Abstract
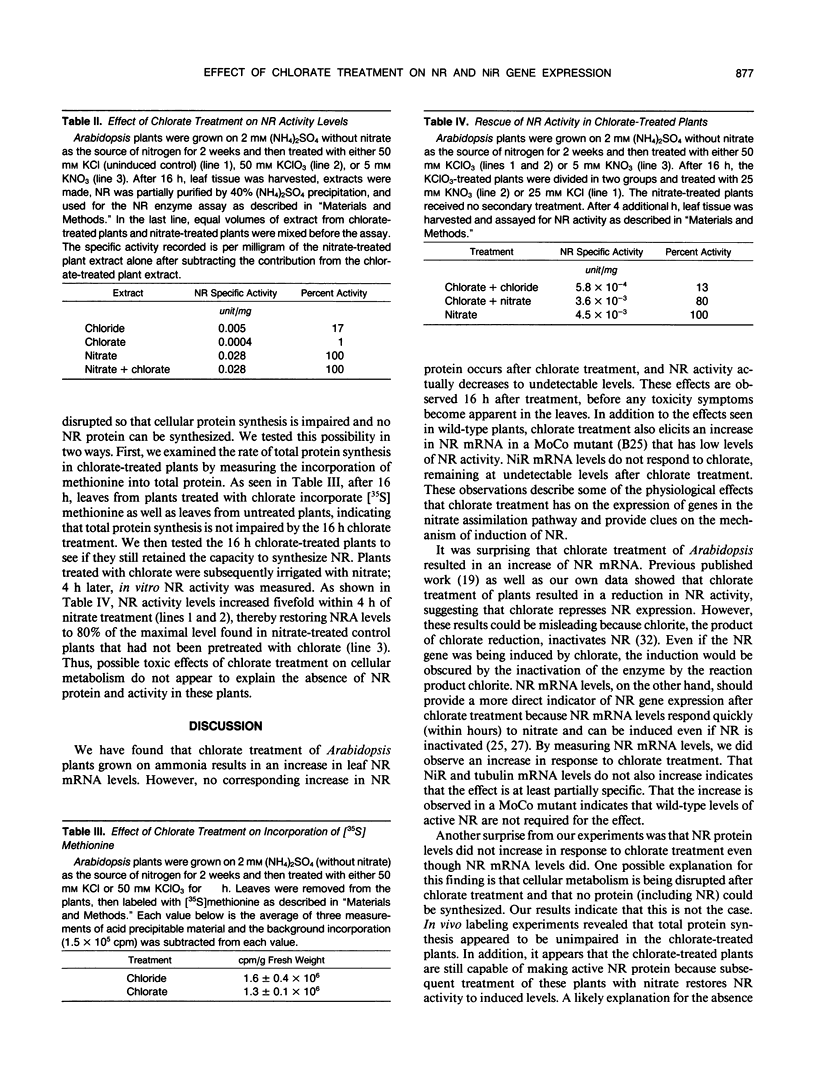
The herbicide chlorate has been used extensively to isolate mutants that are defective in nitrate reduction. Chlorate is a substrate for the enzyme nitrate reductase (NR), which reduces chlorate to the toxic chlorite. Because NR is a substrate (NO3−)-inducible enzyme, we investigated the possibility that chlorate may also act as an inducer. Irrigation of ammonia-grown Arabidopsis plants with chlorate leads to an increase in NR mRNA in the leaves. No such increase was observed for nitrite reductase mRNA following chlorate treatment; thus, the effect seems to be specific to NR. The increase in NR mRNA did not depend on the presence of wild-type levels of NR activity or molybdenum-cofactor, as a molybdenum-cofactor mutant with low levels of NR activity displayed the same increase in NR mRNA following chlorate treatment. Even though NR mRNA levels were found to increase after chlorate treatment, no increase in NR protein was detected and the level of NR activity dropped. The lack of increase in NR protein was not due to inactivation of the cells' translational machinery, as pulse labeling experiments demonstrated that total protein synthesis was unaffected by the chlorate treatment during the time course of the experiment. Chlorate-treated plants still retain the capacity to make functional NR because NR activity could be restored by irrigating the chlorate-treated plants with nitrate. The low levels of NR protein and activity may be due to inactivation of NR by chlorite, leading to rapid degradation of the enzyme. Thus, chlorate treatment stimulates NR gene expression in Arabidopsis that is manifested only at the mRNA level and not at the protein or activity level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. Shotgun DNA sequencing using cloned DNase I-generated fragments. Nucleic Acids Res. 1981 Jul 10;9(13):3015–3027. doi: 10.1093/nar/9.13.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back E., Burkhart W., Moyer M., Privalle L., Rothstein S. Isolation of cDNA clones coding for spinach nitrite reductase: complete sequence and nitrate induction. Mol Gen Genet. 1988 Apr;212(1):20–26. doi: 10.1007/BF00322440. [DOI] [PubMed] [Google Scholar]
- Caboche M., Rouzé P. Nitrate reductase: a target for molecular and cellular studies in higher plants. Trends Genet. 1990 Jun;6(6):187–192. doi: 10.1016/0168-9525(90)90175-6. [DOI] [PubMed] [Google Scholar]
- Cheng C. L., Dewdney J., Nam H. G., den Boer B. G., Goodman H. M. A new locus (NIA 1) in Arabidopsis thaliana encoding nitrate reductase. EMBO J. 1988 Nov;7(11):3309–3314. doi: 10.1002/j.1460-2075.1988.tb03201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D. J. Chlorate toxicity in Aspergillus nidulans. Studies of mutants altered in nitrate assimilation. Mol Gen Genet. 1976 Jul 23;146(2):147–159. doi: 10.1007/BF00268083. [DOI] [PubMed] [Google Scholar]
- Cove D. J. Cholorate toxicity in Aspergillus nidulans: the selection and characterisation of chlorate resistant mutants. Heredity (Edinb) 1976 Apr;36(2):191–203. doi: 10.1038/hdy.1976.24. [DOI] [PubMed] [Google Scholar]
- Crawford N. M., Campbell W. H., Davis R. W. Nitrate reductase from squash: cDNA cloning and nitrate regulation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8073–8076. doi: 10.1073/pnas.83.21.8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford N. M., Smith M., Bellissimo D., Davis R. W. Sequence and nitrate regulation of the Arabidopsis thaliana mRNA encoding nitrate reductase, a metalloflavoprotein with three functional domains. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5006–5010. doi: 10.1073/pnas.85.14.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Nason A. Pyridine Nucleotide-Nitrate Reductase from Extracts of Higher Plants. Plant Physiol. 1953 Apr;28(2):233–254. doi: 10.1104/pp.28.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Marzluf G. A. nit-2, the major positive-acting nitrogen regulatory gene of Neurospora crassa, encodes a sequence-specific DNA-binding protein. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5331–5335. doi: 10.1073/pnas.87.14.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joy K. W. Nitrogen metabolis of Lemna minor. II. Enzymes of nitrate assimilation and some aspects of their regulation. Plant Physiol. 1969 Jun;44(6):849–853. doi: 10.1104/pp.44.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla B., Caddick M. X., Langdon T., Martinez-Rossi N. M., Bennett C. F., Sibley S., Davies R. W., Arst H. N., Jr The regulatory gene areA mediating nitrogen metabolite repression in Aspergillus nidulans. Mutations affecting specificity of gene activation alter a loop residue of a putative zinc finger. EMBO J. 1990 May;9(5):1355–1364. doi: 10.1002/j.1460-2075.1990.tb08250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouteau S., Cherel I., Vaucheret H., Caboche M. Nitrate Reductase mRNA Regulation in Nicotiana plumbaginifolia Nitrate Reductase-Deficient Mutants. Plant Cell. 1989 Nov;1(11):1111–1120. doi: 10.1105/tpc.1.11.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle L. S., Lahners K. N., Mullins M. A., Rothstein S. Nitrate effects on nitrate reductase activity and nitrite reductase mRNA levels in maize suspension cultures. Plant Physiol. 1989 Jul;90(3):962–967. doi: 10.1104/pp.90.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Campbell W. H. Purification of Squash NADH:Nitrate Reductase by Zinc Chelate Affinity Chromatography. Plant Physiol. 1983 Jan;71(1):205–207. doi: 10.1104/pp.71.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P., Vennesland B. Nitrate Reductase and Chlorate Toxicity in Chlorella vulgaris Beijerinck. Plant Physiol. 1972 Oct;50(4):421–424. doi: 10.1104/pp.50.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
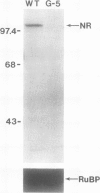
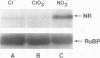
- Wilkinson J. Q., Crawford N. M. Identification of the Arabidopsis CHL3 gene as the nitrate reductase structural gene NIA2. Plant Cell. 1991 May;3(5):461–471. doi: 10.1105/tpc.3.5.461. [DOI] [PMC free article] [PubMed] [Google Scholar]