Abstract
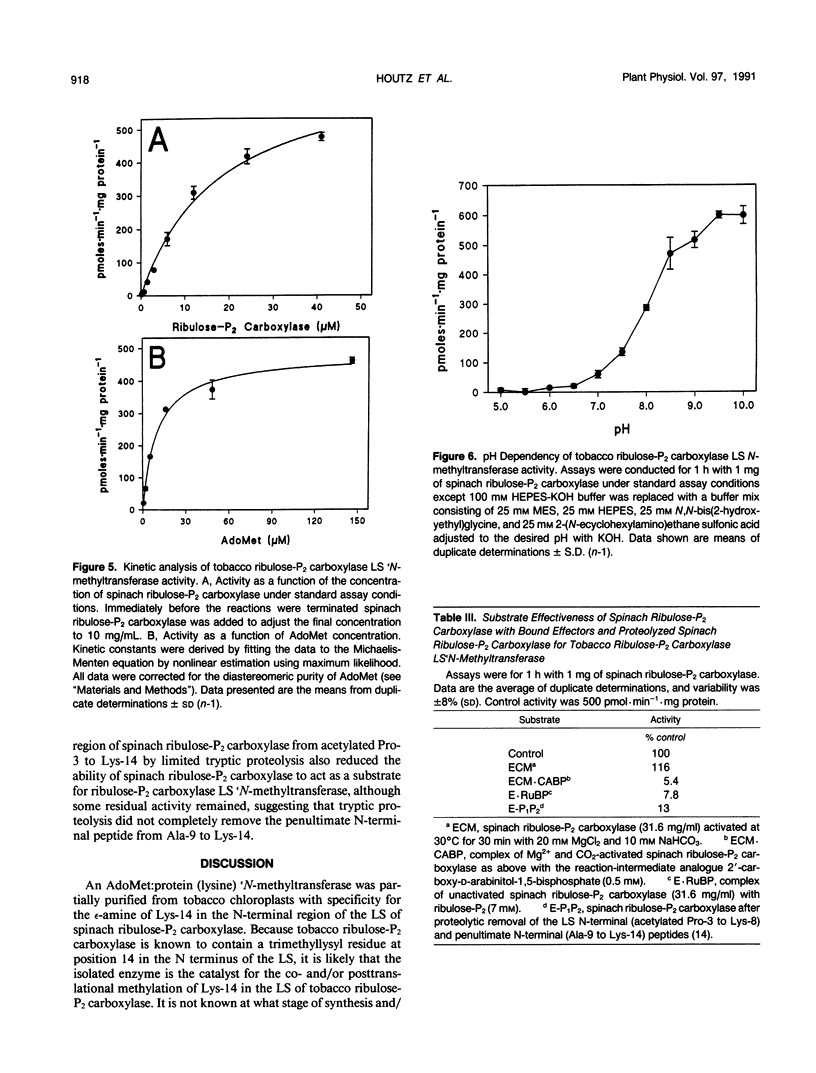
The large subunit (LS) of tobacco (Nicotiana rustica) ribulose-1,5-bisphosphate carboxylase/oxygenase (ribulose-P2 carboxylase) contains a trimethyllysyl residue at position 14, whereas this position is unmodified in spinach ribulose-P2 carboxylase. A protein fraction was isolated from tobacco chloroplasts by rate-zonal centrifugation and anion-exchange fast protein liquid chromatography that catalyzed transfer of methyl groups from S-adenosyl-[methyl-3H]-l-methionine to spinach ribulose-P2 carboxylase. 3H-Methyl groups incorporated into spinach ribulose-P2 carboxylase were alkaline stable but could be removed by limited tryptic proteolysis. Reverse-phase high-performance liquid chromatography of the tryptic peptides released after proteolysis showed that the penultimate N-terminal peptide from the LS of spinach ribulose-P2 carboxylase contained the site of methylation, which was identified as lysine-14. Thus, the methyltransferase activity can be attributed to S-adenosylmethionine:ribulose-P2 carboxylase LS (lysine) `N-methyltransferase, a previously undescribed chloroplast enzyme. The partially purified enzyme was specific for ribulose-P2 carboxylase and exhibited apparent Km values of 10 micromolar for S-adenosyl-l-methionine and 18 micromolar for ribulose-P2 carboxylase, a Vmax of 700 picomoles CH3 groups transferred per minute per milligram protein, and a broad pH optimum from 8.5 to 10.0. S-Adenosylmethionine:ribulose-P2 carboxylase LS (lysine)εN-methyltransferase was capable of incorporating 24 3H-methyl groups per spinach ribulose-P2 carboxylase holoenzyme, forming 1 mole of trimethyllysine per mole of ribulose-P2 carboxylase LS, but was inactive on ribulose-P2 carboxylases that contain a trimethyllysyl residue at position 14 in the LS. The enzyme did not distinguish between activated (Mg2+ and CO2) and unactivated forms of ribulose-P2 carboxylase as substrates. However, complexes of activated ribulose-P2 carboxylase with the reaction-intermediate analogue 2′-carboxy-d-arabinitol-1,5-bisphosphate, or unactivated spinach ribulose-P2 carboxylase with ribulose-1,5-bisphosphate, were poor substrates for tobacco LS εN-methyltransferase.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Black M. T., Meyer D., Widger W. R., Cramer W. A. Light-regulated methylation of chloroplast proteins. J Biol Chem. 1987 Jul 15;262(20):9803–9807. [PubMed] [Google Scholar]
- Chang F. N., Cohen L. B., Navickas I. J., Chang C. N. Purification and properties of a ribosomal protein methylase from Eschericha coli Q13. Biochemistry. 1975 Nov 4;14(22):4994–4998. doi: 10.1021/bi00693a032. [DOI] [PubMed] [Google Scholar]
- Chapman M. S., Suh S. W., Curmi P. M., Cascio D., Smith W. W., Eisenberg D. S. Tertiary structure of plant RuBisCO: domains and their contacts. Science. 1988 Jul 1;241(4861):71–74. doi: 10.1126/science.3133767. [DOI] [PubMed] [Google Scholar]
- DiMaria P., Kim S., Paik W. K. Cytochrome c specific methylase from wheat germ. Biochemistry. 1982 Mar 2;21(5):1036–1044. doi: 10.1021/bi00534a033. [DOI] [PubMed] [Google Scholar]
- Gregori L., Marriott D., Putkey J. A., Means A. R., Chau V. Bacterially synthesized vertebrate calmodulin is a specific substrate for ubiquitination. J Biol Chem. 1987 Feb 25;262(6):2562–2567. [PubMed] [Google Scholar]
- Gregori L., Marriott D., West C. M., Chau V. Specific recognition of calmodulin from Dictyostelium discoideum by the ATP, ubiquitin-dependent degradative pathway. J Biol Chem. 1985 May 10;260(9):5232–5235. [PubMed] [Google Scholar]
- Houtz R. L., Mulligan R. M. Protection of tryptic-sensitive sites in the large subunit of ribulosebisphosphate carboxylase/oxygenase by catalysis. Plant Physiol. 1991 May;96(1):335–339. doi: 10.1104/pp.96.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtz R. L., Stults J. T., Mulligan R. M., Tolbert N. E. Post-translational modifications in the large subunit of ribulose bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1855–1859. doi: 10.1073/pnas.86.6.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettleborough C. A., Parry M. A., Burton S., Gutteridge S., Keys A. J., Phillips A. L. The role of the N-terminus of the large subunit of ribulose-bisphosphate carboxylase investigated by construction and expression of chimaeric genes. Eur J Biochem. 1987 Dec 30;170(1-2):335–342. doi: 10.1111/j.1432-1033.1987.tb13704.x. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Knight S., Andersson I., Brändén C. I. Crystallographic analysis of ribulose 1,5-bisphosphate carboxylase from spinach at 2.4 A resolution. Subunit interactions and active site. J Mol Biol. 1990 Sep 5;215(1):113–160. doi: 10.1016/S0022-2836(05)80100-7. [DOI] [PubMed] [Google Scholar]
- Larimer F. W., Lee E. H., Mural R. J., Soper T. S., Hartman F. C. Intersubunit location of the active site of ribulose-bisphosphate carboxylase/oxygenase as determined by in vivo hybridization of site-directed mutants. J Biol Chem. 1987 Nov 15;262(32):15327–15329. [PubMed] [Google Scholar]
- Lukas T. J., Wiggins M. E., Watterson D. M. Amino Acid sequence of a novel calmodulin from the unicellular alga chlamydomonas. Plant Physiol. 1985 Jul;78(3):477–483. doi: 10.1104/pp.78.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak D. R., Clarke M., Roberts D. M., Watterson D. M. Structural and functional properties of calmodulin from the eukaryotic microorganism Dictyostelium discoideum. Biochemistry. 1984 Jun 19;23(13):2891–2899. doi: 10.1021/bi00308a007. [DOI] [PubMed] [Google Scholar]
- McCurry S. D., Gee R., Tolbert N. E. Ribulose-1,5-bisphosphate carboxylase/oxygenase from spinach, tomato, or tobacco leaves. Methods Enzymol. 1982;90(Pt E):515–521. doi: 10.1016/s0076-6879(82)90178-1. [DOI] [PubMed] [Google Scholar]
- Morino H., Kawamoto T., Miyake M., Kakimoto Y. Purification and properties of calmodulin-lysine N-methyltransferase from rat brain cytosol. J Neurochem. 1987 Apr;48(4):1201–1208. doi: 10.1111/j.1471-4159.1987.tb05647.x. [DOI] [PubMed] [Google Scholar]
- Mulligan R. M., Houtz R. L., Tolbert N. E. Reaction-intermediate analogue binding by ribulose bisphosphate carboxylase/oxygenase causes specific changes in proteolytic sensitivity: the amino-terminal residue of the large subunit is acetylated proline. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1513–1517. doi: 10.1073/pnas.85.5.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtaugh T. J., Rowe P. M., Vincent P. L., Wright L. S., Siegel F. L. Posttranslational modification of calmodulin. Methods Enzymol. 1983;102:158–170. doi: 10.1016/s0076-6879(83)02017-0. [DOI] [PubMed] [Google Scholar]
- Niemi K. J., Adler J., Selman B. R. Protein methylation in pea chloroplasts. Plant Physiol. 1990 Jul;93(3):1235–1240. doi: 10.1104/pp.93.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J., Tolbert N. E., Barker R. Interaction of ribulosebisphosphate carboxylase/oxygenase with transition-state analogues. Biochemistry. 1980 Mar 4;19(5):934–942. doi: 10.1021/bi00546a018. [DOI] [PubMed] [Google Scholar]
- Roberts D. M., Crea R., Malecha M., Alvarado-Urbina G., Chiarello R. H., Watterson D. M. Chemical synthesis and expression of a calmodulin gene designed for site-specific mutagenesis. Biochemistry. 1985 Sep 10;24(19):5090–5098. doi: 10.1021/bi00340a020. [DOI] [PubMed] [Google Scholar]
- Roberts D. M., Rowe P. M., Siegel F. L., Lukas T. J., Watterson D. M. Trimethyllysine and protein function. Effect of methylation and mutagenesis of lysine 115 of calmodulin on NAD kinase activation. J Biol Chem. 1986 Feb 5;261(4):1491–1494. [PubMed] [Google Scholar]
- Robinson S. P., Streusand V. J., Chatfield J. M., Portis A. R. Purification and assay of rubisco activase from leaves. Plant Physiol. 1988 Dec;88(4):1008–1014. doi: 10.1104/pp.88.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe P. M., Wright L. S., Siegel F. L. Calmodulin N-methyltransferase. Partial purification and characterization. J Biol Chem. 1986 May 25;261(15):7060–7069. [PubMed] [Google Scholar]
- Salvucci M. E., Portis A. R., Jr, Ogren W. L. Purification of ribulose-1, 5-bisphosphate carboxylase/oxygenase with high specific activity by fast protein liquid chromatography. Anal Biochem. 1986 Feb 15;153(1):97–101. doi: 10.1016/0003-2697(86)90066-7. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Rasched I., Knippers R. A histone H4-specific methyltransferase. Properties, specificity and effects on nucleosomal histones. Biochim Biophys Acta. 1981 Oct 27;655(3):349–358. doi: 10.1016/0005-2787(81)90045-9. [DOI] [PubMed] [Google Scholar]


