Abstract
Infection with Shiga toxin (Stx)-producing enterohemorrhagic Escherichia coli is increasing among children. In this study, 5-week-old C57BL/6 mice with protein calorie malnutrition (PCM) that had been fed a 5% protein diet for 2 weeks since ablactation were inoculated intragastrically with 2 × 106 CFU of Stx-producing E. coli O157:H7. More than 75% of infected mice with PCM died by 10 days postinfection. Infected mice with PCM developed neurologic symptoms 5 days after infection, while well-nourished control mice receiving a 25% protein diet did not. In the intestinal tracts of infected mice with PCM, inoculated E. coli O157:H7 multiplied between days 2 and 4 of infection, with a peak of growth at day 4. Although the pathogens were not culturable from the stool after day 7, O157 lipopolysaccharide was detectable in the stool by enzyme-linked immunosorbent assay even after day 8. Stx was detectable in the stool after day 2 of infection and increased in proportion to the growth of inoculated organisms. The maximal production of Stx occurred at 4 days postchallenge, and Stx was detectable in the blood on days 3 to 5. In contrast, well-nourished control mice survived the infection, and all of them remained well even after 3 weeks of infection. In these control mice, inoculated E. coli O157:H7 disappeared from the stool before day 3. Stx was not detectable in the stool and blood of infected control mice at any time from day 1 through day 8. Histologically, cerebral hemorrhages seemed to be the cause of acute death of infected mice with PCM. Immunocytochemical staining demonstrated the positive immunoreaction to Stx at the alveus and stratum pyramidale of the hippocampus and in renal tubules of infected malnourished mice. Such immunoreactions were not found in tissues from infected control mice. Histological study of the intestinal epithelium before infection showed that PCM severely affected the development of intestinal epithelia. These findings strongly indicate that PCM-induced nondevelopment of intestinal physical barrier is one of the predisposing factors for infection with Stx-producing E. coli O157:H7 in mice and suggest that our mouse model may explain the high incidence of infection with Stx-producing E. coli O157:H7 in the children whose intestinal epithelia have not yet completely developed.
Shiga toxin (Stx)-producing Escherichia coli O157:H7 has been associated with outbreaks of hemorrhagic colitis throughout developed countries (31). Infection with this pathogen often leads to the development of hemolytic uremic syndrome (HUS) (14, 15) and thrombotic thrombocytopenic purpura (25) in humans. E. coli O157:H7 belongs to the family of enterohemorrhagic E. coli (EHEC) strains, which produce several virulence factors, including intimin (13), Stx1 and Stx2 (formerly known as Shiga-like toxins I and II) (29), and lipopolysaccharide (LPS) (16). Stx and LPS in particular seem to be involved in the pathogenesis of HUS associated with shigellosis (20).
Stx consists of two antigenically distinct toxins, Stx1 and Stx2 (34). Both toxins are cytotoxic for Vero and HeLa cells and paralytic-lethal for mice (32, 34). Stx1 and Stx2 have been known to bind globotriosylceramide glycolipid molecules as functional receptors (22, 39). In piglets, the brain stem, cerebrum, and cerebellum are affected by infection with E. coli producing Stx1 and/or Stx2 (9). It was also reported that intravenously injected Stx1 accumulated in the central nervous system (CNS) much more than in other organs of rabbits (30). More recently, Fujii et al. (11) have shown that Stx has a direct cytotoxic effect on neurons.
In the outbreaks of Stx-producing E. coli O157:H7 infection in Japan, several children manifesting CNS complications died before the development of HUS (16). Tzipori et al. (36) found lesions histologically in the cerebral cortex, thalamus, and cerebellum in a human baby who died after infection with Stx2-producing E. coli. In general, neurologic manifestations are found in up to 30% of patients (7, 27), and CNS complications are an important predictive factor for HUS mortality in children (37).
Although it is clear that EHEC infection is increasing among children and the aged, the specific reason for such age prevalence remains unclear. Recently, we have found out that many children who contracted infection with Stx-producing E. coli O157:H7 in Japan had lived on an unbalanced diet before infection (29a). This finding suggested that malnourished mice might be very susceptible to infection with EHEC. Several mouse models have been reported; however, oral inoculation requires considerably higher doses of EHEC organisms to establish infection in mice than natural infection in humans, possibly because laboratory mice are put on a high-calorie and balanced diet. We tested this hypothesis in mice fed a low-protein diet with several clinical isolates of Stx-producing E. coli O157:H7.
MATERIALS AND METHODS
Animals and diets.
Specific-pathogen-free, 3-week-old female C57BL/6 mice that had been weaned from the breast were purchased from Charles River (Tokyo, Japan). Animals were kept under strict specific-pathogen-free conditions and monitored for common murine pathogens (8) during 2 weeks of quarantine. During this 2-week period, mice were fed a low-protein diet to achieve protein calorie malnutrition (PCM) and were put on the same diet after infection. The low-protein diet (5% protein) in solid form was made isocaloric to the regular casein-based mouse diet (25% protein), and both diets were purchased from Oriental BioService, Inc., Kyoto, Japan. Mice of the same age receiving the casein-based mouse diet served as well-nourished controls. Ingredients of these diets are shown in Table 1.
TABLE 1.
Ingredients of the two diets used in this study
| Ingredient(s) | Content (%)
|
|
|---|---|---|
| Full-protein diet | Low-protein diet | |
| β-Cornstarch | 41.5 | 61.5 |
| Mild casein | 25.0 | 5.0 |
| α-Potato starch | 10.0 | 10.0 |
| Cellulose powder | 8.0 | 8.0 |
| Soybean oil | 6.0 | 6.0 |
| Minerals | 3.5 | 3.5 |
| Sugar | 5.0 | 5.0 |
| Vitamins | 1.0 | 1.0 |
Microorganisms.
All EHEC strains were isolated during an outbreak of hemorrhagic colitis and/or HUS in the western part of Japan in 1996. Strains N-9 (Stx1+ Stx2+) and N-5 (Stx1+ Stx2+), OK-7 (Stx1− Stx2+), and OK-12 (Stx1− Stx2+) were E. coli O157:H7 and isolated from patients with hemorrhagic colitis and HUS. Strains N-10 (E. coli O157:H7, Stx1+ Stx2−) and N-20 (E. coli O157:H−, Stx1+ Stx2−) were clinical isolates from patients with hemorrhagic colitis but not exhibiting HUS. Salmonella soerenga O301 was a gift from M. Tamura, Natural Institute of Infectious Disease (Tokyo, Japan), and its O-antigenic constituents were identical to LPS of E. coli O157 (33). E. coli NP-4 (Stx1− Stx2−), isolated from the stool of a healthy adult, was used as a nonpathogenic strain of E. coli. Characteristics of all bacterial strains used are listed in Table 2.
TABLE 2.
Characteristics of bacterial strains used in this studya
| Bacterial strain | Serotype | stx1/stx2b | eaec | EHECd |
|---|---|---|---|---|
| EHEC | ||||
| N-9 | O157:H7 | +/+ | + | + |
| N-5 | O157:H7 | +/+ | + | + |
| N-10 | O157:H7 | +/− | + | + |
| N-20 | O157:H− | +/− | + | + |
| OK-7 | O157:H7 | −/+ | + | + |
| OK-12 | O157:H7 | −/+ | + | + |
| Controls | ||||
| E. coli NP-4 (isolated from a healthy adult) | −/− | − | − | |
| S. soerenga O301 | −/− | − | − |
DNA hybridization using a probe specific for enteroaggregative E. coli indicated that none of the strains were enteroaggregative.
By PCR using specific primers for stx1 and stx2.
By DNA hybridization using a probe specific for eae.
By DNA hybridization using a probe specific for the EHEC 60-MDa plasmid.
All strains were preserved in gelatin-charcoal disks (42) at −20°C. Before use, one of the preserved disks was dissolved in 1 ml of tryptic soy broth (TSB; Difco Laboratories, Detroit, Mich.), and 100 μl of the broth was inoculated into 10 ml of fresh TSB or plated on TSB agar plates. For infection study, bacteria were harvested by centrifugation from broth culture grown overnight at 37°C, washed once with sterile phosphate-buffered saline (PBS; 10 mM, pH 7.2), and resuspended in sterile PBS at a concentration of 2 × 107 CFU/ml. The number of E. coli in each bacterial cell suspension was determined by plate count. For each infection experiment, one disk randomly selected from the preserved disks of the same batch was used. For genotypic and phenotypic analyses, bacteria grown on TSB agar plates were used, and both analyses were done prior to each infection experiment.
Genotyping of tested organisms.
The presence of stx1 and stx2 genes was detected by PCR using primers specific for each toxin gene according to the method of Kobayashi et al. (19). PCR products were hybridized with the 0.75-kb EcoRI/HindIII fragment of pNTP 705 for stx1 and the 0.85-kb SmaI/PstI fragment of pDEP28 for stx2 (35, 41).
The DNA probe for eae (E. coli attachment and effacement) was the ∼1-kb SalI-KpnI fragment of the recombinant plasmid pCVD 434 (13). The 3.4-kb HindIII fragment of the recombinant plasmid pCVD 419 was used as a probe for the 60-MDa plasmid of EHEC (21).
Probe DNA was prepared by digestion with the appropriate restriction endonucleases. The fragments were separated by low-melting-point agarose gel electrophoresis and isolated from the gel. Each fragment was labeled with digoxigenin as described by the manufacturer (Boehringer, Mannheim, Germany). Hybridization and detection were done by the method of Karpman et al. (16).
Mouse infection protocol.
Mice with PCM were infected intragastrically with desired concentrations of E. coli in a volume of 0.1 ml through a stainless steel catheter with a blunted end (outer diameter, 0.45 mm; Natsume Seisakasyo Co., Tokyo, Japan). Control mice fed a 25% protein diet were infected in the same way, and all infected mice were kept in a biosafety level 3 facility throughout the experiments. All mice were not fasted prior to the inoculation procedure. Infected mice were examined for apparent clinical symptoms every 12 h after infection.
Assessment of disease progression.
The degree of E. coli O157:H7 burden in the stool was assessed either by measuring the amount of O157 LPS or by counting the number of O157 CFU in the stool.
To determine the number of viable E. coli N-9 in the stool, streptomycin-resistant (Smr) mutants of strain N-9 were isolated from a culture on TSB agar supplemented with 100 μg of streptomycin (Meiji Seika Co. Ltd., Tokyo, Japan) per ml. Among 21 Smr mutants, one (SmrN-9) which was comparable with its parental strain N-9 in Stx production was used as a challenge strain. SMrN-9 was cultured overnight in TSB containing streptomycin (100 μg/ml) at 37°C, after which the organism was washed once with PBS by centrifugation (6,000 × g for 8 min). The bacterial suspension was adjusted to 2 × 107 CFU/ml in PBS. After intragastric inoculation, the number of viable SmrN-9 in the stool was measured by plate count using sorbitol IPA bile salts (SIB) agar (Kyokuto Seiyaku, Co. Ltd., Tokyo, Japan) supplemented with 100 μg of streptomycin per ml (SM-SIB). Sorbitol-negative colonies formed on SM-SIB agar were tested for agglutinability with O157 antibody reagent (Denka Seiken, Tokyo, Japan). Only the colonies which were sorbitol negative and agglutinable with O157 antibody were counted.
The amount of O157 LPS was determined by the enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (Premier E. coli O157; Meridian Diagnostic, Inc., Cincinnati, Ohio), which was kindly provided by Ortho-Clinical Diagnostics (Tokyo, Japan). To estimate the number of E. coli O157 from the ELISA results, calibration curves were constructed for strain SMrN-9. Briefly, twofold serial dilutions of a bacterial suspension (5 × 108 CFU/ml), made in homogenates of normal mouse feces, were subjected to ELISA after each suspension was diluted fourfold with the washing buffer supplied by the manufacturer. The test was performed as instructed by the manufacturer. The exact number of E. coli in each bacterial dilution was determined by plate count. By measuring both the absorbance (at 450 nm) yielded in this ELISA and the number of CFU, a calibration curve was made, and the amount of O157 LPS in the stool was expressed as the equivalent number of strain SMrN-9 organisms. This method was applicable to stool samples which contained O157 organisms at concentrations of 9 × 102 to 5 × 106 CFU/mg when a stool sample was diluted fourfold with the washing buffer before assay. When the reading for a stool sample was >3.00 at 450 nm with this kit, the sample was further diluted with the washing buffer.
Stool samples were obtained by manually pressing the lower abdomen of mice and placed in a sample tube containing PBS (50 mg of sample/ml) after its weight was determined. Then each stool sample was extensively homogenized with a plastic pestle and mixed with 3 volumes of the washing buffer. A 100-μl volume of the diluted homogenate was assayed with the ELISA kit. For colony counting, individual stool samples were homogenized in PBS containing 100 μg of streptomycin per ml (SM-PBS) (50 mg of sample/ml) and then further diluted with SM-PBS before being plated on SM-SIB agar.
Measurement of Stx in the blood and stool.
Blood was obtained from the ophthalmic arteries or by cardiac puncture of infected mice. Serum was separated from clotted blood by centrifugation. Semiquantitation of Stx in the blood and stool was performed by ELISA using a commercially available kit (Premier EHEC; Meridian Diagnostics). Since this kit can detect both Stx1 and Stx2 but cannot distinguish between them toxin levels in the stool and the serum were expressed as the amount of Stx1 after a standard curve was constructed with the purified Stx1 (28) incorporated into feces of normal mice or normal mouse serum. With this kit, the limits of detection for Stx1 were 10 pg/mg of feces and 20 pg/ml of serum, respectively.
Stool samples were homogenized in PBS at a concentration of 50 mg/ml and then fivefold diluted with the sample dilution buffer supplied by the manufacturer. A 100-μl volume of diluted homogenates was assayed with the ELISA kit. Serum samples were 20-fold concentrated by ultrafiltration, 20 μl of each concentrated sample was mixed with 4 volumes of the sample dilution buffer, and then, 100 μl of each mixture was assayed with the ELISA kit.
Histolopathological studies.
Samples for histopathological studies were prepared as described previously (18). Briefly, tissues were fixed in 10% buffered formalin before embedment in paraffin. Hematoxylin-and-eosin (HE), Alcian blue (pH 2.5)-periodic acid Schiff (PAS), or Luxol fast blue (LFB)-HE staining of sections from paraffin blocks was used. With LFB-HE staining, myelinated nerve fibers stain blue and demyelinated portions are stained with eosin (pink).
Immunocytochemical staining.
Sections were deparaffinized, and the endogenous peroxidase activities were blocked by incubation in a hydrogen peroxide solution (Dako Japan, Tokyo, Japan) for 20 min. A murine monoclonal anti-Stx1 and -Stx2 antibody (immunoglobulin G [IgG]; 1 μg/ml; Toxin Technology, Inc., Sarasota, Fla.) was applied at 4°C overnight after the sections were treated with normal goat serum for 1 h at 37°C. As controls, sections prepared from the same animals were incubated at 4°C overnight with normal mouse IgG (1 μg/ml; Organon Teknika, Durham, N.C.), or sections from infected well-nourished mice were treated with the monoclonal anti-Stx1 and -Stx2 IgG antibody at 4°C overnight. All sections were then treated for 1 h at 37°C with a 1:200 dilution of biotinylated anti-mouse IgG F(ab′)2 (Organon Teknika). Thereafter, a streptavidin-peroxidase complex (Histofine SAB-PO kit; Nichirei Co. Ltd., Tokyo, Japan) diluted 1:600 was applied to all slides for 1 h at 37°C. The peroxidase-diaminobenzidine method was used for colorization. The sections were counterstained with hematoxylin.
Blood tests.
Individual serum samples were used to measure blood urea nitrogen and creatinine levels. For the measurement of leukocyte and platelet count, individual blood samples were collected in test tubes containing EDTA powders. Blood samples obtained from mice orally infected with E. coli NP-4 were included in the assay.
RESULTS
Susceptibility of mice with PCM to E. coli O157:H7.
Since E. coli O157:H7 N-9 was the highest Stx producer among the strains tested in our preliminary study, groups of 10 mice were inoculated intragastrically with 2 × 105 to 2 × 107 CFU of strain N-9. Results are summarized in Table 3. Only mice with PCM were susceptible to strain N-9 at these dose ranges: at a dose of 2 × 106 CFU, 90% of infected mice with PCM succumbed to infection by day 10 postinfection. An intragastric inoculation of strain SMrN-9 also killed 85% of mice with PCM at a dose of about 2 × 106 CFU. When mice with PCM were given drinking water containing streptomycin (100 mg/liter) from 3 days before infection with 2 × 106 CFU of strain SMrN-9, the mortality rate did not increase to a significant extent: about 90% of the mice died by 10 days postinfection. In contrast, all of the control mice receiving a full-protein (25%) diet were alive 3 weeks after infection with either N-9 or SMrN-9. In addition, streptomycin treatment did not alter the colonization rate in control mice.
TABLE 3.
Susceptibility of PCM mice to E. coli O157:H7 strain N-9
| Mice fed witha: | Inoculum size (CFU) | Mortality (%) at 10 days postinfectionb
|
|
|---|---|---|---|
| N-9 | SMrN-9 | ||
| Low-protein diet | 2 × 105 | 55 | 50 |
| 2 × 106 | 90 | 85 | |
| 2 × 107 | 95 | 90 | |
| Full-protein diet | 2 × 105 | 0 | 0 |
| 2 × 106 | 0 | 0 | |
| 2 × 107 | 0 | 0 | |
Groups of 10 mice were given either a low-protein diet (PCM group) or a full-protein diet from 2 weeks before and during the experiment.
Experiments were repeated four times, and the mortality rate is expressed as the mean of four tests.
Infected mice with PCM developed no gastrointestinal symptoms such as bloody, loose, or watery stools. For less than 40% of the mice, stool was semisolid during the first 4 days but normal thereafter. Systemic symptoms that developed in infected mice with PCM within 48 to 96 h after infection included lethargy, ruffled fur, restlessness, anorexia, and shivering. After 120 h, these mice developed neurologic symptoms including hindleg weakness or paralysis and jerky rhythmic motions. Only mice developing neurologic symptoms died by day 10 postinfection. In contrast, well-nourished controls developed no symptoms even after infection with 2 × 107 CFU of strain N-9 or SMrN-9, and all of the animals were well throughout 3 weeks of observation.
Based on the mortality rate of infected mice with PCM, strains N-10 and N-20, producing only Stx1, were less virulent than strains N-9 and N-5, producing Stx1 and Stx2, and strains OK-7 and OK-12, producing only Stx2 (Table 4). S. soerenga was completely avirulent in mice with PCM even though it possesses O antigen identical with that of E. coli O157. These results indicate that acute death of infected mice with PCM may be attributable to Stx2 rather than Stx1.
TABLE 4.
Mortality rates in malnourished C57BL/6 mice after oral infection with various strains of E. coli O157 used in this study
| Bacterial strain (serotype) | Mortality (%) at 10 days postinfectiona |
|---|---|
| EHEC | |
| N-9 (O157:H7) | 90 |
| N-5 (O157:H7) | 75 |
| N-10 (O157:H7) | 40 |
| N-20 (O157:H−) | 35 |
| OK-7 (O157:H7) | 65 |
| OK-12 (O157:H7) | 60 |
| Controls | |
| E. coli NP-4 (isolated from a healthy adult) | 0 |
| S. soerenga (O301) | 0 |
Groups of 10 mice with PCM were infected intragastrically with 2 × 106 CFU of Stx-producing E. coli or of nonpathogenic E. coli or S. soerenga. Each group consisted of 10 mice. Data were obtained from two separate experiments and are expressed as the mean of two results.
Quantitation of E. coli and Stx in infected mice.
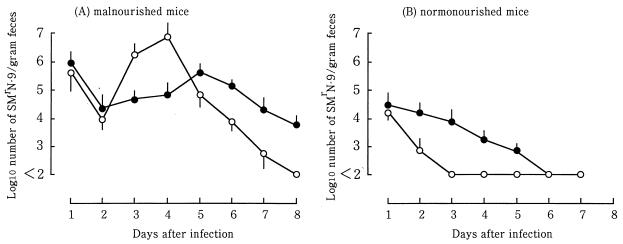
When 2 × 106 CFU of strain SMrN-9 was intragastrically inoculated into mice with PCM, the number of CFU of strain SMrN-9 in the stool decreased between days 1 and 2 of infection (Fig. 1A). Over the next 48 h, the level rapidly increased by approximately 3 logs, reaching about 107 CFU/g of feces on day 4. After day 4, viable counts fell steadily, reaching uncountable levels (less than 100 CFU/g of feces) at 8 days postinfection. Changes in the quantity of O157 LPS in the stool, as expressed by the equivalent number of SMrN-9, paralleled those in the number of CFU of strain SMrN-9 during the first 2 days (Fig. 1A). Between days 2 and 4, the amount of O157 LPS was lower than the number of CFU of SMrN-9. The quantity of O157 LPS slightly increased on day 5 and then gradually decreased. Even on day 8, when SMrN-9 organisms were no longer recovered from the stool, O157 LPS was still detectable at a level equivalent to 104 SMrN-9 organisms.
FIG. 1.
Quantitation of viable count and LPS of strain SMrN-9 in stool of infected mice. The number of CFU was determined by plate count and expressed as log10 CFU per gram of feces (○). The amount of LPS was measured by ELISA and expressed as the equivalent number (log10) of SMrN-9 organisms per gram feces (•). At each time point, three mice were used and individual samples were assayed in triplicate. Each point represents the geometric mean value of three separate experiments. The bar at each point indicates 1 standard error of the geometric mean value. There was a significant difference between the geometric mean CFU and the geometric mean LPS quantity on days 3, 4, and 7 in malnourished mice as determined by Student’s unpaired t test (P < 0.05). In well-nourished (normonourished) mice, there was a significant difference between these two mean values only on day 2 (P < 0.05).
Stx was not detectable (less than 10 pg/mg of feces) in the stools of mice with PCM on day 1, despite the presence of considerably high numbers of viable SMrN-9 in the stool (Fig. 1A). The amount of Stx rapidly increased in the stool, in proportion to the growth of strain SMrN-9 in the stool, between days 2 and 3 of infection. After a maximum production of Stx on day 4, the amount of Stx decreased as the number of CFU of E. coli decreased. Between days 6 and 7, the amount of Stx dropped to undetectable levels in the stool. Although all of infected mice were lethargic and anorexic after day 3 of infection, blood cultures from them were all negative from day 1 through day 8.
In contrast, strain SMrN-9 did not multiply in the intestinal tracts of mice fed a full-protein diet after the animals were inoculated with 2 × 106 CFU. The number of CFU in the stool dropped to undetectable levels (<100 CFU/g of feces) between days 2 and 3 (Fig. 1B), while O157 LPS slowly decreased and remained at a level equivalent to 103 SMrN-9 organisms on day 5. However, Stx was not detected in the stool from days 1 through 8. To achieve the same level of bacterial secretion in control mice on day 1 as that in mice with PCM, the oral inoculum dose had to be increased to 5 × 109 CFU (data not shown). With this inoculum dose, infected control mice continued to secrete SMrN-9 organisms at about 5 × 105 to 6 × 106 CFU/g of feces throughout the 21-day experiment, but only 10% of them succumbed to the infection. Although Stx was detectable in the stool on days 3 and 4 of infection, no marked pathological changes were observed in the brain and kidney. In addition, Stx was not detected in the blood from days 1 through 21. These findings indicate that the minimal infectious dose for mice with PCM was at least 1,000 times lower than that for control mice.
Stx was measurable in the blood of infected mice with PCM between days 3 and 5 at amounts ranging from 20 pg/ml (on day 3) to 34 pg/ml (on day 4) (Table 5). In contrast, Stx was not detectable in the blood of infected control mice from days 1 through 8.
TABLE 5.
Quantitation of Stx in stool and serum samples of malnourished mice after infection with strain SMrN-9
| Days after infectiona | Malnourished mice
|
|
|---|---|---|
| Stool (pg/mg) | Serum (pg/ml) | |
| 1 | NDb | ND |
| 2 | 18.5 ± 2.4 | ND |
| 3 | 32.4 ± 4.3 | 20 ± 3 |
| 4 | 67.8 ± 9.3 | 34 ± 5 |
| 5 | 24.6 ± 3.5 | 26 ± 5 |
| 6 | 12.6 ± 2.2 | ND |
| 7 | ND | ND |
| 8 | ND | ND |
Mice were intragastrically infected with 2 × 106 CFU of strain SMrN-9. At each time point, three mice randomly selected from each group were sacrificed by extracting blood. Each group consisted of 24 mice. Data were obtained from two different experiments, and results are expressed as the mean ± standard deviation for six samples.
ND, not detectable.
Blood examination.
The number of leukocytes increased in the peripheral blood of infected mice with PCM after 48 h of infection (1.7-fold increase), reaching a maximum at 4 days postinfection (2.8-fold increase). The number of platelets increased slightly but not significantly during the first 2 days of infection (1.3-fold increase), returning to the normal range in the next 2 days.
With respect to serum biochemistry, only the urea nitrogen level was elevated after 4 days of infection (1.9-fold increase), with a maximum on day 6 (2.7-fold increase). The creatinine level in serum stayed within the normal range.
In contrast, no increase in leukocyte and platelet counts was found in blood samples from infected control mice, regardless of the times when blood was sampled after infection. Both urea nitrogen and creatinine levels remained within the normal range in these mice from days 1 through 6. Oral infection with 5 × 107 CFU of E. coli NP-4 induced no abnormal changes in the blood cell count and serum biochemistry in infected mice with PCM (data not shown).
Histological examination.
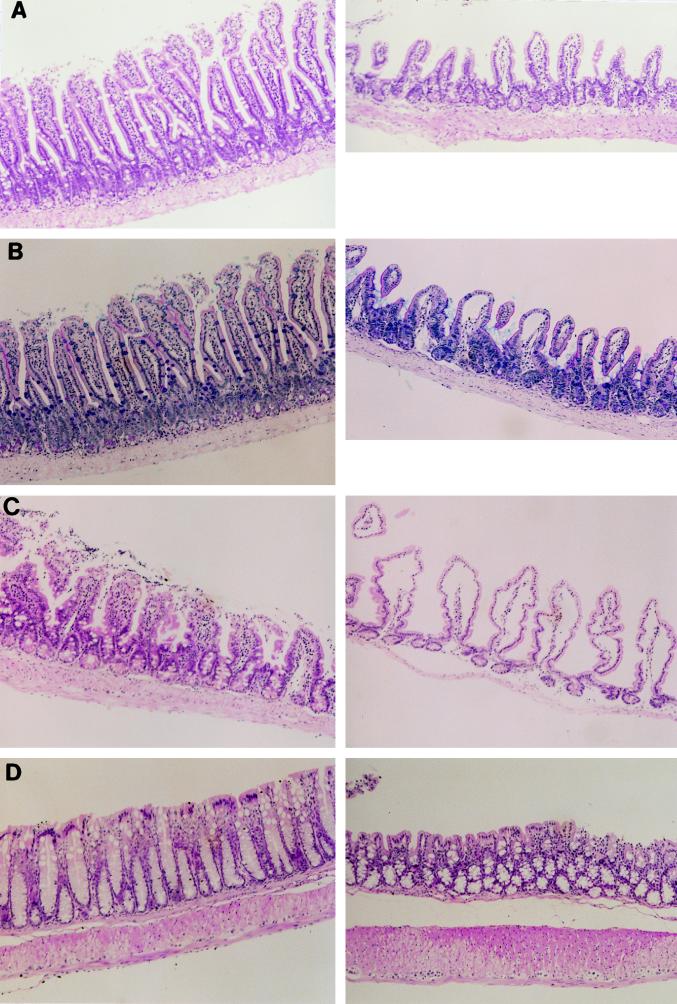
The tissue specimens obtained from mice with PCM were examined for pathological changes before infection. All tissue specimens except those from intestinal tracts obtained from mice with PCM appeared normal. The epithelium of the entire small intestine was poorly developed and edematous in mice with PCM: villi were shorter, and the lamina propria contained fewer cells compared with intestinal epithelia of well-nourished mice (Fig. 2A). Very few goblet cells were found in the epithelial cells of mice with PCM, as demonstrated by Alcian blue-PAS staining (Fig. 2B); this would probably cause a thinner surface coat of mucopolysaccharide. The cecal epithelial layer of mice with PCM was very thin, and the lamina propria was nearly devoid of cellular components (mainly lymphocytes) (Fig. 2C). The colonic epithelial layer of mice with PCM was also shorter, thinner, and irregular compared with control colonic epithelia (Fig. 2D). Moreover, goblet cells were localized mainly at the epithelial base of the colon in malnourished mice, while these cells were rich in the apical side of the epithelia in control mice.
FIG. 2.
Photomicrographs of intestines from mice with PCM. (A) HE staining of the jejunum from a control mouse (left) and a mouse with PCM (right). The villi were short and the lamina propria was edematous in the mouse with PCM. (B) Alcian blue-PAS staining of the jejunum from a control mouse (left) and a mouse with PCM (right). There were few Alcian blue-positive goblet cells at epithelial cell layers of the mouse with PCM. (C) HE staining of the cecum from a control mouse (left) and a mouse with PCM (right). Cellular components were not found at the lamina propria of cecal epithelia in the mouse with PCM. (D) HE staining of the colon from a control mouse (left) and a mouse with PCM (right). The colonic epithelial layer of the mouse with PCM was short, thin, and irregular, and goblet cells were mainly localized at the epithelial base. Magnification, ×126.
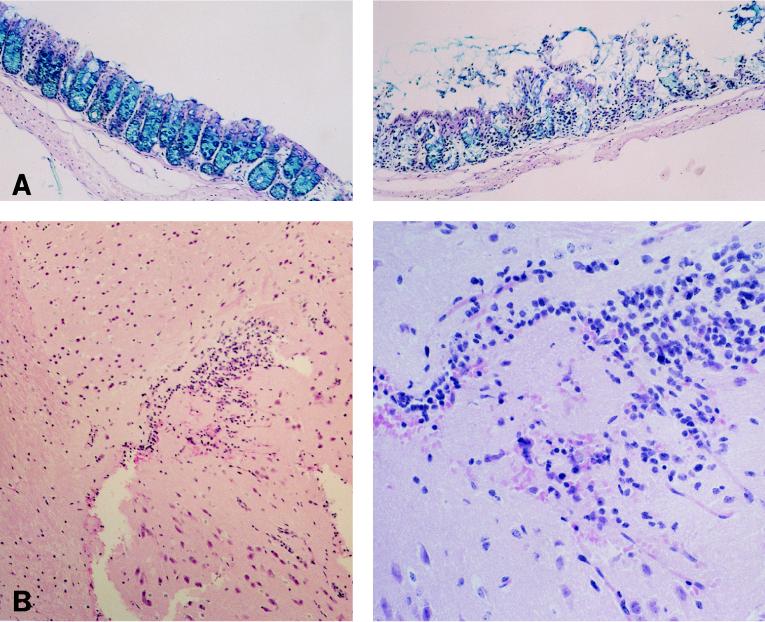
Infected mice with PCM showed minimal renal tubular degeneration, and glomeruli of these mice had remained normal by light microscopy when the tissue specimens were examined after day 6 of infection. When the intestine was removed on day 4 from infected malnourished mice shedding semisolid stool, severe erosive changes with lack of the Alcian blue-positive material were observed in the colon and cecum (Fig. 3A); hemorrhage was not noticed in any of the mice. In contrast, histopathologic changes were not observed in the intestines from infected control mice when the animals were sacrificed between days 4 and 6 of infection (Fig. 3A).
FIG. 3.
Photomicrographs of colon and brain from infected mice with PCM. (A) The colon was removed from a control mouse (left) and a mouse with PCM (right) at 6 days postinfection. Erosive changes of colonic epithelia were noticed in the mouse with PCM. Sections were stained with Alcian blue-PAS. Magnification, ×150. (B) Section of brain removed from a moribound mouse with PCM at 9 days postinfection. Left, section of cerebral cortex of the temporal lobe showing cell infiltration with hemorrhagic change (HE staining; magnification, ×150); right, high magnification (×450) of the same lesion. (C) Section of brain prepared from the same specimen as shown in panel B, showing occlusion of the vascular lumen by fibrinlike material (arrow) at the cerebral cortex (HE staining; magnification, ×450).
The most marked pathological findings were observed in the brains of moribund mice with PCM at 9 days postinfection. Hemorrhages with cell infiltration were observed in the cerebral cortex of the temporal lobe (Fig. 3B). In addition, edematous swelling of capillary endothelia, occasionally associated with pyknosis of endothelial cells, was noticed in the cerebral cortex and cerebellum of each mouse with PCM after day 5 of infection. Particularly, luminal occlusion by fibrinoid material was found in cerebral capillaries (Fig. 3C). In contrast, no histopathological changes were observed in the brains of infected control mice from days 4 through day 10.
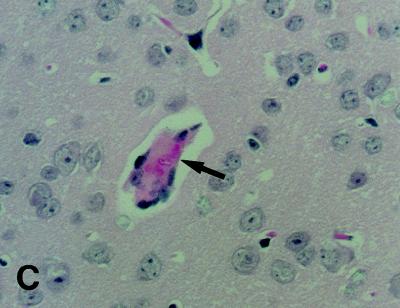
Immunocytochemical detection of Stx.
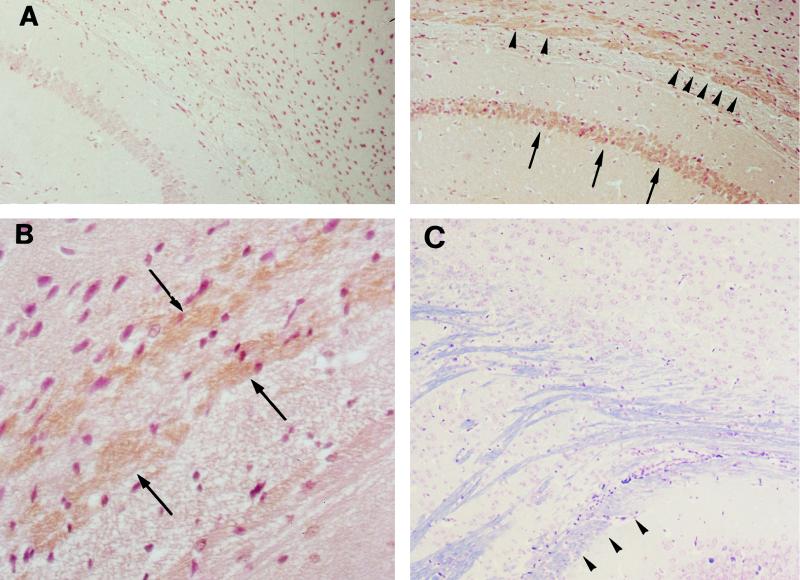
Brain tissue preparations were obtained from infected mice between days 5 and 9 of infection. Immunoreactions of Stx were not found at the hemorrhagic lesion in the cerebral cortex. In the hippocampus of infected mice with PCM, however, immunoreactions of Stx were detected at the alveus and the stratum pyramidale (Fig. 4A and B). Immunoreactions of Stx were noticed at the hippocampus as early as 5 days after infection, although the monoclonal antibody used in this study did not differentiate between Stx1 and Stx2. With LFB-HE staining, myelinated nerve fibers staining blue were noticed at the alveus of the hippocampus (Fig. 4C). However, demyelinated nerve fibers, which must be stained with eosin, were not noticed at the hippocampus tissues of moribound mice with PCM (on day 9 of infection) by LFB-HE staining. In the kidney, cortical tubular epithelia were weakly positive for immunoreaction of Stx (data not shown). In contrast, immunoreactions of Stx were negative in the kidney and brain of each infected mouse fed a full-protein diet when the animals were sacrificed at 9 days postinfection. In addition, inoculation of nonpathogenic E. coli NP-4 into mice with PCM did not induce immunoreactions of Stx in the brain and kidney.
FIG. 4.
Photomicrographs of immunoreactions of Stx in brain tissues of mice with PCM. (A) Stx was detected at the hippocampus of a mouse with PCM (right) by a monoclonal antibody to Stx1 and Stx2 at 9 days postinfection, and immunoreactions of Stx were noticed along the alveus (arrowheads) and the stratum pyramidale (arrows). No immunoreactions were observed at these areas when normal mouse IgG was used as the primary antibody (data not shown), and also the hippocampus of the infected control mouse was negative for immunoreactions of Stx (left). These sections were poststained with hematoxylin. Magnification, ×150. (B) High magnification (×450) of the section in panel A, showing the positive immunoreaction to Stx at the alveus (arrows). (C) LFB-HE staining of the hippocampus of a moribound mouse with PCM (day 9), showing the presence of myelinated nerve fibers staining blue at the alveus (arrowheads). Demyelinated fibers staining pink were not noticed at this area. Magnification, ×150.
DISCUSSION
Our findings indicate that the nondevelopment of intestinal epithelia, induced by PCM, is one of the predisposing factors to infection with EHEC in mice; among E. coli O157:H7 strains tested, Stx2-producing E. coli O157:H7 is highly pathogenic for mice with PCM. In control well-nourished mice, Stx-producing E. coli O157:H7 did not multiply in the intestinal tract when the inoculum was 2 × 106 CFU. In addition, we confirmed the results of a previous report (2) demonstrating that Stx affects the CNS rather than the kidney in mice, which may be the major cause of the acute death of infected mice with PCM.
The reason why mice with PCM are highly susceptible to Stx-producing E. coli O157:H7 was not clarified in this study. Chan et al. (6) have shown that PCM selectively affects the cellular immune responses to Mycobacterium tuberculosis. However, Stx-producing E. coli O157:H7 causes a rapid development of infection in mice with PCM, resulting in acute death of the infected mice. In such rapid progression of the disease, the cellular immunity diminished by PCM may not account for the early resistance of mice with PCM to Stx-producing E. coli O157:H7. Rather, a nonspecific defense system may be affected by PCM. For example, physical barriers of the intestinal epithelia may be disturbed by PCM, since the development of intestinal epithelia is poor in mice with PCM in comparison with that in mice fed a full-protein diet. This difference may mimic the difference between a mucosal membrane in an intact animal and in a tissue culture model; real mucosal surfaces are covered with a thick layer of mucus, but cultured cells lack a mucus layer. In humans, both children and the aged are especially prone to infection with Stx-producing E. coli. This seems to be attributable to either the nondevelopment or diminution of intestinal physical barriers in these age brackets, since the structure of the intestine usually attains the adult shape at about 10 years of age.
In most mouse models for Stx-producing E. coli, high doses of the bacteria are required for the establishment of infection. Without streptomycin treatment, the minimal infectious dose was more than 1,000-fold lower for mice with PCM than for mice fed a full-protein diet. Streptomycin treatment is known to reduce the number of facultative anaerobic bacteria in the intestines (26), thereby enhancing the colonization of infecting pathogens. In this study, however, streptomycin treatment did not enhance the mortality rate of infected mice with PCM, which means that PCM not only affects the development of intestinal wall but also alters bacterial flora in the intestines.
Fujii et al. (11) used mitomycin (MMC) in their mouse model for infection with Stx-producing E. coli O157:H7. MMC is known to function as an agent not only to activate temperate phages encoding Stx to result in an increased yield of the toxins (2) but also to induce microangiopathy (3, 38). Although the precise role of MMC in their mouse model has not been determined, their speculation is that MMC disturbs mucosal epithelia and subsequently increases intestinal permeability (11). Judging from histological study, PCM may be a substitute for treatment of MMC in our mouse model. In fact, the minimal level of tumor necrosis factor alpha (TNF-α) was measurable in the blood of mice with PCM after infection, while TNF-α was not detectable in infected mice fed a full-protein diet (20a). It is therefore a plausible assumption that not only Stx but also O157 LPS is absorbed through intestinal epithelia of malnourished mice to the extent that it can induce TNF-α production in the bloodstream. It has been reported that LPS augments the in vivo effect of Stx2 (5) and that the in vitro cytotoxicity of Stx to vascular endothelial cells is enhanced in the presence of LPS (24), TNF-α (23), and interleukin-1β (17, 23). Based on these reports, the enhanced absorption of O157 LPS and Stx by the increased permeability of intestinal mucosal epithelia is likely to cause the progression of infection with Stx-producing E. coli O157:H7 in mice with PCM.
On day 1 of infection, a large number of strain SMrN-9 cells were isolated from the stool of infected mice with PCM, while Stx was not detected in the stool of any mice with PCM on day 1. The quantity of O157 LPS, as expressed by the equivalent number of strain SMrN-9 cells, remained almost at the same level as the number of CFU of strain SMrN-9 during the first 2 days. These facts may imply that most of the inoculated SMrN-9 organisms passed through the intestinal tract not only without producing Stx to a detectable level but also without bacteriolysis. In mice with PCM, the fecal Stx level rose as the number of CFU of strain SMrN-9 increased after day 2. Interestingly, the number of viable SMrN-9 organisms was larger only by about 0.5 log on day 4 than on day 1, while Stx was detectable in the stool on day 4 but not on day 1. These findings indicate that the increase in the quantity of Stx in the stool may be due to the multiplication of the bacteria. After maximal multiplication on day 4, the number of CFU of SMrN-9 decreased linearly in accordance with the decrease in the quantity of Stx in the stool. After that, bacteriolysis of these organisms may occur, probably releasing O157 LPS but not Stx to a great extent; this may be the reason why only O157 LPS remained at detectable levels in the stool after day 6 of infection. Based on the data obtained from the measurement of Stx and the number of CFU of SMrN-9 in the stool, it is likely that SMrN-9 organisms produce in vivo Stx to detectable levels during multiplication.
Histological changes in the kidney of infected mice with PCM were noticed only at the tubules and were not remarkable. In humans with EHEC-mediated HUS, not only cortical necrosis but also severe glomerular damage was observed (10). However, there was no light microscopic evidence of glomerular damage in infected mice with PCM, which is in agreement with a report by Wadolkowski et al. (40). It has been proposed that the failure of mice to show glomerular damage may be due to the absence of a functional receptor specific for Stx (12). Although the presence of the receptors for Stx has been demonstrated in the extract of mouse kidneys (40), further work is necessary to determine why renal damage is limited in mice.
Histopathologically, cerebral hemorrhage may be the major cause of acute death of infected mice with PCM, although immunoreactions of Stx were not found at the lesion. On the other hand, degenerative changes such as the demyelination of nerve fibers were not demonstrated by LFB-HE staining. Since infected mice with PCM usually die by 10 days postinfection, degenerative changes may not become apparent histopathologically. An immunocytochemical study using a monoclonal antibody to Stx1 and Stx2 demonstrated immunoreactions of Stx at the alveus and the stratum pyramidale of the hippocampus (Fig. 4A and B). These findings support the report by Fujii et al. (11) demonstrating that severe neurological disorder developed in rabbits injected with Stx is associated with the signal hyperintensity in the hippocampus as revealed by magnetic resonance imaging. Although it is not known what pathological changes occur after Stx binds the nerve fibers at the hippocampus, the function of the hippocampus is likely to be altered by the presence of Stx. The hippocampus belongs to the limbic system in the brain, and it communicates with the hypothalamus via afferent nerves. Alteration of function of the hippocampus therefore affects the function of the autonomic nervous system. Moreover, temporal lobe epilepsy is often seen with lesions of the hippocampus (4), and epilepsy occurred in patients who developed HUS after infection with Stx-producing E. coli in Japan (1). These facts implicate the involvement of hyppocampus lesions in the encephalopathy caused by Stx-producing E. coli.
The present study showed that PCM enhances the susceptibility of mice to intragastric infection with a relatively low dose of Stx-producing E. coli O157:H7, but PCM does not affect the resistance of mice to Stx-nonproducing E. coli or S. soerenga. The enhanced susceptibility of malnourished mice is due to the impairment of intestinal physical barriers as well as the alteration of intestinal bacterial flora, which may allow E. coli O157:H7 more easily to cause the lesion in the intestine. Based on the results obtained from our mouse model, the higher incidence of infection with Stx-producing E. coli O157:H7 in children and the aged than in other age brackets may be due to the poor development or diminution of intestinal epithelia.
ACKNOWLEDGMENTS
This work was supported in part by a Research Grant for International Medical Cooperation.
We thank K. Ohkura for valuable information and Ortho-Clinical Diagnostics (Tokyo, Japan) for providing ELISA kits.
REFERENCES
- 1.Akashi S, Joh K, Tsuji A, Hoshi H, Hayakawa T, Ihara J, Abe T, Hatori M, Mori T, Nakayama T. A severe outbreak of haemorrhagic colitis and haemolytic uraemic syndrome associated with Escherichia coli O157:H7 in Japan. Eur J Pediatr. 1994;153:650–655. doi: 10.1007/BF02190685. [DOI] [PubMed] [Google Scholar]
- 2.Al-Jumaili I, Burke D A, Scotland S M, Al-Mardini H M, Record C O. A method of enhancing verocytotoxin production by Escherichia coli. FEMS Microbiol Lett. 1992;93:121–126. doi: 10.1016/0378-1097(92)90516-q. [DOI] [PubMed] [Google Scholar]
- 3.Antman K H, Skarin A T, Mayer R J, Hargreaves H K, Canellos G P. Microangiopathic hemolytic anemia and cancer: a review. Medicine (Baltimore) 1979;58:377–384. doi: 10.1097/00005792-197909000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Babb T L, Brown W J. Pathological finding in epilepsy. In: Engl J, editor. Surgical treatment of the epilepsies. New York, N.Y: Raven Press; 1987. pp. 511–540. [Google Scholar]
- 5.Barrett T J, Potter M E, Wachsmuth I K. Bacterial endotoxin both enhances and inhibits the toxicity of Shiga-like toxin II in rabbits and mice. Infect Immun. 1989;57:3434–3437. doi: 10.1128/iai.57.11.3434-3437.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan J, Tian Y, Tanaka K E, Tsang M S, Yu K, Salgame P, Carroll D, Kress Y, Teitelbaum R, Bloom B R. Effects of protein calorie malnutrition on tuberculosis in mice. Proc Natl Acad Sci USA. 1996;93:14857–14861. doi: 10.1073/pnas.93.25.14857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cimolai N, Morrison B J, Carter J E. Risk factors for the central nervous system manifestations of gastroenteritis-associated hemolytic uremic syndrome. Pediatrics. 1992;90:616–621. [PubMed] [Google Scholar]
- 8.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2253. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis D H, Moxley R A, Andraos C Y. Edema disease-like brain lesions in gnotobiotic piglets infected with Escherichia coli serotype O157:H7. Infect Immun. 1989;57:1339–1342. doi: 10.1128/iai.57.4.1339-1342.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii J, Kinoshita Y, Kita T, Higure A, Takeda T, Tanaka N, Yoshida S. Magnetic resonance imaging and histopathological study of brain lesions in rabbits given intravenous verotoxin 2. Infect Immun. 1996;64:5053–5060. doi: 10.1128/iai.64.12.5053-5060.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii J, Kita T, Yoshida S, Kakeda T, Kobayashi H, Nakata N, Ohsato K, Mizuguchi Y. Direct evidence of neuron impairment by oral infection with verotoxin-producing Escherichia coli O157:H− in mitomycin-treated mice. Infect Immun. 1994;62:3447–3453. doi: 10.1128/iai.62.8.3447-3453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamano S, Nakanishi Y, Nara T, Seki T, Ohtani T, Oishi T, Joh K, Oikawa T, Muramatsu Y, Ogawa Y, Akashi S. Neurological manifestations of hemorrhagic colitis in the outbreak of Escherichia coli O157:H7 infection in Japan. Acta Paediatr. 1993;82:454–458. doi: 10.1111/j.1651-2227.1993.tb12721.x. [DOI] [PubMed] [Google Scholar]
- 13.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effecing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karmali M A, Petric M, Lim C, Fleming P C, Arbus G S, Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985;151:775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 15.Karmali M A, Steele B T, Petric M, Lim C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet. 1983;i:619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 16.Karpman D, Connell H, Svensson M, Scheutz F, Alm P, Svanborg C. The role of lipopolysaccharide and Shiga-like toxin in a mouse model of Escherichia coli O157:H7 infection. J Infect Dis. 1997;175:611–620. doi: 10.1093/infdis/175.3.611. [DOI] [PubMed] [Google Scholar]
- 17.Kaye S A, Louise C B, Boyd B, Lingwood C A, Obrig T G. Shiga toxin-associated hemolytic uremic syndrome: interleukin-1β, enhancement of Shiga toxin cytotoxicity toward human vascular endothelial cells in vitro. Infect Immun. 1993;61:3886–3891. doi: 10.1128/iai.61.9.3886-3891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kita E, Katsui N, Emoto M, Yanagase Y, Kashiba S. Hepatic lesions in experimental Campylobacter jejuni. J Gen Microbiol. 1986;132:3095–3103. doi: 10.1099/00221287-132-11-3095. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi K, Seto K, Makino M. Detection of two Shiga-like toxins from Escherichia coli O157:H7 isolates by the polymerase chain reaction method. Nippon Saikingaku-Zasshi (Jpn J Bacteriol) 1990;45:649–652. doi: 10.3412/jsb.45.649. [DOI] [PubMed] [Google Scholar]
- 20.Koster F, Levin J, Walker L, Tung K S K, Gilman R H, Rahaman M, Mjid A, Islam S, Williams R C. Hemolytic uremic syndrome after shigellosis. Relation to endotoxemia and circulating immune complexes. N Engl J Med. 1978;298:927–933. doi: 10.1056/NEJM197804272981702. [DOI] [PubMed] [Google Scholar]
- 20a.Kurioka, T., et al. Unpublished data.
- 21.Levine M M, Xu J G, Kaper J B, Lior H, Prado V, Tall B, Nataro J, Karch H, Wachsmuth I K. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1987;156:175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- 22.Lingwood C A, Law H, Richardson S, Petric M, Brunton J L, DeGrandis S, Karmali M A. Glycolipid binding of purified and recombinant Escherichia coli produced verotoxin in vitro. J Biol Chem. 1987;262:8834–8839. [PubMed] [Google Scholar]
- 23.Louise C B, Obrig T G. Shiga toxin-associated hemolytic uremic syndrome: combined cytotoxic effects of Shiga toxin, interleukin-1β, and tumor necrosis factor alpha on human vascular endothelial cells in vitro. Infect Immun. 1991;59:4173–4179. doi: 10.1128/iai.59.11.4173-4179.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louise C B, Obrig T G. Shiga toxin-associated hemolytic uremic syndrome: combined cytotoxic effects of Shiga toxin and lipopolysaccharide (endotoxin) on human vascular endothelial cells in vitro. Infect Immun. 1992;60:1536–1543. doi: 10.1128/iai.60.4.1536-1543.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrison D M, Tyrrell D L J, Jewell L D. Colonic biopsy in verotoxin-induced hemorrhagic colitis and thrombotic thrombocytopenic purpura (TTP) Am J Clin Pathol. 1986;86:108–112. doi: 10.1093/ajcp/86.1.108. [DOI] [PubMed] [Google Scholar]
- 26.Myhal M L, Laux D C, Cohen P S. Relative colonizing abilities of human fecal and K-12 strains of Escherichia coli in the large intestines of streptomycin-treated mice. Eur J Clin Microbiol. 1982;1:186–192. doi: 10.1007/BF02019621. [DOI] [PubMed] [Google Scholar]
- 27.Neild G H. Haemolytic-uraemic syndrome in practice. Lancet. 1994;343:398–401. doi: 10.1016/s0140-6736(94)91228-9. [DOI] [PubMed] [Google Scholar]
- 28.Noda M, Yutsudo T, Nakabayashi N, Hirayama T, Takeda Y. Purification and some properties of Shiga-like toxin from Escherichia coli O157:H7 that is immunologically identical to Shiga toxin. Microb Pathog. 1987;2:339–349. doi: 10.1016/0882-4010(87)90076-3. [DOI] [PubMed] [Google Scholar]
- 29.O’Brien A D, Holmes R K. Shiga and Shiga-like toxins. Microbiol Rev. 1987;51:206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.Ohkura, K. (Department of Pediatrics, Kobe Municipal Hospital, Kobe, Japan). Personal communication.
- 30.Richardson S E, Rotman T A, Jay V, Smith C R, Becker L E, Petric M, Olivieri N F, Karmali M A. Experimental verocytotoxemia in rabbits. Infect Immun. 1992;60:4154–4167. doi: 10.1128/iai.60.10.4154-4167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riley L W, Remis R S, Helegerson S D, McGee H B, Wells J G, Davis B R, Hebert R J, Olcott E S, Johnson L M, Hargrett N T, Blake P A, Cohen M L. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med. 1983;308:681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 32.Scotland S M, Smith H R, Rowe B. Two distinct toxin active on Vero cells from Escherichia coli. Lancet. 1985;ii:885–886. doi: 10.1016/s0140-6736(85)90146-1. [DOI] [PubMed] [Google Scholar]
- 33.Shimada T, Kosako Y, Isshiki Y, Hisatsune K. Enterohemorrhagic Escherichia coli O157:H7 possesses somatic (O) antigen identical with that of Salmonella O301. Cur Microbiol. 1992;25:215–217. doi: 10.1007/BF01570721. [DOI] [PubMed] [Google Scholar]
- 34.Strockbine N A, Marques L R M, Newland J W, Smith H W, Holmes R K, O’Brien A D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986;53:135–140. doi: 10.1128/iai.53.1.135-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas A, Smith H R, Willshaw G A, Rowe B. Non radioactively labelled polynucleotide and oligonucleotide DNA probes for selectively detecting Escherichia coli strains producing Vero cytotoxins VT1, VT2, and VT2 variant. Mol Cell Probes. 1990;5:129–135. doi: 10.1016/0890-8508(91)90007-7. [DOI] [PubMed] [Google Scholar]
- 36.Tzipori S, Chow C W, Powell H R. Cerebral infection with Escherichia coli O157:H7 in humans and gnotobiotic piglets. J Clin Pathol. 1988;41:1099–1103. doi: 10.1136/jcp.41.10.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Upadhyaya K, Barwick K, Fishaut M, Kashgarian M, Siegel N J. The importance of nonrenal involvement in hemolytic-uremic syndrome. Pediatrics. 1980;65:115–120. [PubMed] [Google Scholar]
- 38.Valavaara R, Nordman E. Renal complication of mitomycin C therapy with special reference to the total dose. Cancer. 1985;55:47–50. doi: 10.1002/1097-0142(19850101)55:1<47::aid-cncr2820550108>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 39.Waddell T, Head S, Petric M, Cohen A, Lingwood C. Globotriosyl ceramide is specifically recognized by the Escherichia coli verotoxin 2. Biochem Biophys Res Commun. 1988;152:674–679. doi: 10.1016/s0006-291x(88)80091-3. [DOI] [PubMed] [Google Scholar]
- 40.Wadolkowski E A, Sung L M, Burris J A, Samuel J E, O’Brien A D. Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect Immun. 1990;58:3959–3964. doi: 10.1128/iai.58.12.3959-3965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willshaw G A, Smith H R, Scotland S M, Rowe B. Cloning of genes determining the production of Vero cytotoxicity by Escherichia coli. J Gen Microbiol. 1985;131:3047–3053. doi: 10.1099/00221287-131-11-3047. [DOI] [PubMed] [Google Scholar]
- 42.Yamai S, Obara Y, Nikkawa T, Shimoda Y, Miyamoto Y. Preservation of Neisseria gonorrhoeae by the gelatin-disc method. Br J Vener Dis. 1979;55:90–93. doi: 10.1136/sti.55.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]