proportion of alveolar macrophages which had ingested apoptotic cells or bodies was then determined at the same time points. Microscopic analysis on cytospin preparations showed increased numbers of macrophages containing apoptotic bodies, which started 7 days after infection and reached a plateau between 14 and 21 days (Table 1). No significant changes in the proportion of macrophages engulfing apoptotic cells were noted in BAL cells from freshly isolated variant 18323H−-infected mice (Table 1).
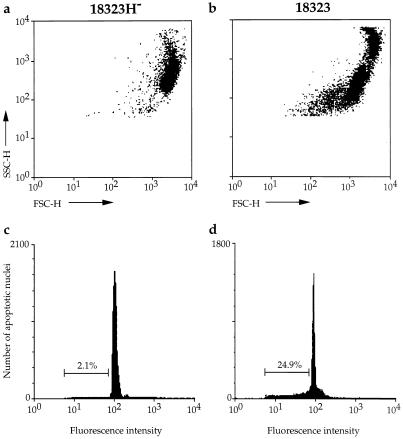
Changes in the morphology of cells undergoing apoptosis affect their light scattering properties, as shown in flow cytometry profiles (Fig. 3a and b). Indeed, a decrease in forward scatter occurred in BAL cells from mice infected with parental strain 18323 compared to those from animals infected with the freshly isolated variant 18323H− (Fig. 3a and b). Propidium iodide-fluorescence analysis of BAL cells from mice infected with the freshly isolated variant 18323H− disclosed a typical sharp diploid DNA peak and an minor more flattened one in the hypodiploid range, corresponding to the apoptotic nuclei (Fig. 3c). In contrast, BAL cells collected from animals infected with freshly isolated parental strain 18323 contained a reduced proportion of nuclei with diploid DNA content and an enhanced proportion of apoptotic nuclei (Fig. 3d).
FIG. 3.
Flow cytometry analysis of apoptosis in BAL cells from B. pertussis-infected mice. Changes in light scattering properties (a and b) and DNA fluorescence flow cytometry profiles (c and d) of BAL cells collected 14 days after infection with variant 18323H− (a and c) or with parental strain 18323 (b and d). Percentages values of apoptotic nuclei for each experimental condition are indicated in the corresponding panels.
Identification of apoptotic cells in BAL fluids from B. pertussis-infected mice.
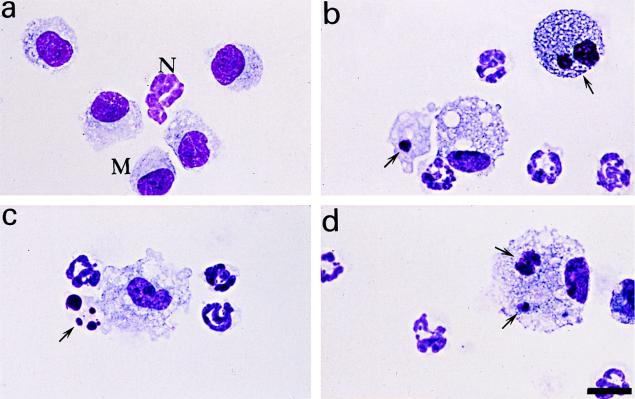
Microscopic analysis of BAL fluids from mice infected with variant 18323H− revealed normal morphology of alveolar macrophages and neutrophils (Fig. 4a). In contrast, BAL cells from 18323-infected mice showed several of the characteristic features of apoptosis, including chromatin condensation and cell shrinkage (data not shown). In some cases, the apoptotic process reached late stages accompanied by an intense cell damage and by a loss of the nucleus morphology (Fig. 4b and c). Macrophage engulfment of apoptotic cells or bodies was also occasionally noted (Fig. 4d).
FIG. 4.
Morphological analysis of BAL cells after B. pertussis infection. BAL fluids were collected from mice infected with the parental strain 18323 or with variant 18323H−, and cytospin preparations were made. Slides were stained with Diff-Quick dye and examined in an oil immersion light microscope. (a) Normal morphology of macrophages (M) and a neutrophil (N) after infection with variant 18323H−. Also shown are apoptotic macrophages (arrows; b) and neutrophil (c) after infection with the parental strain 18323 and macrophage engulfment of an apoptotic cell and of an apoptotic body (arrows) after infection with the parental strain 18323 (d). Scale bar, 12 μm.
Apoptotic death of BAL cells was confirmed by using the TUNEL technique and fluorescence microscopy. Significant higher proportions of apoptotic cells were observed after infection with parental strain 18323, compared to variant 18323H−, at all time points (Table 2). By combining this technique and morphological analysis, we established that in vivo infection with parental strain 18323, but not with variant 18323H−, induced both neutrophil and alveolar macrophages apoptosis (Fig. 5; Table 2). The proportion of apoptotic neutrophils in BAL fluids rose at all time points and reached values ranging between 6.8 and 10.9% of total neutrophil numbers (Table 2). Apoptotic macrophages also increased in number after parental strain 18323 infection, and a peak of approximately 45.5% of total macrophage counts was observed at 14 days (Table 2).
TABLE 2.
Identification of apoptotic cells in BAL fluids from B. pertussis-infected mice
| Days after infection | Apoptotic cells in BAL fluids (%)a
|
|||||
|---|---|---|---|---|---|---|
|
B. pertussis 18323H−
|
B. pertussis 18323
|
|||||
| Total cells | Neutrophils | Macrophages | Total cells | Neutrophils | Macrophages | |
| 2 | 3.9 ± 0.7 | 3.3 ± 0.9 | 3.9 ± 0.9 | 11.6 ± 1.0b | 8.1 ± 1.7b | 25.9 ± 3.6b |
| 7 | 2.7 ± 0.2 | 0 | 2.7 ± 0.2 | 11.9 ± 0.8b | 10.9 ± 0.5b | 40.0 ± 2.4b |
| 14 | 3.1 ± 0.3 | 0 | 3.2 ± 0.3 | 12.4 ± 2.7b | 6.8 ± 2.8b | 45.5 ± 10.4b |
| 21 | 2.8 ± 0.3 | 0 | 2.9 ± 0.3 | 10.9 ± 1.8b | 9.0 ± 2.8b | 17.3 ± 1.2b |
Apoptotic cells were detected in BAL fluids by the TUNEL technique. Percentages of total apoptotic cells were determined after counting 500 to 1,000 cells/slide in randomly selected fields, using an oil immersion fluorescence microscope. In parallel, percentages of apoptotic neutrophils and alveolar macrophages over the total number of each cell type were determined. Results are expressed as means ± SEM of 6 to 12 experiments.
P < 0.05 compared to B. pertussis 18323H−-infected animals.
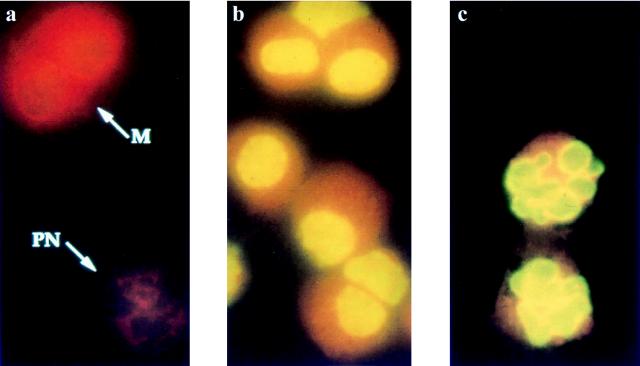
FIG. 5.
Identification of apoptotic cells in BAL fluids from B. pertussis-infected mice. Cytospin preparations of BAL cells were stained by the TUNEL technique combined with fluorescence microscopy; fluorescein isothiocyanate was used for detection. (a) Neutrophils (PN) and macrophages (M) show normal red nuclei after infection with variant 18323H− (arrows). Macrophages (b) and neutrophils (c) contain apoptotic green nuclei after infection with the parental strain 18323. Magnification, ×222.
Detection of apoptotic cells in the lung tissue from B. pertussis-infected mice.
To verify whether the results for BAL cells have a tissue counterpart, in situ apoptosis detection by the TUNEL technique was performed on lung sections. A significant rise in the number of apoptotic nuclei was observed in parental strain 18323-infected, compared to variant 18323H−-infected, mice at 7 and 14 days (Table 1; Fig. 6). Although an intense peribronchial or perivascular inflammatory infiltrate was observed after administration of parental strain 18323 (data not shown), apoptotic cells were enumerated exclusively in the alveolar compartment (Fig. 6).
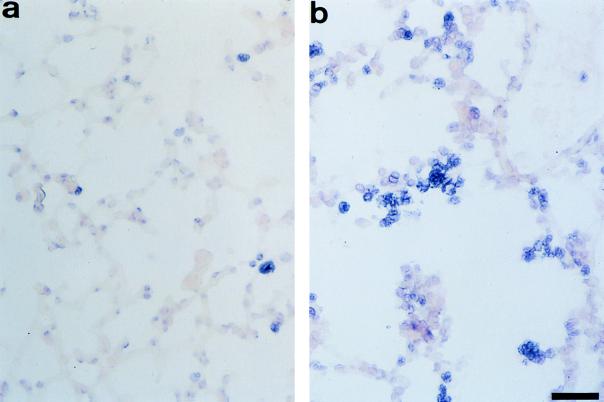
FIG. 6.
Detection of apoptotic cells in lung sections from B. pertussis-infected mice. Cryostat lung sections were stained by the TUNEL technique followed by detection with alkaline phosphatase (fast blue substrate). Tissues were then counterstained with nuclear red dye. (a) Negative red cells after infection with variant 18323H− (arrows). (b) Positive blue apoptotic cells in the alveolar spaces after infection with the parental strain 18323. Scale bar, 40 μm.
Role of AC-Hly in B. pertussis-induced cellular accumulation in BAL fluids and in apoptosis of alveolar macrophages.
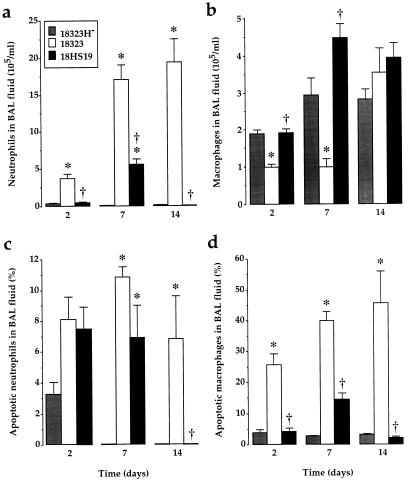
Cellular infiltration and apoptosis were examined at 2, 7, and 14 days in animals infected with freshly isolated variant 18323H−, parental strain 18323, and AC-Hly-deficient mutant 18HS19. Mutant 18HS19-infected animals displayed reduced neutrophil accumulation in the BAL fluid at 2, 7, and 14 days compared to parental strain 18323-infected animals. Neutrophil numbers returned to basal values at 14 days (Fig. 7a). Interestingly, the drop in macrophage counts observed at 2 and 7 days after infection with parental strain 18323 was undetectable in mutant 18HS19-treated animals (Fig. 7b).
FIG. 7.
Cell numbers and apoptotic death in BAL fluids from mice infected with B. pertussis parental strain or mutants. BAL fluids were collected 2, 7, and 14 days after infection with variant 18323H−, with parental strain 18323, or with mutant 18HS19. Neutrophils (a) and macrophages (b) were enumerated on cytospin preparations after Diff Quick staining. The proportions of apoptotic neutrophils (c) and macrophages (d) over the total number of each cell type were determined on cytospin preparations after TUNEL technique staining combined with morphological analysis. Results are expressed as means ± SEM of 6 to 12 experiments for each time point. ∗, P < 0.05 compared to variant 18323H−-infected mice. †, P < 0.05 compared to parental strain 18323-infected mice.
Flow cytometry analysis disclosed decreased proportions of total apoptotic nuclei in BAL fluids from mutant 18HS19-infected, compared to parental strain 18323-infected, mice at all time points (Table 1). At 14 days, similar proportions of apoptotic nuclei were found in mutant 18HS19- and variant 18323H−-infected mice (Table 1). In parallel, the percentage of BAL macrophages having ingested apoptotic cells or bodies was significantly reduced at 14 days in mutant 18HS19-infected mice (Table 1).
The observations concerning lung tissue sections were compatible with those for BAL fluids, since apoptotic cell death was suppressed at 7 and 14 days in mutant 18HS19-treated mice (Table 1).
Compared to parental strain 18323-treated mice, animals infected with mutant 18HS19 displayed a dramatic decrease in the proportions of apoptotic macrophages at 2, 7, and 14 days, as assessed by the TUNEL technique and fluorescence microscopy (Fig. 7d). Accordingly, apoptotic macrophage counts in variant 18323H−- and mutant 18HS19-infected animals were not statistically different (Fig. 7d). Infection with mutant 18HS19 failed to modify the proportion of apoptotic neutrophils in BAL fluids at 2 and 7 days (Fig. 7c). Resolution of BAL neutrophilia 14 days after infection with mutant 18HS19 precluded the determination of apoptotic neutrophils.
DISCUSSION
In this study, we examined the ability of B. pertussis to induce apoptosis of bronchopulmonary cells in vivo, using a murine respiratory model that we described previously (7). Apoptosis was assessed in BAL cells and in lung tissue sections by flow cytometry and by specific labeling of the DNA fragments by the TUNEL technique, respectively. The proportion of alveolar macrophages engulfing apoptotic BAL cells was also determined, since this phenomenon is one of the characteristic features of the apoptotic process (8). Morphological criteria were used to identify the cell types undergoing apoptotic death. To determine the bacterial factor(s) involved in this process, infections were performed not only with parental strain 18323 but also with a variant deficient in the expression of toxins and adhesins (18323H−) and a mutant deficient in the expression of AC-Hly (18HS19), since this factor has been proven to be necessary and sufficient for inducing macrophage apoptosis in vitro (11, 13). In parallel to apoptosis, bacterial colonization in the lung and inflammatory cell accumulation in BAL fluids were monitored, as indices of the progress of the respiratory infection.
Our findings show a time-dependent increase in the number of neutrophils and of lymphocytes in BAL fluids of mice infected with parental strain 18323 but not with variant 18323H−, thus extending our previous report (10). However, the intensity of total cell and neutrophil accumulation in the BAL fluid of parental strain 18323-infected mice described here was markedly greater than that which we have reported previously (10). This difference may result from changes in bacterial culture conditions, since in the present study mice were infected with freshly isolated B. pertussis strains and not subcultured in vitro exclusively. The in vivo isolation procedure was previously shown to enhance the expression of AC-Hly and PT (7). In the present study, we demonstrate that these growth conditions prolong airway persistence of B. pertussis, thus emphasizing their importance to study bacterial virulence and pathogenesis.
In the present study, B. pertussis-induced BAL neutrophilia involves AC-Hly expression, since i.n. infection with a mutant deficient in AC-Hly expression was followed by a marked reduction of neutrophilic infiltrate at 2, 7, and 14 days. The observation that the AC-Hly-deficient mutant 18HS19 was still capable of inducing a detectable, although moderate, neutrophil accumulation in the BAL fluid differs again from our previous findings showing suppression of neutrophilia in BAL fluids at 7 days in mutant 18HS19-infected mice (10). As suggested above, this difference may be linked to the isolation of bacteria from lungs, since this procedure was shown to increase the expression of PT (7), a toxin which is involved in neutrophil recruitment (10).
In addition to neutrophil influx, infection with the parental strain 18323 increased macrophage accumulation in BAL fluid at 21 days. However, at early stages of the infection, i.e., 2 and 7 days, a significant decrease in their numbers was observed in BAL fluids from mice infected with the parental strain 18323 compared with those treated with variant 18323H− or mutant 18HS19. This finding suggests that AC-Hly may activate alveolar macrophages, promote their subsequent adhesion to pulmonary epithelial cells, and thus reduce their recovery by BAL. Alternatively, AC-Hly may accelerate macrophage removal from the alveolar spaces by inducing their apoptosis and the subsequent phagocytosis by other surrounding phagocytic cells.
In this study, we present the first evidence that B. pertussis induces in vivo host cell apoptosis, as determined by the detection of apoptotic nuclei and by the enumeration of macrophages ingesting apoptotic bodies. Apoptotic cells were found both in BAL fluids and in lung tissue. The sparse localization of apoptotic cells in the alveolar compartments indicates that they may be resident lung cells, such as fibroblasts, pneumocytes, interstitial and alveolar macrophages, and/or infiltrating neutrophils. In contrast, apoptotic cells in BAL fluids were clearly identified as neutrophils and macrophages, partially extending our demonstration of in vitro murine macrophage apoptosis induced by B. pertussis (13). Parallel evaluation of the numbers of inflammatory cells recovered by BAL and of incidence of apoptosis disclosed that approximately 11 and 45% of neutrophils and macrophages, respectively, were apoptotic. In a recent study, Cox et al. (4) demonstrated the presence of an intense accumulation of neutrophils in the BAL fluid of lipopolysaccharide-treated rats. However, only 2% of these neutrophils showed an apoptotic morphology and no dead macrophages were detected, indicating that the extent of the apoptotic process and possibly its role played in vivo are greater during airway infection with B. pertussis. It should be noted that the increase of apoptotic cells observed is more important in lung tissue than in BAL fluid at day 7, compared to day 2, in mice infected with the parental strain 18323, probably because BAL fluids contain some apoptotic cells which form aggregates or which stick to the alveolar wall.
Infection with variant 18323H−-, or with the parental strain 18323, which had not been freshly reisolated from lungs of infected mice failed to promote significant apoptosis of bronchopulmonary cells. This finding suggests that the apoptotic process is dependent not only on the presence of toxins and adhesins but also on their amounts. Several B. pertussis factors may be involved in induction of inflammatory cell apoptosis in the lung. One of the best candidates is AC-Hly, since its addition to murine macrophages was shown to promote their death in vitro (11, 13). In confirmation, we demonstrate here that apoptotic death of alveolar macrophages is largely compromised 2, 7, and 14 days after infection with the AC-Hly-deficient mutant 18HS19. This result parallels the observation that lower numbers of macrophages were found at 2 and 7 days in BAL fluids of parental strain 18323-infected mice than in those of mutant 18HS19-infected mice. This finding suggests an early role of AC-Hly in the removal of resident alveolar macrophages from the alveolar spaces by inducing their apoptosis.
Contrary to our observations concerning alveolar macrophages, infection with the mutant 18HS19 did not modify BAL neutrophil apoptosis. This result indicates that AC-Hly, although implicated in the recruitment of neutrophils into the airways, is not involved in their removal by apoptosis. In keeping with these findings, we did not observe any apoptosis upon addition of high amounts of purified AC-Hly to human peripheral blood neutrophils from healthy donors (data not shown). The fact that AC-Hly is not implicated in neutrophil apoptosis suggests that another bacterial product may be responsible for this phenomenon.
Previous studies have shown that AC-Hly is required for the initiation of infection (12, 23). We can now hypothesize that in addition to ciliatosis induced by tracheal cytotoxin, which prevents the host organism from removing bacteria from the respiratory tract, and the adherence of bacteria to the respiratory cells via the expression of its adhesins (9, 17), B. pertussis uses AC-Hly to prolong its persistence and to favor its multiplication in the host cells present in the airways by promoting phagocytic cell apoptosis. Alternatively, apoptosis-induced clearance of redundant neutrophils and macrophages by B. pertussis might represent a way for host cells to remove two well-known cellular reservoirs for this pathogen (20, 22) and thus limit the infection.
Programmed host cell death in response to bacterial infection has now been reported for a number of bacterial pathogens (26, 27). In a recent review, Zychlinsky et al. (26) proposed at least three possible roles for apoptosis in bacterial diseases: massive cell destruction, inhibition of apoptosis, and initiation of inflammation. In the cases of Listeria and Shigella, apoptosis may play a role in the initiation of inflammation. Apoptotic hepatic cells from L. monocytogenes-infected mice produce neutrophil chemoattractants (19). S. flexneri expresses IpaB, which binds to the interleukin 1β-converting enzyme or to a related protein, binding which is accompanied by the release of mature interleukin 1β (26). In the case of B. pertussis, we speculate that apoptosis may also play a role in the initiation of inflammation, although the mechanisms of induction are still unknown. In contrast to S. flexneri, B. pertussis does not need to be intracellular to induce cell apoptosis (11). It secretes an AC-Hly that is able to penetrate the host cell, bind calmodulin, and then increase the intracellular cyclic AMP concentration, which can lead to apoptosis. The induction or suppression of this process may represent a crucial step in the evolution of host-pathogen interaction and in the progress of infection. The extent to which the induction of apoptotic cell death in the lung during B. pertussis infection is beneficial for the bacteria or for the host remains to be established.
ACKNOWLEDGMENTS
We are grateful to G. Milon and B. B. Vargaftig for helpful discussion and I. Old for correcting the English.
P. Gueirard is supported by a grant from the Caisse Nationale d’Assurance Maladie et Maternité des Travailleurs non Salariés des Professions non Agricoles. Financial support for this work was provided by Institut Pasteur Fondation.
REFERENCES
- 1.Ameisen J C, Estaquier J, Idziorek T. From AIDS to parasite infection: pathogen-mediated subversion of programmed cell death as a mechanism for immune dysregulation. Immunol Rev. 1994;142:9–51. doi: 10.1111/j.1600-065x.1994.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 2.Boschwitz J S, Batanghari J W, Kedem H, Relman D A. Bordetella pertussis infection of human monocytes inhibits antigen-dependent CD4 T cell proliferation. J Infect Dis. 1997;176:678–686. doi: 10.1086/514090. [DOI] [PubMed] [Google Scholar]
- 3.Confer D L, Eaton J W. Phagocyte impotence caused by an invasive bacterial adenylate cyclase. Science. 1982;217:948–950. doi: 10.1126/science.6287574. [DOI] [PubMed] [Google Scholar]
- 4.Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol. 1995;12:232–237. doi: 10.1165/ajrcmb.12.2.7865221. [DOI] [PubMed] [Google Scholar]
- 5.Douglas R S, Tarshis A D, Pletcher C H, Nowell P C, Moore J S. A simplified method for the coordinate examination of apoptosis and surface phenotype of murine lymphocytes. J Immunol Methods. 1995;188:219–228. doi: 10.1016/0022-1759(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 6.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guiso N, Szatanik M, Rocancourt M. Protective activity of Bordetella adenylate cyclase-hemolysin against bacterial colonization. Microb Pathog. 1991;11:423–431. doi: 10.1016/0882-4010(91)90038-c. [DOI] [PubMed] [Google Scholar]
- 8.Haanen C, Vermes I. Apoptosis and inflammation. Med Inflamm. 1995;4:5–15. doi: 10.1155/S0962935195000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewlett E L. Pertussis: current concepts of pathogenesis and prevention. Pediatr Infect Dis J. 1997;16:S78–S84. doi: 10.1097/00006454-199704001-00002. [DOI] [PubMed] [Google Scholar]
- 10.Khelef N, Bachelet C M, Vargaftig B B, Guiso N. Characterization of murine lung inflammation after infection with parental Bordetella pertussis and mutants deficient in adhesins or toxins. Infect Immun. 1994;62:2893–2900. doi: 10.1128/iai.62.7.2893-2900.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khelef N, Guiso N. Induction of macrophage apoptosis by Bordetella pertussis adenylate cyclase-hemolysin. FEMS Microbiol Lett. 1995;134:27–32. doi: 10.1111/j.1574-6968.1995.tb07909.x. [DOI] [PubMed] [Google Scholar]
- 12.Khelef N, Sakamoto H, Guiso N. Both adenylate cyclase and hemolytic activities are required by Bordetella pertussis to initiate infection. Microb Pathog. 1992;12:227–235. doi: 10.1016/0882-4010(92)90057-u. [DOI] [PubMed] [Google Scholar]
- 13.Khelef N, Zychlinsky A, Guiso N. Bordetella pertussis induces apoptosis: role of adenylate cyclase-hemolysin. Infect Immun. 1993;61:4064–4071. doi: 10.1128/iai.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ladant D, Brézin C, Alonso J M, Crenon I, Guiso N. Bordetella pertussis adenylate cyclase purification, characterization and radioimmunoassay. J Biol Chem. 1987;261:16264–16269. [PubMed] [Google Scholar]
- 15.Merrick J C, Edelson T, Bhardwaj V, Swanson P E, Unanue E R. Lymphocyte apoptosis during early phase of Listeria infection in mice. Am J Pathol. 1997;151:758–792. [PMC free article] [PubMed] [Google Scholar]
- 16.Pittman M. Genus Bordetella. In: Krieg N R, Holt J G, editors. Bergey’s manual of systemic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins Co.; 1984. pp. 388–393. [Google Scholar]
- 17.Rappuoli R. Pathogenicity mechanisms of Bordetella. Curr Top Microbiol Immunol. 1994;192:319–336. doi: 10.1007/978-3-642-78624-2_14. [DOI] [PubMed] [Google Scholar]
- 18.Rogel A, Hanski E. Distinct steps in the penetration of adenylate cyclase toxin of Bordetella pertussis into sheep erythrocytes. J Biol Chem. 1992;12:232–237. [PubMed] [Google Scholar]
- 19.Rogers H W, Callery M P, Deck B, Unanue E R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 20.Saukkonen K, Cabellos C, Burroughs M, Prasad S, Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991;173:1143–1149. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stainer D W, Scholte M J. A simple chemically defined medium for the production of Phase I Bordetella pertussis. J Gen Microbiol. 1971;63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 22.Steed L L, Morey S, Friedman R L. Intracellular survival of virulent Bordetella pertussis in human polymorphonuclear leukocytes. J Leukocyte Biol. 1991;50:321–330. doi: 10.1002/jlb.50.4.321. [DOI] [PubMed] [Google Scholar]
- 23.Weiss A A, Goodwin M S. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun. 1989;57:3757–3764. doi: 10.1128/iai.57.12.3757-3764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams G T. Programmed cell death: a fundamental protective response to pathogens. Trends Microbiol. 1994;2:463–464. doi: 10.1016/0966-842x(94)90648-3. [DOI] [PubMed] [Google Scholar]
- 25.Wolff J G, Cook H, Goldhammer A R, Berkowitz S A. Calmodulin activates prokaryotic adenylate cyclase. Proc Natl Acad Sci USA. 1980;77:3841–3844. doi: 10.1073/pnas.77.7.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]
- 27.Zychlinsky A, Sansonetti P J. Apoptosis in bacterial pathogenesis. J Clin Invest. 1997;100:493–496. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zychlinsky A, Thirumamai K, Arondel J, Cantey R, Aliparantis A O, Sansonetti P J. In vivo apoptosis in Shigella flexneri infections. Infect Immun. 1996;64:5357–5365. doi: 10.1128/iai.64.12.5357-5365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]