Abstract
Background
We assessed the laboratory diagnosis and treatment of invasive fungal disease (IFD) in Italy to detect limitations and potential for improvement.
Methods
The survey was available online at www.clinicalsurveys.net/uc/IFI management capacity/, and collected variables such as (a) institution profile, (b) perceptions of IFD in the respective institution, (c) microscopy, (d) culture and fungal identification, (e) serology, (f) antigen detection, (g) molecular tests, (h) susceptibility testing and (i) therapeutic drug monitoring (TDM).
Results
The laboratory capacity study received responses from 49 Italian centres, with an equitable geographical distribution of locations. The majority of respondents (n = 36, 73%) assessed the occurrence of IFD as moderate-high, with Aspergillus spp. being the pathogen of highest concern, followed by Candida spp. and Mucorales. Although 46 (94%) of the institutions had access to microscopy, less than half of them performed direct microscopy on clinical specimens always when IFD was suspected. Cultures were available in all assessed laboratories, while molecular testing and serology were available in 41 (83%), each. Antigen detection tests and antifungal drugs were also generally accessible (> 90%) among the participating institutions. Nevertheless, access to TDM was limited (n = 31, 63%), with a significant association established between therapeutic drug monitoring availability and higher gross domestic product per capita.
Conclusions
Apart from TDM, Italy is adequately prepared for the diagnosis and treatment of IFD, with no significant disparities depending on gross domestic product. Future efforts may need to focus on enhancing the availability and application of direct microscopic methods, as well as TDM, to promote optimal treatment and better patient outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s15010-023-02084-x.
Keywords: Italy; Mycology; Therapeutic drug monitoring; Antifungals; Diagnostic capacity, microscopy; Culture, serology; Antigen; Molecular test
Introduction
Invasive fungal diseases (IFDs) are a significant clinical threat, affecting a wide spectrum of patients, notably those who are immunocompromised or undergoing invasive procedures due to their associated high morbidity and mortality rates [1–6]. Despite their prevalence, IFD are still under-diagnosed in many countries, owing to a lack of knowledge among healthcare practitioners [7, 8] and the use of suboptimal diagnostic tools at healthcare facilities [9, 10]. Moreover, once identified, treatment of IFD remains particularly difficult [11] due to the scarcity of effective antifungal therapies [12, 13], along with associated toxicity [14] and constant monitoring requirements [15, 16], resulting in a huge unmet demand for patients and an increased healthcare burden.
Recently, the European Confederation of Medical Mycology (ECMM) released the results of a study done in 45 European countries to define the diagnostic methodologies used in clinical microbiology laboratories for IFD diagnosis and to examine the availability of antifungal therapies [17]. The study found significant differences between laboratories and countries, prompting national investigations to better understand specific concerns and support focused policy development [17].
We conducted a survey to determine the spectrum of diagnostic tests presently utilized by Italian institutions for IFD diagnosis and to define the current antifungal armamentarium available.
Methods
This was a multi-centre questionnaire-based study that took place between March and November 2022, with data collected using an online electronic case report form hosted at www.clinicalsurveys.net/uc/IFI management capacity/. TIVIAN GmbH, Cologne, Germany (EFS Summer 2021). Each participant's answers were validated before the analysis to guarantee data correctness and completeness. The survey assessed variable topics relevant for diagnosis and treatment of IFD, including (a) institution profile, (b) perceptions on incidence and relevance of IFD at the particular institution, (c) microscopy, (d) culture and fungal identification, (e) serology, (f) antigen detection, (g) molecular assays, and (h) therapeutic drug monitoring (TDM). Participants were asked to respond dichotomously, stating whether or not the relevant method was accessible at their individual locations. In the case of serology, antigen detection, molecular testing, and TDM laboratories were approached to specify whether these methods were accessible onsite or at an outsourced institution. A Likert scale was used to estimate the prevalence of IFD, with responses ranging from 1 (extremely low) to 5 (very high) (Supplementary Table 1). Researchers from all regions of Italy, who are daily involved in the management of patients with IFIs (e.g., infectious disease specialists, microbiologists, internists), were contacted by mass email, which targeted not only the authors' close collaborators but also members of key scientific organizations such as the International Society of Human and Animal Mycology (ISHAM; www.isham.org), the European Confederation for Medical Mycology (ECMM; www.ecmm.info), [18] Epidemiological Surveillance of Infections in Haematological Diseases of Italy (SEIFEM; www. seifem.org), and the Italian Society of Anti-infective Therapy (SITA; www. sitaonline.net). To obtain the most accurate response, we screened online scientific repositories (ClinicalTrials.gov [19], EU Clinical Trials Register [20], Google Scholar [21], PubMed [19], and ScienceDirect [22]), as well as mycology publications. This approach was used to identify a broader pool of potential participants who are experts in the field of IFIs. In addition, online calls were launched on the social networks LinkedIn® and Twitter®.
The participating institutions were classified according to their region's gross domestic product (GDP) per capita to evaluate whether there were any statistically significant variations in the availability of diagnostic tests or antifungals between Italian regions. A cut-off point was created, based on the Italian average GDP (29,661.5 € for year 2019) [23] dividing the country into regions with GDP of lower than 30,000 € and greater than 30,000 € (Supplementary Table 1). The data were summarized with frequencies and percentages. Proportions were presented in contingency tables and compared using Fisher’s exact test (variables with at least one cell with an expected value of 5) and the X2 test (variables with all cells having an expected value of > 5), where applicable. P values < 0.05 were deemed statistically significant. For statistical analysis, SPSS v27.0 was employed (SPSS, IBM Corp., Chicago, IL, United States).
Results
Throughout the study period, 49 researchers from 49 different institutions in 15/20 Italian regions responded to the online survey (Fig. 1). Among the responders, 26 (53.1%) were linked with university hospitals or national research institutions, 22 (44.9%) to non-university public hospitals, and one (2.0%) to a private hospital (Table 1). Patients with COVID-19 (n = 46, 93.9%), solid tumors (n = 43, 87.8%), haematological malignancies (n = 42, 85.7%), patients living with HIV or AIDS (n = 39, 79.6%), and patients who had undergone hematopoietic stem cell transplantations (n = 32, 65.3%) or solid organ transplantations (n = 29, 59.2%) were treated at the majority of institutions. Virtually all institutions (n = 43, 87.8%) were able to administer total parenteral nutrition, if required.
Fig. 1.
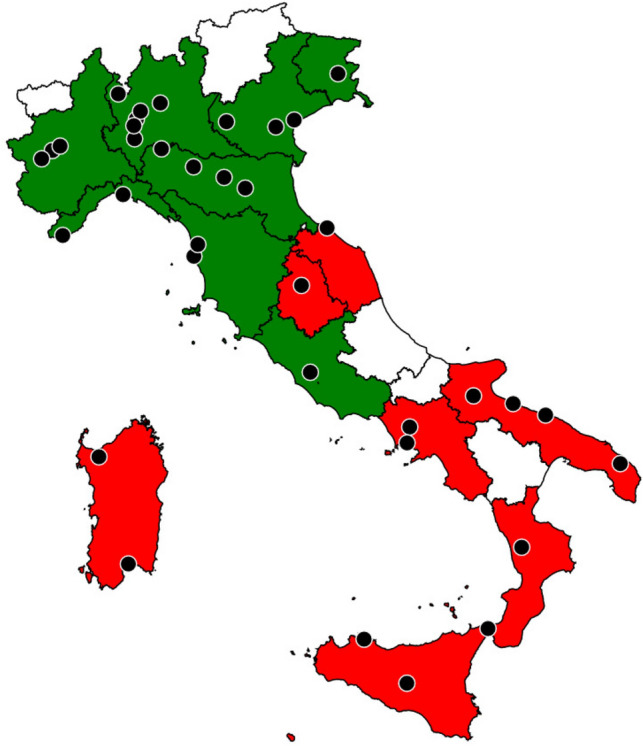
Map of participating institutions per region. Regions with a gross domestic product < 30,000 € are coloured in red. Regions with a gross domestic product > 30,000 € are coloured in green. Regions whose centres have not been included are coloured in white. If more than one participating centre from the same city, a single point is pictured
Table 1.
Baseline characteristic of participating institutions in Italy
| Overall (n = 49) | GDP | |||||
|---|---|---|---|---|---|---|
| < 30,000 € (n = 19) | > 30,000 € (n = 30) | |||||
| n | % | n | % | n | % | |
| Type of institution | ||||||
| University hospital/National research institute | 26 | 53.1 | 8 | 42.1 | 18 | 60.0 |
| Public hospital | 22 | 44.9 | 11 | 57.9 | 11 | 36.7 |
| Private hospital | 1 | 2.0 | 0 | 0.0 | 1 | 3.3 |
| Target patients | ||||||
| COVID-19 | 46 | 93.9 | 17 | 89.5 | 29 | 96.7 |
| Diabetes mellitus | 43 | 87.8 | 16 | 84.2 | 27 | 90.0 |
| Parenteral nutrition | 43 | 87.8 | 16 | 84.2 | 27 | 90.0 |
| Oncology | 43 | 87.8 | 15 | 78.9 | 28 | 93.3 |
| Hematology | 42 | 85.7 | 15 | 78.9 | 27 | 90.0 |
| HIV/AIDS | 39 | 79.6 | 14 | 73.7 | 25 | 83.3 |
| Stem cell transplantation | 32 | 65.3 | 9 | 47.4 | 23 | 76.7 |
| Neonatal ICU | 29 | 59.2 | 12 | 63.2 | 17 | 56.7 |
| Solid organ transplantation | 29 | 59.2 | 9 | 47.4 | 20 | 66.7 |
| Access to onsite microbiology laboratory? | 49 | 100.0 | 19 | 100.0 | 30 | 100.0 |
| Mycological diagnostic procedures performed? | ||||||
| Always in our institution | 29 | 59.2 | 12 | 63.2 | 17 | 56.7 |
| Part in our institution/part outsourced | 20 | 40.8 | 7 | 36.8 | 13 | 43.3 |
| IFD incidence | ||||||
| Very low | 2 | 4.1 | 2 | 10.5 | 0 | 0.0 |
| Low | 11 | 22.4 | 6 | 31.6 | 5 | 16.7 |
| Moderate | 25 | 51.0 | 11 | 57.9 | 14 | 46.7 |
| High | 11 | 22.4 | 0 | 0.0 | 11 | 36.7 |
| Very high | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Most important pathogen(s) | ||||||
| Aspergillus spp. | 45 | 91.8 | 17 | 89.5 | 28 | 93.3 |
| Candida spp. | 44 | 89.8 | 18 | 94.7 | 26 | 86.7 |
| Mucorales | 14 | 28.6 | 4 | 21.1 | 10 | 33.3 |
| Fusarium spp. | 10 | 20.4 | 2 | 10.5 | 8 | 26.7 |
| Cryptococcus spp. | 9 | 18.4 | 4 | 21.1 | 5 | 16.7 |
| Histoplasma spp. | 2 | 4.1 | 1 | 5.3 | 1 | 3.3 |
COVID-19 coronavirus disease 2019, € Euros, GDP gross domestic product, HIV/AIDS human immunodeficiency virus/acquired immunodeficiency syndrome, ICU intensive care unit, IFD invasive fungal disease, spp. species
All Italian institutions reported having a microbiology laboratory, with 29 (59.2%) of them always performing mycological diagnoses on-site. Some tests were done on-site at the remaining 20 (40.8%) institutions, while others were outsourced to external laboratories (Table 1).
When considering epidemiology, a significant proportion of respondents classified the incidence of IFD at their institutions as either moderate (n = 25, 51.0%) or high (n = 11, 22.4%). The fungal pathogens most commonly causing concern were Aspergillus spp. (n = 45, 91.8%), Candida spp. (n = 44, 89.8%), Mucorales (n = 14, 28.6%), and Fusarium spp. (n = 10, 20.4%).
When questioned about microscopy performance (Table 2), 95% (n = 46) of the institutions reported having at least one or more microscopy methods available. The most accessible tools were China/India ink (n = 34, 69.4%), silver and Giemsa stains (n = 24, 49.0%, each), followed by potassium hydroxide (n = 15, 30.6%), and calcofluor white (n = 12, 24.5%). In terms of clinical suspicion of IFD, fewer than half of the Italian institutions (n = 21, 42.9%) always conducted direct microscopy of clinical specimens, 18.4% (n = 9) did so frequently, 24.5% (n = 12) sometimes, and 12.2% (n = 6) seldom. Only one institution stated that when an IFD was suspected, it never used direct microscopy.
Table 2.
Comparison of available diagnostic techniques for mycological diagnosis in Italy
| Overall (n = 49) | GDP | ||||||
|---|---|---|---|---|---|---|---|
| < 30,000 € (n = 19) | > 30,000 € (n = 30) | ||||||
| n | % | n | % | n | % | ||
| Microscopy | 46 | 93.9 | 17 | 89.5 | 29 | 96.7 | |
| Stains | |||||||
| China/India ink | 34 | 69.4 | 14 | 73.7 | 20 | 66.7 | |
| Silver stain | 24 | 49.0 | 8 | 42.1 | 16 | 53.3 | |
| Giemsa stain | 24 | 49.0 | 7 | 36.8 | 17 | 56.7 | |
| Potassium hydroxide | 15 | 30.6 | 6 | 31.6 | 9 | 30.0 | |
| Calcofluor white | 12 | 24.5 | 4 | 21.1 | 8 | 26.7 | |
| Direct microscopy frequency when IFD suspected | |||||||
| Never | 1 | 2.0 | 1 | 5.3 | 0 | 0.0 | |
| Rarely | 6 | 12.2 | 4 | 21.1 | 2 | 6.7 | |
| Sometimes | 12 | 24.5 | 6 | 31.6 | 6 | 20.0 | |
| Often | 9 | 18.4 | 1 | 5.3 | 8 | 26.7 | |
| Always | 21 | 42.9 | 7 | 36.8 | 14 | 46.7 | |
| Access to fluorescent dye | 19 | 38.8 | 8 | 42.1 | 11 | 36.7 | |
| Direct examination in body fluids for suspected cryptococcosis | 39 | 79.6 | 15 | 78.9 | 24 | 80.0 | |
| India ink | 34 | 69.4 | 14 | 73.7 | 20 | 66.7 | |
| Other dyes | 5 | 10.2 | 1 | 5.3 | 4 | 13.3 | |
| Direct microscopy for suspected mucormycosis | 26 | 53.1 | 12 | 63.2 | 14 | 46.7 | |
| Silver stain for suspected pneumocystosis | 21 | 42.9 | 8 | 42.1 | 13 | 43.3 | |
| Culture and fungal identification | 49 | 100.0 | 19 | 100.0 | 30 | 100.0 | |
| Blood cultures for suspected fungemia | 49 | 100.0 | 19 | 100.0 | 30 | 100.0 | |
| Fungal culture media | |||||||
| Sabouraud dextrose agar | 27 | 55.1 | 10 | 52.6 | 17 | 56.7 | |
| Sabouraud dextrose agar + chloramphenicol | 26 | 53.1 | 11 | 57.9 | 15 | 50.0 | |
| Sabouraud dextrose agar + gentamicin | 22 | 44.9 | 10 | 52.6 | 12 | 40.0 | |
| Chromogen | 21 | 42.9 | 7 | 36.8 | 14 | 46.7 | |
| Selective agar (chloramphenicol + cycloheximide) | 17 | 34.7 | 6 | 31.6 | 11 | 36.7 | |
| Potato dextrose agar | 13 | 26.5 | 6 | 31.6 | 7 | 23.3 | |
| Agar Niger | 10 | 20.4 | 6 | 31.6 | 4 | 13.3 | |
| Lactrimel agar | 4 | 8.2 | 2 | 10.5 | 2 | 6.7 | |
| Available tests for specific identification | 46 | 93.9 | 18 | 94.7 | 28 | 93.3 | |
| MALDI–TOF–MS | 40 | 81.6 | 14 | 73.7 | 26 | 86.7 | |
| Automated identification (i.e., VITEK®) | 33 | 67.3 | 14 | 73.7 | 19 | 63.3 | |
| Biochemical tests (conventional mycology) | 28 | 57.1 | 13 | 68.4 | 15 | 50.0 | |
| DNA sequencing | 26 | 53.1 | 8 | 42.1 | 18 | 60.0 | |
| Mounting medium | 4 | 8.2 | 0 | 0.0 | 4 | 13.3 | |
| Antifungal susceptibility tests? | 45 | 91.8 | 16 | 84.2 | 29 | 96.7 | |
| For yeasts and moulds | 32 | 65.3 | 13 | 68.4 | 19 | 63.3 | |
| For yeasts | 13 | 26.5 | 3 | 15.8 | 10 | 33.3 | |
| Available antifungal susceptibility test technologies | |||||||
| Broth microdilution, using EUCAST standards | 23 | 46.9 | 9 | 47.4 | 14 | 46.7 | |
| VITEK® | 18 | 36.7 | 8 | 42.1 | 10 | 33.3 | |
| E-test® | 18 | 36.7 | 5 | 26.3 | 13 | 43.3 | |
| Broth microdilution, using CLSI standards | 18 | 36.7 | 3 | 15.8 | 15 | 50.0* | |
| Maximum identification capability | |||||||
| Yeasts | 49 | 100.0 | 19 | 100.0 | 30 | 100.0 | |
| Genus/species | 20 | 40.8 | 11 | 57.9 | 9 | 30.0 | |
| Genus/species/complex | 19 | 38.8 | 4 | 21.1 | 15 | 50.0 | |
| Genus/species/complex/cryptic species | 8 | 16.3 | 4 | 21.1 | 4 | 13.3 | |
| Genus | 2 | 4.1 | 0 | 0.0 | 2 | 6.7 | |
| Moulds | 49 | 100.0 | 19 | 100.0 | 30 | 100.0 | |
| Genus/species | 41 | 83.7 | 17 | 89.5 | 24 | 80.0 | |
| Genus | 8 | 16.3 | 2 | 10.5 | 6 | 20.0 | |
| Serology | 41 | 83.7 | 16 | 84.2 | 25 | 83.3 | |
| Aspergillus spp. | 39 | 79.6 | 16 | 84.2 | 23 | 76.7 | |
| Onsite | 31 | 63.3 | 12 | 63.2 | 19 | 63.3 | |
| Outsourced | 8 | 16.3 | 4 | 21.1 | 4 | 13.3 | |
| Candida spp. | 28 | 57.1 | 13 | 68.4 | 15 | 50.0 | |
| Onsite | 20 | 40.8 | 9 | 47.4 | 11 | 36.7 | |
| Outsourced | 8 | 16.3 | 4 | 21.1 | 4 | 13.3 | |
| Histoplasma spp. | 25 | 51.0 | 8 | 42.1 | 17 | 56.7 | |
| Onsite | 14 | 28.6 | 4 | 21.1 | 10 | 33.3 | |
| Outsourced | 11 | 22.4 | 4 | 21.1 | 7 | 23.3 | |
| Antigen detection | 49 | 100.0 | 19 | 100.0 | 30 | 100.0 | |
| Aspergillus overall | 42 | 85.7 | 17 | 89.5 | 25 | 83.3 | |
| Aspergillus galactomannan ELISA | 47 | 95.9 | 19 | 100.0 | 28 | 93.3 | |
| Onsite | 39 | 79.6 | 16 | 84.2 | 23 | 76.7 | |
| Outsourced | 8 | 16.3 | 3 | 15.8 | 5 | 16.7 | |
| Aspergillus galactomannan LFA | 18 | 36.7 | 8 | 42.1 | 10 | 33.3 | |
| Onsite | 14 | 28.6 | 6 | 31.6 | 8 | 26.7 | |
| Outsourced | 4 | 8.2 | 2 | 10.5 | 2 | 6.7 | |
| Aspergillus LFD | 13 | 26.5 | 6 | 31.6 | 7 | 23.3 | |
| Onsite | 10 | 20.4 | 4 | 21.1 | 6 | 20.0 | |
| Outsourced | 3 | 6.1 | 2 | 10.5 | 1 | 3.3 | |
| 1–3-Beta-d-glucan | 42 | 85.7 | 14 | 73.7 | 28 | 93.3 | |
| Onsite | 27 | 55.1 | 10 | 52.6 | 17 | 56.7 | |
| Outsourced | 15 | 30.6 | 4 | 21.1 | 11 | 36.7 | |
| Cryptococcus overall | 38 | 77.6 | 13 | 68.4 | 25 | 83.3 | |
| Cryptococcus LAT | 38 | 77.6 | 15 | 78.9 | 23 | 76.7 | |
| Onsite | 34 | 69.4 | 12 | 63.2 | 22 | 73.3 | |
| Outsourced | 4 | 8.2 | 3 | 15.8 | 1 | 3.3 | |
| Cryptococcus LFA | 20 | 40.8 | 9 | 47.4 | 11 | 36.7 | |
| Onsite | 15 | 30.6 | 5 | 26.3 | 10 | 33.3 | |
| Outsourced | 5 | 10.2 | 4 | 21.1 | 1 | 3.3 | |
| Candida antigen | 22 | 44.9 | 11 | 57.9 | 11 | 36.7 | |
| Onsite | 15 | 30.6 | 7 | 36.8 | 8 | 26.7 | |
| Outsourced | 7 | 14.3 | 4 | 21.1 | 3 | 10.0 | |
| Histoplasma | 18 | 36.7 | 7 | 36.8 | 11 | 36.7 | |
| Onsite | 8 | 16.3 | 2 | 10.5 | 6 | 20.0 | |
| Outsourced | 10 | 20.4 | 5 | 26.3 | 5 | 16.7 | |
| Molecular tests | 41 | 83.7 | 16 | 84.2 | 25 | 83.3 | |
| Pneumocystis PCR | 35 | 71.4 | 14 | 73.7 | 21 | 70.0 | |
| Onsite | 29 | 59.2 | 11 | 57.9 | 18 | 60.0 | |
| Outsourced | 6 | 12.2 | 3 | 15.8 | 3 | 10.0 | |
| Aspergillus PCR | 32 | 65.3 | 12 | 63.2 | 20 | 66.7 | |
| Onsite | 23 | 46.9 | 8 | 42.1 | 15 | 50.0 | |
| Outsourced | 9 | 18.4 | 4 | 21.1 | 5 | 16.7 | |
| Candida PCR | 25 | 51.0 | 9 | 47.4 | 16 | 53.3 | |
| Onsite | 15 | 30.6 | 4 | 21.1 | 11 | 36.7 | |
| Outsourced | 10 | 20.4 | 5 | 26.3 | 5 | 16.7 | |
| PCR for other fungi | 19 | 38.8 | 8 | 42.1 | 11 | 36.7 | |
| Onsite | 9 | 18.4 | 3 | 15.8 | 6 | 20.0 | |
| Outsourced | 10 | 20.4 | 5 | 26.3 | 5 | 16.7 | |
| Mucorales PCR | 18 | 36.7 | 9 | 47.4 | 9 | 30.0 | |
| Onsite | 9 | 18.4 | 3 | 15.8 | 6 | 20.0 | |
| Outsourced | 9 | 18.4 | 6 | 31.6 | 3 | 10.0 | |
*This difference was considered as statistically significance, p = 0.032
CLSI Clinical and Laboratory Standards Institute, DNA deoxyribonucleic acid, ELISA enzyme-linked immunosorbent assay, E test® epsilometer test, EUCAST European Committee on Antimicrobial Susceptibility Testing, € euros, GDP gross domestic product, IFD invasive fungal disease, LAT latex agglutination test, LFA lateral flow assay, LFD lateral flow device, MALDI—TOF, matrix-assisted laser desorption/ionization—time-of-flight mass spectrometer, PCR polymerase chain reaction, spp. species
All institutions had access to fungal culture medium and were able to run blood cultures to rule out fungemia. In 40 (81.6%) institutions, matrix-assisted laser desorption/ionisation time-of-flight spectrometry (MALDI-TOF-MS) was the most regularly utilized technology for species-level identification (Table 2). At 67.3% (n = 33) of the institutions, automated identification utilizing a VITEK® system was available, whereas conventional biochemical testing was accessible in 57.1% (n = 28). Access to molecular methods was relatively limited, with just over half of the institutions (n = 26, 53.1%) offering deoxyribonucleic acid (DNA) sequencing.
Only yeast susceptibility testing was accessible in 26.5% (n = 13) of the laboratories, whereas testing for both yeast and moulds was available in 65.3% (n = 32). Broth microdilution according to the European Committee on Antimicrobial Susceptibility Testing Standards (EUCAST) was available in less than half of the institutions (n = 23, 46.9%), whereas access to broth microdilution according to the Clinical and Laboratory Standards Institute (CLSI) was reported in 36.7% (n = 18). Furthermore, 36.7% (n = 18) of the institutions employed the E test® or VITEK® automated system.
Serological testing was available at all participating facilities. The majority of institutions (n = 39, 79.6%) had access to Aspergillus spp. serology, followed by Candida spp. (n = 28, 57.1%) and Histoplasma spp. (n = 25, 51%). Serological testing was mostly available on-site, with roughly one-fifth of the institutions reporting serological antibody detection outsourcing.
The availability of antigen detection for several fungi, either on site or by outsourcing to other laboratories, was quite high in our survey, as indicated in Table 2. Antigen detection for Aspergillus spp. by galactomannan enzyme immunoassay was accessible at 47 (95.9%) institutions, with eight (16.3%) exclusively through an outsourced laboratory. Similarly, 42 (85.7%) institutions performed the test either on-site (n = 27, 55.1%) or through outsourcing (n = 15, 30.6%). The availability of Cryptococcus spp. latex testing was more limited, with 34 (69.4%) facilities performing the test domestically and 4 (8.2%) relying on an outsourced laboratory.
In 41 (83.7%) of the facilities, access to polymerase chain reaction (PCR) or other molecular tests was mentioned. Molecular testing targeted Pneumocystis spp. (n = 35, 71.4%), Aspergillus spp. (n = 32, 65.3%), or Candida spp. (n = 25, 51.0%) more often.
The availability of antifungal medication in Italy is shown in Table 3. Fluconazole and itraconazole (n = 45, 91.8% each) were the most often available triazoles, followed by voriconazole (n = 44, 89.4%). In 45 (91.8%) of the institutions, at least one echinocandin was accessible, mostly micafungin (n = 45; 91.8%). Flucytosine, terbinafine, and amphotericin B formulations (particularly liposomal formulation [n = 43, 87.8%] and lipid complex formulation [n = 15, 30.6%]) were available in 44 (89.8%), 24 (49.0%), and 7 (14.3%) facilities, respectively. When therapeutic drug monitoring (TDM) was evaluated, it was primarily accessible on-site for voriconazole (n = 23, 46.9%), while it was available in an outsourced laboratory in eight (16.3%) institutions. Posaconazole, itraconazole, and 5-flucytosine TDM were available at 22 (44.9%), 14 (28.6%), and 6 (12.2%) institutions, respectively, both on-site and in an outsourced laboratory.
Table 3.
Comparison of available drugs for antifungal treatment in Italy
| Overall (n = 49) | GDP | |||||
|---|---|---|---|---|---|---|
| < 30,000 € (n = 19) | > 30,000 € (n = 30) | |||||
| n | % | n | % | n | % | |
| Available antifungals | ||||||
| Amphotericin B | 44 | 89.8 | 18 | 94.7 | 26 | 86.7 |
| Deoxycholate | 9 | 18.4 | 1 | 5.3 | 8 | 26.7 |
| Lipid complex | 15 | 30.6 | 6 | 31.6 | 9 | 30.0 |
| Liposomal | 43 | 87.8 | 17 | 89.5 | 26 | 86.7 |
| Echinocandins | 45 | 91.8 | 18 | 94.7 | 27 | 90.0 |
| Anidulafungin | 36 | 73.5 | 15 | 78.9 | 21 | 70.0 |
| Caspofungin | 36 | 73.5 | 13 | 68.4 | 23 | 76.7 |
| Micafungin | 45 | 91.8 | 18 | 94.7 | 27 | 90.0 |
| Triazoles | 45 | 91.8 | 18 | 94.7 | 27 | 90.0 |
| Fluconazole | 45 | 91.8 | 18 | 94.7 | 27 | 90.0 |
| Isavuconazole | 39 | 79.6 | 16 | 84.2 | 23 | 76.7 |
| Itraconazole | 44 | 89.8 | 18 | 94.7 | 26 | 86.7 |
| Posaconazole | 41 | 83.7 | 18 | 94.7 | 23 | 76.7 |
| Voriconazole | 44 | 89.8 | 17 | 89.5 | 27 | 90.0 |
| Flucytosine | 24 | 49.0 | 11 | 57.9 | 13 | 43.3 |
| Terbinafine | 7 | 14.3 | 2 | 10.5 | 5 | 16.7 |
| Therapeutic drug monitoring | 31 | 63.3 | 7 | 36.8 | 24 | 80.0* |
| Flucytosine | 6 | 12.2 | 1 | 5.3 | 5 | 16.7 |
| Onsite | 5 | 10.2 | 1 | 5.3 | 4 | 13.3 |
| Outsourced | 1 | 2.0 | 0 | 0.0 | 1 | 3.3 |
| Itraconazole | 14 | 28.6 | 3 | 15.8 | 11 | 36.7 |
| Onsite | 10 | 20.4 | 2 | 10.5 | 8 | 26.7 |
| Outsourced | 4 | 8.2 | 1 | 5.3 | 3 | 10.0 |
| Posaconazole | 22 | 44.9 | 3 | 15.8 | 19 | 63.3* |
| Onsite | 16 | 32.7 | 2 | 10.5 | 14 | 46.7 |
| Outsourced | 6 | 12.2 | 1 | 5.3 | 5 | 16.7 |
| Voriconazole | 31 | 63.3 | 7 | 36.8 | 24 | 80.0** |
| Onsite | 23 | 46.9 | 6 | 31.6 | 17 | 56.7 |
| Outsourced | 8 | 16.3 | 1 | 5.3 | 7 | 23.3 |
GDP gross domestic product, US$ United States dollar
*This difference was considered as statistically significance, p = 0.005
**This difference was considered as statistically significance, p < 0.001
When the diagnostic capacity of participating institutions was compared by the GDP of their affiliated regions, only the use of broth microdilution using CLSI standards and therapeutic drug monitoring of antifungal agents revealed significant differences, with institutions located in regions with a GDP greater than 30,000 euros reporting more frequently (Tables 2 and 3).
Discussion
This is Italy’s first nationwide assessment on the diagnostic and therapeutic capacities for patients with invasive fungal infections. The ECMM study group recently published a survey in 45 European nations, including Italy [17]. Nevertheless, limited numbers of Italian hospitals took part (n = 38), whereas the current study included 49 hospitals of various sizes and geographical regions. Our results, which provide a more comprehensive representation of the Italian condition at a national-level, show that our institutions seem well-prepared to manage IFD, with remarkably uniform adherence to current norms regardless of regional financing. Yet, the research indicated several diagnostic and treatment gaps, necessitating future attempts to improve IFD management and concentrate funding in relevant areas.
In terms of IFD epidemiology, one of the most significant factors to consider is the self-perception of the most relevant infections indicated by Italian respondents. According to the findings of this survey, Aspergillus spp. was the most important species, followed by Candida spp., Mucorales, and Fusarium spp. These findings contrast with the occurrence of infections caused by these pathogens in Italy in 2008 [24, 25], as well as recent European studies, which revealed a considerable concern, primarily for Candida spp. [17]. The enhanced awareness of Aspergillus spp. is probable due to the high number of patients with COVID-19 or haematological malignancies who are seen at participating institutions and are at a higher risk of acquiring invasive aspergillosis [26–28]. Yet, the rising worry in Italy regarding the development of azole-resistant Aspergillus spp. strains and the related high death rate may have contributed to the reported results [29–32].
This survey also suggests that, despite the presence of onsite microbiology laboratories in all Italian centres, approximately 40% of clinicians lacked complete access to all necessary tests within their own laboratories. This resulted in the need to send samples outside their hospitals for diagnostic testing. Consequently, concerns arise regarding the potential impact on turnaround times, particularly for centres managing complex patients, such as those with haematological malignancies or undergoing transplants.
Despite the fact that microscopy stains were accessible in more than 90% of centres, direct microscopy findings were always done on fewer than half of IFD suspects. This is in contrast to existing guidelines, which indicate that clinical specimens from patients suspected of having IFD should be analysed as soon as possible and that direct microscopy results should be reported as soon as possible to help in the diagnosis and management of IFD [33–35]. Moreover, our investigation discovered even poorer accessibility to direct testing employing optical brighteners, with calcofluor white staining available in less than 25% of the facilities studied. This conclusion cannot be attributable to cost concerns, as higher availability of this technology was reported in Asia/Pacific areas with similar GDP (60%) [17] and in Europe (~ 38%) in general [7, 9]. The restricted availability of this staining, which allows for the quick and presumptive identification of fungal species, might be addressed by advocating with key Italian institutions to increase the availability of calcofluor white stain, notably for the diagnosis of aspergillosis [35] and mucormycosis [12].
All of the Italian institutions surveyed were able to process materials for culture and fungal identification. Remarkably, more than 80% of respondents had access to the MALDI-TOF–MS technology for fast fungal identification, which is substantially higher than the proportion reported in Africa (17.5%) [36], Asia (43.0%) [17], and other European countries (~ 61.0%) [7, 9]. This high level of accessibility is good since MALDI-TOF-MS decreases response times [37], has proven to be cost-effective [37], does not require sophisticated technical expertise, and can definitely be useful for identifying Candida spp., Aspergillus spp., as well as rarer pathogenic species [38].
Regular antifungal susceptibility testing (AFST) is critical in the treatment of IFD patients. AFST not only assists in determining the most appropriate therapy for individual patients, but it also provides insight into local antifungal resistance patterns within healthcare facilities, which is critical in guiding the selection of optimal empirical treatment when an IFD is suspected [29]. Routine AFST is also useful for detecting inherent and recently acquired resistances [29]. According to our findings, most Italian laboratories can perform susceptibility testing for yeasts and moulds. Access to CLSI microdilution, on the other hand, was unevenly distributed, with only 15.8% of areas with GDP 30,000 euros having access to this approach, compared to 50% of regions with GDP > 30,000 euros (p = 0.001). In any case, the availability of EUCAST microdilution, Europe's preferred antifungal testing technique [39], was similar across Italian regions. The variance in CLSI microdilution availability might be attributed to places with lower economic resources investing in cost-effective and time-efficient commercial methods, such as E test® or VITEK®, which have demonstrated a high correlation with standard procedures [29].
In terms of antifungal therapies, the primary antifungals were widely available and extensively dispersed throughout the country. The rate was approximately 90% not just for the World Health Organization (WHO)-defined essential medications [40], but also for additional pharmaceuticals such as isavuconazole (a first-line therapy together with voriconazole for invasive aspergillosis) or posaconazole (indicated for prophylaxis of fungal infections in high-risk haematological patients and for the treatment of mucormycosis). Certain systemic antifungals, such as 5-flucytosine and terbinafine, are likely unavailable due to low demand, as IFD needing these medications as first-line therapy is uncommon in Europe (e.g., disseminated cryptococcosis or scedosporiosis) [41–44].
Our research found numerous concerning impediments to TDM availability among participating universities. To begin, in comparison to recent European and Spanish surveys access to TDM in Italy was lower and generally inadequate, particularly for flucytosine (12.2%), itraconazole (28.6%), posaconazole (44.9%), and voriconazole (63.3%), for which routine dosing is now recommended by several guidelines [45]. Second, over half of the participating institutions sent blood samples to an outside laboratory for antifungal TDM, which might contribute to delays in findings and, eventually, dose modifications. Finally, there was an unequal distribution of TDM methodology throughout the country, with a substantial positive association between TDM availability and GDP. Given the potential benefits of TDM-guided dosage, such as enhanced therapeutic effectiveness [16, 46], reduced toxicity risk [15], cost savings, and decreased emergence of antifungal-resistant strains [47], addressing the limitations in TDM availability in Italy is deemed imperative. The limited accessibility to molecular tests observed in our survey underscores a further potential challenge that must be addressed in the next future.
The fact that the survey was done by email with a voluntary answer style that may result in an undetected response bias is the principal weakness of our study. In this regard, the majority of responses were from university hospitals or national research institutions, which often see a greater volume of patients and have more financial resources at their disposal. Thus, the results of this study may not be applicable to unspecialized clinical settings. Moreover, we did not collect data on Gram staining during the bacteriological processing of specimens. Although Gram stain is not specific to fungi, it theoretically has the potential to provide relevant clinical information that could impact the treatment approach for patients with suspected IFD. Lastly, it is crucial to note that our analysis was constrained by the impossibility to include centres from all areas of Italy. Yet, to the best of our knowledge, this survey is the broadest study ever undertaken to investigate the diagnostic capacities of invasive fungal infections in Italian laboratories.
In conclusion, our data show that overall diagnostic capacities for IFD in Italy are adequate, with no significant variations based on GDP except for TDM. Future efforts, in our opinion, should focus on enhancing the availability and usage of direct microscopic methods, as well as therapeutic medication monitoring, in order to achieve optimal treatment and improve patient outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all participating institutions for their utmost contributions and support to the project during a pandemic situation and to all the individuals and associations that have disseminated the link to the survey.
Orazio Romeo, Alberto Enrico Maraolo, Nicholas Geremia, Maurizio Sanguinetti, Teresa Maria Fasciana, Mario Cruciani, Simone Mornese Pinna, Giuliana Lo Cascio, Goffredo Angioni, Laura Magnasco, Vivian Tullio, Francesco Barchiesi, Lucia Prezioso, Silvana Sanna, Andrea Cortegiani, Daniela Dalla Gasperina, Francesca Farina, Anna Grancini, Emanuele Pontali, Francesco Forfori, Marco Berruti, Caterina Buquicchio, Patrizia Danesi, Fabio Forghieri, Maddalena Giannella, Daniela Angela Zeme, Nicola Coppola, Lidia Dalfino, Gianpaolo Nadali, Claudio Farina, Teresa Santantonio, Giuliana Guadagnino, Maria Calabrò, Patrizia Zappasodi, Giammarco Raponi, Assunta Sartor, Manuela Ceccarelli, Federico Itri, Olimpia Finizio, Carlo Pallotto, Luisa Verga, Maria Enza Mitra, Annarosa Cuccaro, Alessandra Mularoni, Mario Virgilio Papa, Gianluigi Lombardi, Maria Ilaria Del Principe, Michelina Dargenio, Sita Giovani (Young Investigators Group of the Società Italiana Terapia Antinfettiva, International Society of Human and Animal Mycology (ISHAM), Epidemiological Surveillance of Infections in Haematological Diseases of Italy (SEIFEM), European Confederation for Medical Mycology (ECMM).
Authors contributions
AV, MH, OAC and JSG: contributed to study design. AV and JSG: conceived the study idea, collected and validated the data, did the statistical plan and analysis, and drafted the first version of the manuscript. All authors contributed to data interpretation, manuscript writing and review of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This manuscript was conducted as part of our routine work, and no external funding was obtained for the research.
Availability of data and materials
We ensure data availability for this manuscript, and all relevant data used in the research will be made accessible upon request to promote transparency and reproducibility.
Declarations
Conflict of interest
Antonio Vena (AV) has participated on advisory boards and/or received speaker fees from Angelini, Menarini, Merck Sharp and Dohme, Pfizer, and Shionogi. He has also received study grants from Merck Sharp and Dohme and Astellas, outside of the submitted work. Matteo Bassetti (MB) has participated on advisory boards and/or has received speaker fees from Achaogen, Angelini, Astellas, Bayer, Basilea, Biomerieux, Cidara, Gilead Sciences, Menarini, Merck Sharp and Dohme, Nabriva, Paratek, Pfizer Inc., Roche, Melinta Therapeutics, Shionogi, Tetraphase, VenatoRx, and Vifor. He has also received study grants from Angelini, Basilea, Astellas, Shionogi, Cidara, Melinta, Gilead Sciences, Pfizer, and Merck Sharp and Dohme, outside of the submitted work. Laura Mezzogori (LM) has no conflict of interest to disclose. Francesco Marchesi (FM) has no conflict of interest to disclose. Martin Hoenigl (MH) received research funding from Gilead, Astellas, MSD, IMMY, Scynexis, F2G and Pfizer, outside of the submitted work. Daniele Roberto Giacobbe (DRG) has received investigator-initiated grants from Pfizer Inc, Shionogi, Gilead Italia, as well as speaker fees and/or advisory board fees from Pfizer and Tillotts Pharma, outside of the submitted work. Silvia Corcione (SC) has no conflict of interest to disclose. Michele Bartoletti (MB) has no conflict of interest to disclose. Jannik Stemler (JS) has received research support by the Ministry of Education and Research (BMBF) and Basilea Pharmaceuticals Inc., outside the submitted work; has received speaker honoraria by Pfizer Inc., Gilead and AbbVie, outside the submitted work; has been a consultant to Gilead, Produkt&Markt GmbH, Alvea Vax. and Micron Research, outside the submitted work; and has received travel grants by German Society for Infectious Diseases (DGI e.V.) and Meta-Alexander Foundation, outside the submitted work. Livio Pagano (LP) was Board member of Gilead Science, MSD, Pfizer, Stemline, Basilea, Janssen-Cilab, Novartis, Jazz Pharmaceutical, Cidara and has been speaker for Gilead Sciences, Kiowa Kirin, MSD, Pfizer Pharmaceuticals, Astellas Pharma, Novartis, Jazz Pharmaceutical, Janseen-Cilag. Consultant for Menarini, Cidara, outside of the submitted work. Oliver A. Cornely (OAC) reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; Consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis, Seres; Honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, Pfizer; Payment for expert testimony from Cidara; Participation on a Data Safety Monitoring Board or Advisory Board from Actelion, Allecra, Cidara, Entasis, IQVIA, Janssen, MedPace, Paratek, PSI, Shionogi; A patent at the German Patent and Trade Mark Office (DE 10 2021 113 007.7), outside of the submitted work. Jon Salmanton-García (JSG) reports speaker honoraria from Gilead and Pfizer, outside of the submitted work.
Ethical approval
Not applicable.
Footnotes
Members of the group “Young Investigators Group of the Società Italiana Terapia Antinfettiva, International Society of Human and Animal Mycology (ISHAM), Epidemiological Surveillance of Infections in Haematological Diseases of Italy (SEIFEM), European Confederation for Medical Mycology (ECMM)” are listed in the acknowledgements.
Contributor Information
Antonio Vena, Email: anton.vena@gmail.com.
Jon Salmanton-García, Email: jon.salmanton-garcia@uk-koeln.de.
References
- 1.Colombo AL, de Almeida Junior JN, Slavin MA, Chen SC, Sorrell TC. Candida and invasive mould diseases in non-neutropenic critically ill patients and patients with haematological cancer. Lancet Infect Dis. 2017;17(11):e344–e356. doi: 10.1016/S1473-3099(17)30304-3. [DOI] [PubMed] [Google Scholar]
- 2.Bassetti M, Peghin M, Vena A. Challenges and solution of invasive aspergillosis in non-neutropenic patients: a review. Infect Dis Ther. 2018;7(1):17–27. doi: 10.1007/s40121-017-0183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Munoz P, Vena A, Ceron I, et al. Invasive pulmonary aspergillosis in heart transplant recipients: two radiologic patterns with a different prognosis. J Heart Lung Transplant. 2014;33(10):1034–1040. doi: 10.1016/j.healun.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi (Basel) 2017;3(4):57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruping MJ, Vehreschild JJ, Cornely OA. Patients at high risk of invasive fungal infections: when and how to treat. Drugs. 2008;68(14):1941–1962. doi: 10.2165/00003495-200868140-00002. [DOI] [PubMed] [Google Scholar]
- 6.Fortun J, Muriel A, Martin-Davila P, et al. Caspofungin versus fluconazole as prophylaxis of invasive fungal infection in high-risk liver transplantation recipients: a propensity score analysis. Liver Transpl. 2016;22(4):427–435. doi: 10.1002/lt.24391. [DOI] [PubMed] [Google Scholar]
- 7.Driemeyer C, Falci DR, Hoenigl M, et al. The current state of Clinical Mycology in Eastern and South-Eastern Europe. Med Mycol. 2022;60(4):myac017. doi: 10.1093/mmy/myac017. [DOI] [PubMed] [Google Scholar]
- 8.Valerio M, Vena A, Bouza E, et al. How much European prescribing physicians know about invasive fungal infections management? BMC Infect Dis. 2015;15:80. doi: 10.1186/s12879-015-0809-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salmanton-Garcia J, Au WY, Hoenigl M, et al. The current state of laboratory mycology in Asia/Pacific: a survey from the European Confederation of Medical Mycology (ECMM) and International Society for Human and Animal Mycology (ISHAM) Int J Antimicrob Agents. 2023;61(3):106718. doi: 10.1016/j.ijantimicag.2023.106718. [DOI] [PubMed] [Google Scholar]
- 10.Mikulska M, Magnasco L, Signori A, et al. Sensitivity of serum beta-d-glucan in candidemia according to candida species epidemiology in critically ill patients admitted to the intensive care unit. J Fungi (Basel) 2022;8(9):921. doi: 10.3390/jof8090921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vena A, Bouza E, Corisco R, et al. Efficacy of a "Checklist" intervention bundle on the clinical outcome of patients with candida bloodstream infections: a quasi-experimental pre-post study. Infect Dis Ther. 2020;9(1):119–135. doi: 10.1007/s40121-020-00281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421. doi: 10.1016/S1473-3099(19)30312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoenigl M, Salmanton-Garcia J, Walsh TJ, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis. 2021;21(8):e246–e257. doi: 10.1016/S1473-3099(20)30784-2. [DOI] [PubMed] [Google Scholar]
- 14.Mellinghoff SC, Bassetti M, Dorfel D, et al. Isavuconazole shortens the QTc interval. Mycoses. 2018;61(4):256–260. doi: 10.1111/myc.12731. [DOI] [PubMed] [Google Scholar]
- 15.Guinea J, Escribano P, Marcos-Zambrano LJ, et al. Therapeutic drug monitoring of voriconazole helps to decrease the percentage of patients with off-target trough serum levels. Med Mycol. 2016;54(4):353–360. doi: 10.1093/mmy/myv099. [DOI] [PubMed] [Google Scholar]
- 16.Vena A, Munoz P, Mateos M, et al. Therapeutic drug monitoring of antifungal drugs: another tool to improve patient outcome? Infect Dis Ther. 2020;9(1):137–149. doi: 10.1007/s40121-020-00280-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salmanton-Garcia J, Hoenigl M, Gangneux JP, et al. The current state of laboratory mycology and access to antifungal treatment in Europe: a European Confederation of Medical Mycology survey. Lancet Microbe. 2023;4(1):e47–e56. doi: 10.1016/S2666-5247(22)00261-0. [DOI] [PubMed] [Google Scholar]
- 18.Hoenigl M, Gangneux JP, Segal E, et al. Global guidelines and initiatives from the European Confederation of Medical Mycology to improve patient care and research worldwide: new leadership is about working together. Mycoses. 2018;61(11):885–894. doi: 10.1111/myc.12836. [DOI] [PubMed] [Google Scholar]
- 19.United States National Library of Medicine (NLM). ClinicalTrials.gov. https://clinicaltrials.gov/ (Last accessed February 1, 2022).
- 20.European Medicines Agency. EU Clinical Trials Register. https://www.clinicaltrialsregister.eu/ (Last accessed February 1, 2023).
- 21.Google Scholar. http://scholar.google.com/ (Last accessed February 1, 2023).
- 22.Elsevier. ScienceDirect. https://www.sciencedirect.com/ (Last accessed February 1, 2023).
- 23.ASR Lombardia. Annuario Statistico Regionale. PIL a prezzi di mercato per abitante – Regionale. https://www.asr-lombardia.it/asrlomb/it/8001pil-prezzi-di-mercato-abitante-regionale (Last accessed March 13, 2023).
- 24.Montagna MT, Caggiano G, Lovero G, et al. Epidemiology of invasive fungal infections in the intensive care unit: results of a multicenter Italian survey (AURORA Project) Infection. 2013;41(3):645–653. doi: 10.1007/s15010-013-0432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montagna MT, Giglio O, Napoli C, et al. Invasive fungal infections in patients with hematologic malignancies (aurora project): lights and shadows during 18-months surveillance. Int J Mol Sci. 2012;13(1):774–787. doi: 10.3390/ijms13010774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prigitano A, Esposto MC, Grancini A, et al. Azole resistance in Aspergillus isolates by different types of patients and correlation with environment—an Italian prospective multicentre study (ARiA study) Mycoses. 2021;64(5):528–536. doi: 10.1111/myc.13241. [DOI] [PubMed] [Google Scholar]
- 27.Mikulska M, Furfaro E, Dettori S, et al. Aspergillus-PCR in bronchoalveolar lavage—diagnostic accuracy for invasive pulmonary aspergillosis in critically ill patients. Mycoses. 2022;65(4):411–418. doi: 10.1111/myc.13428. [DOI] [PubMed] [Google Scholar]
- 28.Verweij PE, Bruggemann RJM, Azoulay E, et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021;47(8):819–834. doi: 10.1007/s00134-021-06449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bassetti M, Vena A, Bouza E, et al. Antifungal susceptibility testing in Candida, Aspergillus and Cryptococcus infections: are the MICs useful for clinicians? Clin Microbiol Infect. 2020;26(8):1024–1033. doi: 10.1016/j.cmi.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 30.van der Linden JW, Arendrup MC, Warris A, et al. Prospective multicenter international surveillance of azole resistance in Aspergillus fumigatus. Emerg Infect Dis. 2015;21(6):1041–1044. doi: 10.3201/eid2106.140717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmanton-Garcia J, Sprute R, Stemler J, et al. COVID-19-associated pulmonary aspergillosis, March-August 2020. Emerg Infect Dis. 2021;27(4):1077–1086. doi: 10.3201/eid2704.204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect Dis. 2021;73(11):e3606–e3614. doi: 10.1093/cid/ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813–1821. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bassetti M, Azoulay E, Kullberg BJ, et al. EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the intensive care unit working group. Clin Infect Dis. 2021;72(Suppl 2):S121–S127. doi: 10.1093/cid/ciaa1751. [DOI] [PubMed] [Google Scholar]
- 35.Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1–e38. doi: 10.1016/j.cmi.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Driemeyer C, Falci DR, Oladele RO, et al. The current state of clinical mycology in Africa: a European Confederation of Medical Mycology and International Society for Human and Animal Mycology survey. Lancet Microbe. 2022;3(6):e464–e470. doi: 10.1016/S2666-5247(21)00190-7. [DOI] [PubMed] [Google Scholar]
- 37.Dhiman N, Hall L, Wohlfiel SL, Buckwalter SP, Wengenack NL. Performance and cost analysis of matrix-assisted laser desorption ionization-time of flight mass spectrometry for routine identification of yeast. J Clin Microbiol. 2011;49(4):1614–1616. doi: 10.1128/JCM.02381-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barker KR, Kus JV, Normand AC, et al. A Practical Workflow for the Identification of Aspergillus, Fusarium, Mucorales by MALDI-TOF MS: database, medium, and incubation optimization. J Clin Microbiol. 2022;60(12):e0103222. doi: 10.1128/jcm.01032-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.yeasts ECoASTEEDdEDMftdobdmicoaaf. https://unire.unige.it/handle/123456789/5894?show=full
- 40.WHO; World Health Organization model list of essential medicines 22nd list. 2021. https://apps.who.int/iris/rest/bitstreams/1374779/.Accessed 22 Mar 2022
- 41.https://apps.who.int/iris/rest/bitstreams/1374779/retrieve (Last accessed February 3, 2023).
- 42.Rivoisy C, Vena A, Schaeffer L, et al. Prosthetic Valve Candida spp. endocarditis: new insights into long-term prognosis-the ESCAPE study. Clin Infect Dis. 2018;66(6):825–32. doi: 10.1093/cid/cix913. [DOI] [PubMed] [Google Scholar]
- 43.Vena A, Munoz P, Guinea J, et al. Fluconazole resistance is not a predictor of poor outcome in patients with cryptococcosis. Mycoses. 2019;62(5):441–449. doi: 10.1111/myc.12847. [DOI] [PubMed] [Google Scholar]
- 44.Jenks JD, Seidel D, Cornely OA, et al. Voriconazole plus terbinafine combination antifungal therapy for invasive Lomentospora prolificans infections: analysis of 41 patients from the FungiScope(R) registry 2008–2019. Clin Microbiol Infect. 2020;26(6):784 e1–e5. doi: 10.1016/j.cmi.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Vena A, Bouza E, Alvarez-Uria A, et al. The misleading effect of serum galactomannan testing in high-risk haematology patients receiving prophylaxis with micafungin. Clin Microbiol Infect. 2017;23(12):1000 e1–e4. doi: 10.1016/j.cmi.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 46.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother. 2014;69(5):1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Munoz P, Bouza E. The current treatment landscape: the need for antifungal stewardship programmes. J Antimicrob Chemother. 2016;71(suppl 2):ii5–ii12. doi: 10.1093/jac/dkw391. [DOI] [PubMed] [Google Scholar]
- 48.Ioannidis K, Papachristos A, Skarlatinis I, et al. Do we need to adopt antifungal stewardship programmes? Eur J Hosp Pharm. 2020;27(1):14–18. doi: 10.1136/ejhpharm-2017-001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We ensure data availability for this manuscript, and all relevant data used in the research will be made accessible upon request to promote transparency and reproducibility.


