Abstract
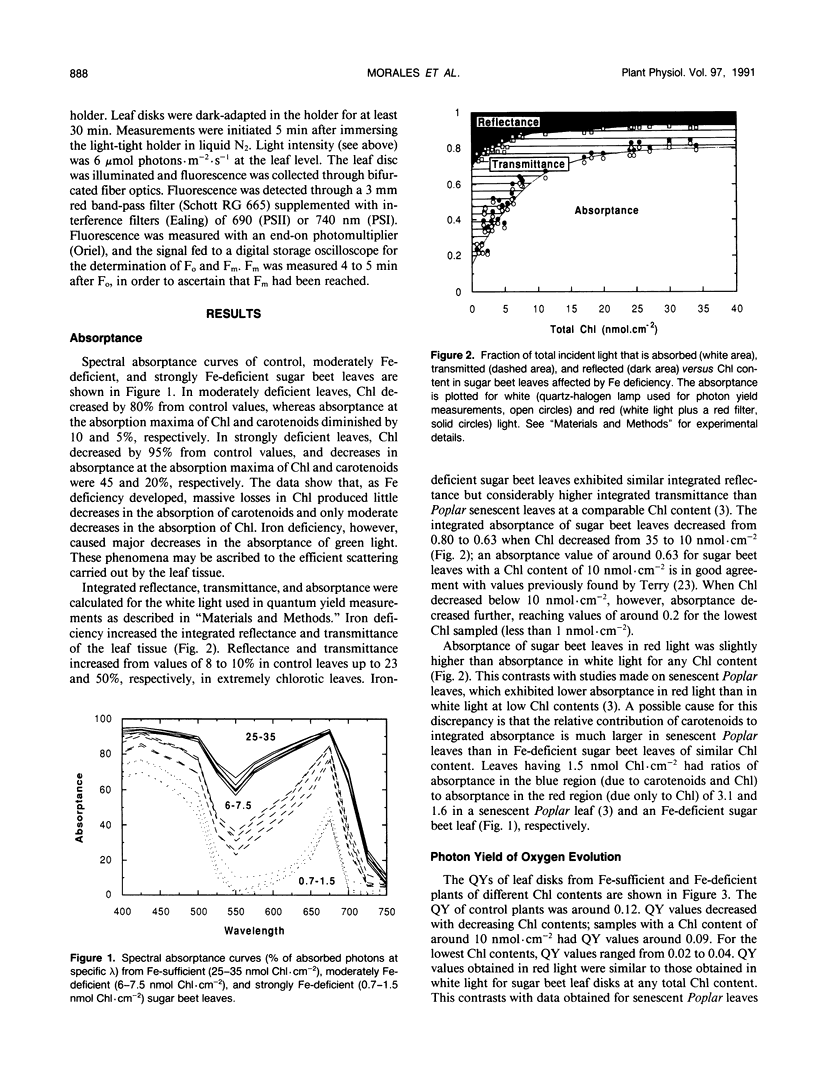
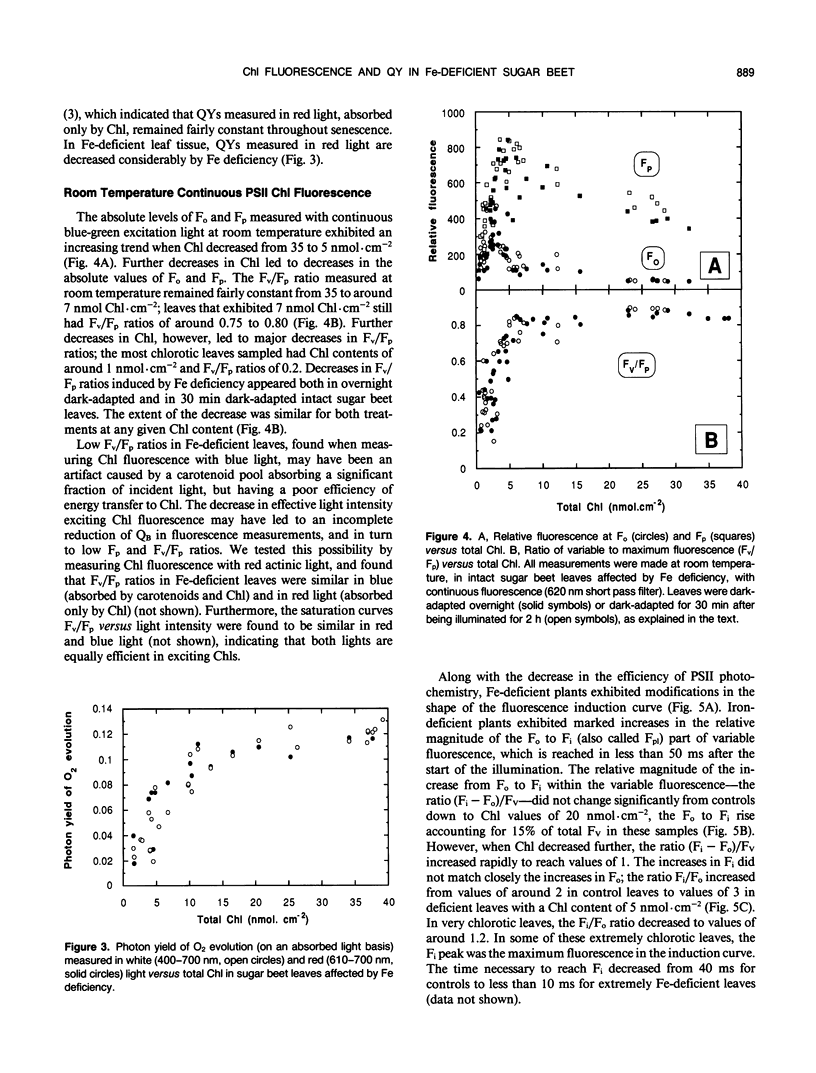
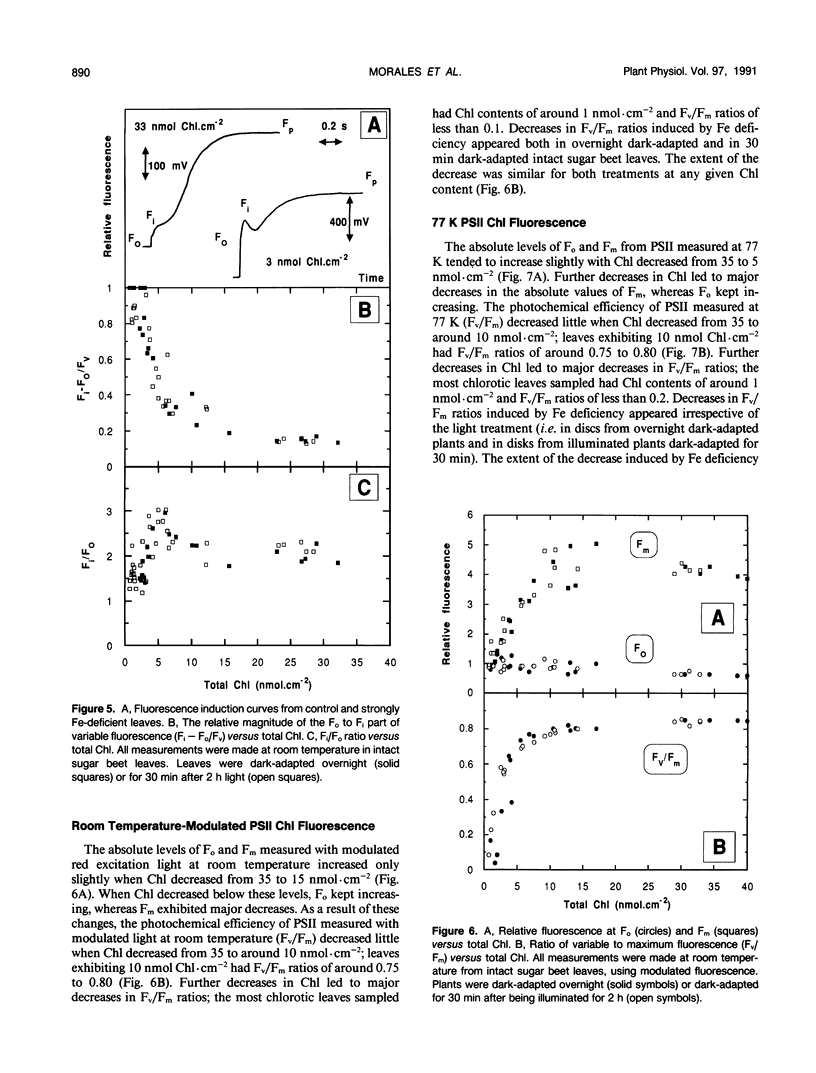
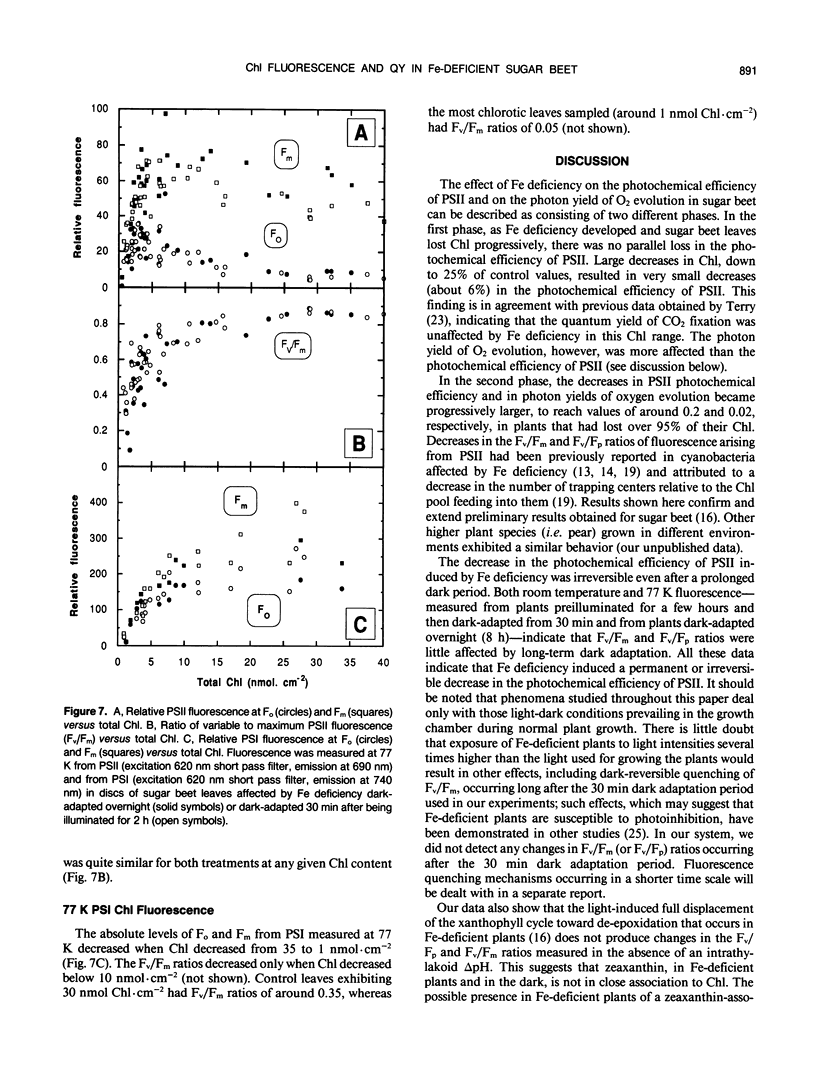
The response of sugar beet (Beta vulgaris L.) leaves to iron deficiency can be described as consisting of two phases. In the first phase, leaves may lose a large part of their chlorophyll while maintaining a roughly constant efficiency of photosystem II photochemistry; ratios of variable to maximum fluorescence decreased by only 6%, and photon yields of oxygen evolution decreased by 30% when chlorophyll decreased by 70%. In the second phase, when chlorophyll decreased below a threshold level, iron deficiency caused major decreases in the efficiency of photosystem II photochemistry and in the photon yield of oxygen evolution. These decreases in photosystem II photochemical efficiency were found both in plants dark-adapted for 30 minutes and in plants dark-adapted overnight, indicating that photochemical efficiency cannot be repaired in that time scale. Decreases in photosystem II photochemical efficiency and in the photon yield of oxygen evolution were similar when measurements were made (a) with light absorbed by carotenoids and chlorophylls and (b) with light absorbed only by chlorophylls. Leaves of iron-deficient plants exhibited a room temperature fluorescence induction curve with a characteristic intermediate peak I that increases with deficiency symptoms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. W., Winter K., Schreiber U., Schramel P. Photosynthesis and Chlorophyll Fluorescence Characteristics in Relationship to Changes in Pigment and Element Composition of Leaves of Platanus occidentalis L. during Autumnal Leaf Senescence. Plant Physiol. 1990 Apr;92(4):1184–1190. doi: 10.1104/pp.92.4.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTLER W. L. Chloroplast development: energy transfer and structure. Arch Biochem Biophys. 1961 Feb;92:287–295. doi: 10.1016/0003-9861(61)90351-4. [DOI] [PubMed] [Google Scholar]
- Barr R., Arntzen C. J. The Occurence of delta-Tocopherylquinone in Higher Plants and Its Relation to Senescence. Plant Physiol. 1969 Apr;44(4):591–598. doi: 10.1104/pp.44.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben G. Y., Osmond C. B., Sharkey T. D. Comparisons of Photosynthetic Responses of Xanthium strumarium and Helianthus annuus to Chronic and Acute Water Stress in Sun and Shade. Plant Physiol. 1987 Jun;84(2):476–482. doi: 10.1104/pp.84.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boese S. R., Huner N. P. Effect of growth temperature and temperature shifts on spinach leaf morphology and photosynthesis. Plant Physiol. 1990 Dec;94(4):1830–1836. doi: 10.1104/pp.94.4.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Govindjee Chlorophyll a fluorescence transient as an indicator of active and inactive Photosystem II in thylakoid membranes. Biochim Biophys Acta. 1990 Feb 2;1015(2):180–188. doi: 10.1016/0005-2728(90)90018-y. [DOI] [PubMed] [Google Scholar]
- Chylla R. A., Whitmarsh J. Inactive Photosystem II Complexes in Leaves : Turnover Rate and Quantitation. Plant Physiol. 1989 Jun;90(2):765–772. doi: 10.1104/pp.90.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEDHEER J. C. Effect of changes in chlorophyll concentration on photosynthetic properties. I. Fluorescence and absorption of greening bean leaves. Biochim Biophys Acta. 1961 Aug 19;51:494–504. doi: 10.1016/0006-3002(61)90605-9. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Organization and Function of Chlorophyll in Membranes of Cyanobacteria during Iron Starvation. Plant Physiol. 1983 Oct;73(2):250–256. doi: 10.1104/pp.73.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales F., Abadía A., Abadía J. Characterization of the Xanthophyll Cycle and Other Photosynthetic Pigment Changes Induced by Iron Deficiency in Sugar Beet (Beta vulgaris L.). Plant Physiol. 1990 Oct;94(2):607–613. doi: 10.1104/pp.94.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale P. J., Melis A. Activation of a Reserve Pool of Photosystem II in Chlamydomonas reinhardtii Counteracts Photoinhibition. Plant Physiol. 1990 Apr;92(4):1196–1204. doi: 10.1104/pp.92.4.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riethman H. C., Sherman L. A. Purification and characterization of an iron stress-induced chlorophyll-protein from the cyanobacterium Anacystis nidulans R2. Biochim Biophys Acta. 1988 Sep 14;935(2):141–151. doi: 10.1016/0005-2728(88)90211-3. [DOI] [PubMed] [Google Scholar]
- Terry N. Limiting Factors in Photosynthesis: I. USE OF IRON STRESS TO CONTROL PHOTOCHEMICAL CAPACITY IN VIVO. Plant Physiol. 1980 Jan;65(1):114–120. doi: 10.1104/pp.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Las Rivas J., Abadía A., Abadía J. A New Reversed Phase-HPLC Method Resolving All Major Higher Plant Photosynthetic Pigments. Plant Physiol. 1989 Sep;91(1):190–192. doi: 10.1104/pp.91.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]


