Abstract
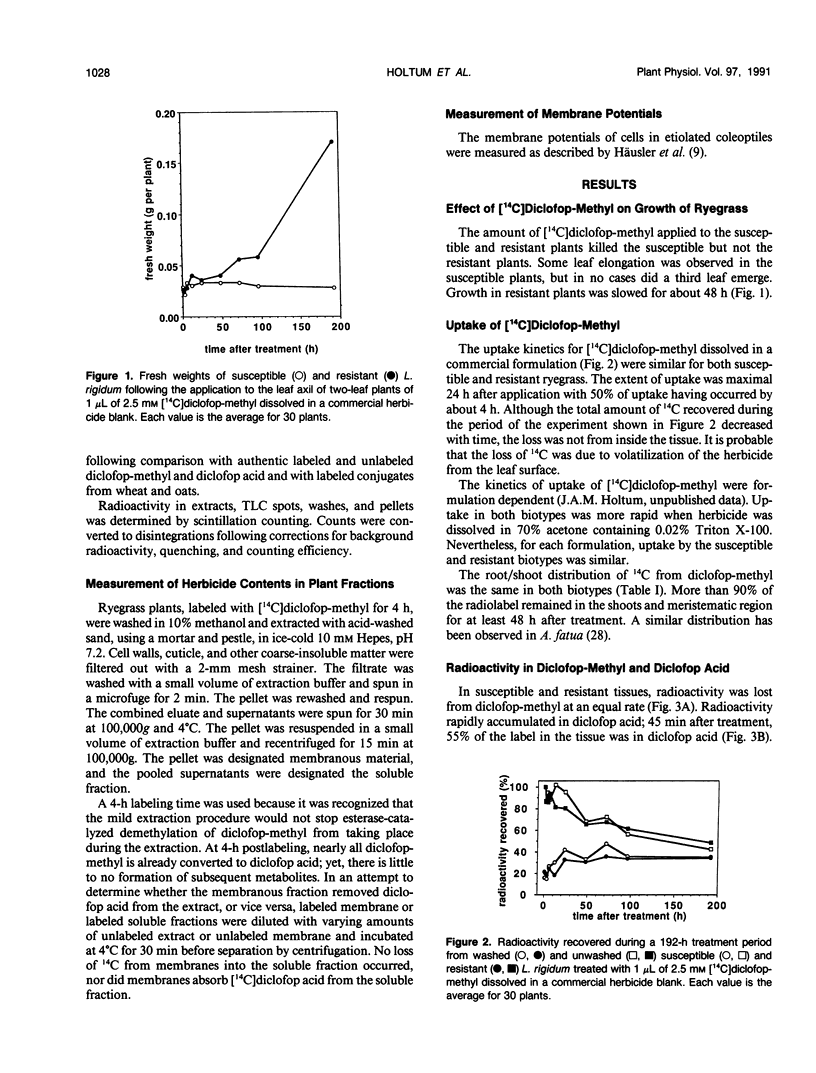
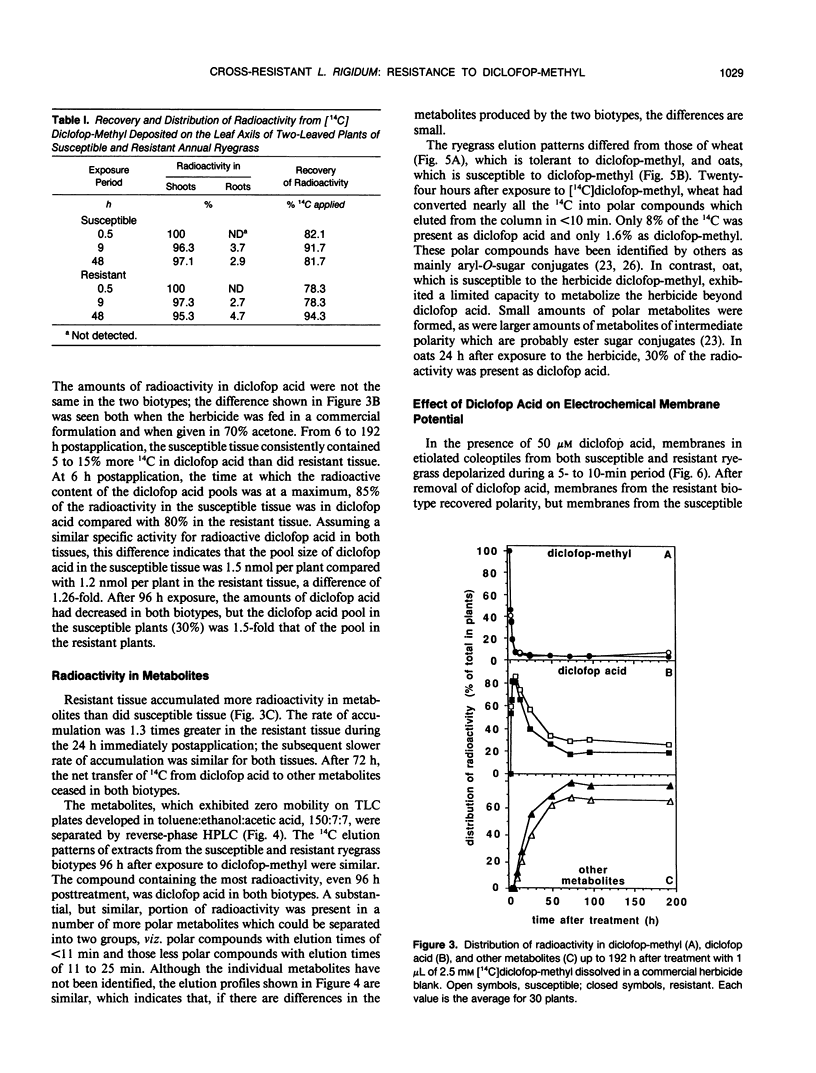
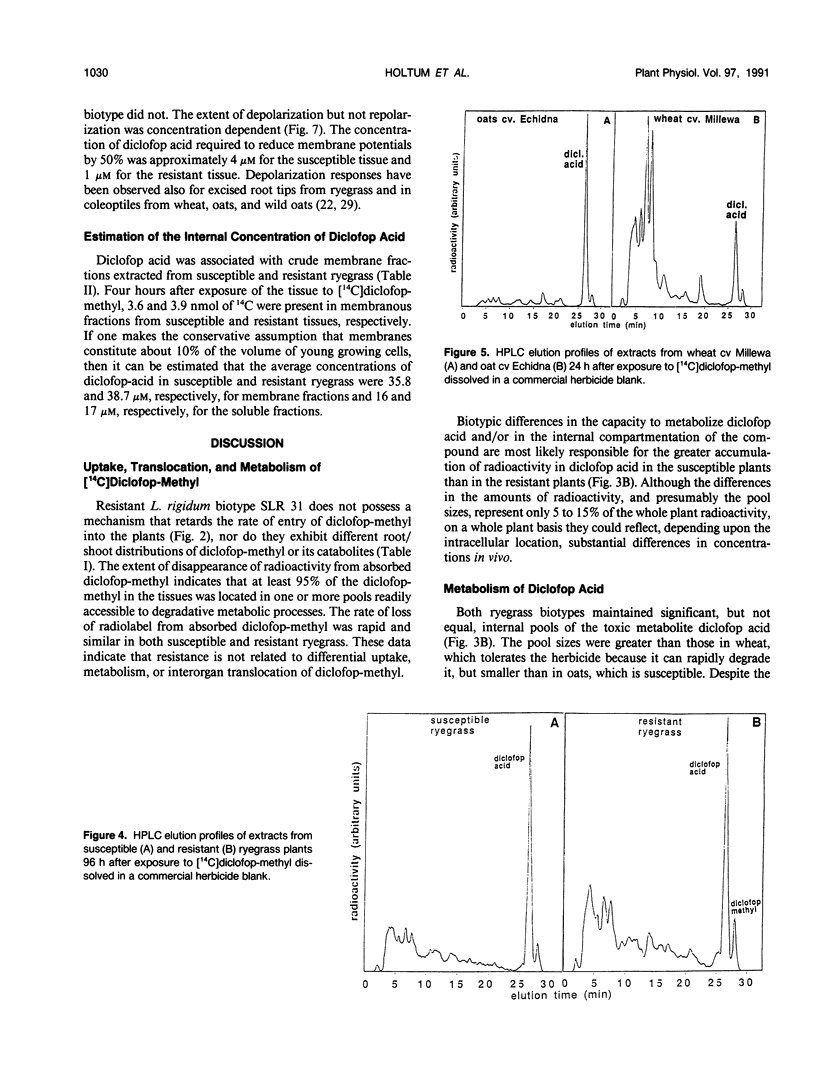
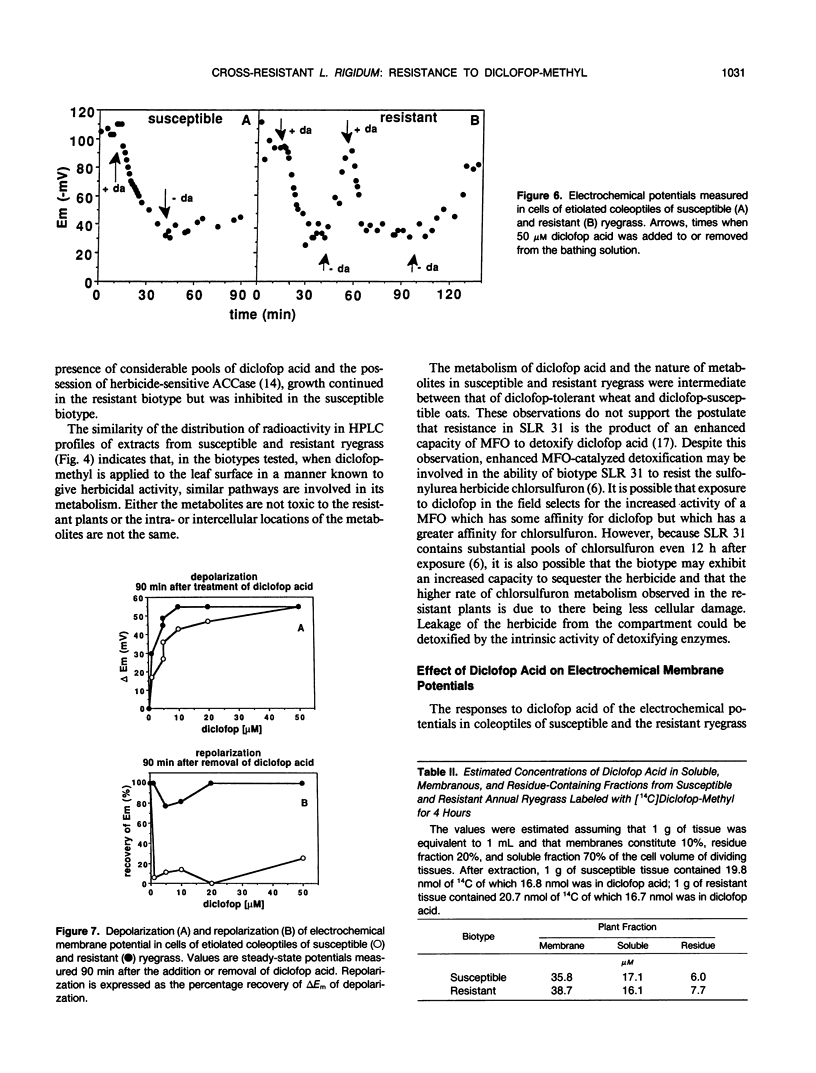
Annual ryegrass (Lolium rigidum) biotype SLR 31 is resistant to the postemergent graminicide methyl-2-[4-(2,4-dichlorophenoxy)phenoxy]-propanoate (diclofop-methyl). Uptake of [14C](U-phenyl)diclofop-methyl and root/shoot distribution of radioactivity in susceptible and resistant plants were similar. In both biotypes, diclofop-methyl was rapidly demethylated to the biocidal metabolite diclofop acid which, in turn, was metabolized to ester and aryl-O-sugar conjugates. Susceptible plants accumulated 5 to 15% more radioactivity in dicloflop acid than did resistant plants. Resistant plants had a slightly greater capacity to form nonbiocidal sugar conjugates. Despite these differences, resistant plants retained 20% of 14C in the biocidal metabolite diclofop acid 192 hours after treatment, whereas susceptible plants, which were close to death, retained 30% in diclofop acid. The small differences in the pool sizes of the active and inactive metabolites are by themselves unlikely to account for a 30-fold difference in sensitivity to the herbicide at the whole plant level. Similar high-pressure liquid chromatography elution patterns of conjugates from both susceptible and resistant biotypes indicated that the mechanisms and the products of catabolism in the biotypes are similar. It is suggested that metabolism of diclofop-methyl by the resistant biotype does not alone explain resistance observed at the whole-plant level. Diclofop acid reduced the electrochemical potential of membranes in etiolated coleoptiles of both biotypes; 50% depolarization required 1 to 4 μm diclofop acid. After removal of diclofop acid, membranes from the resistant biotype recovered polarity, whereas membranes from the susceptible biotype did not. Internal concentrations of diclofop acid 4 h after exposing plants to herbicide were estimated to be 36 to 39 micromolar in a membrane fraction and 16 to 17 micromolar in a soluble fraction. Such concentrations should be sufficient to fully depolarize membranes. It is postulated that differences in the ability of membranes to recover from depolarization are correlated with the resistance response of biotype SLR 31.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettoni G., Loiodice F., Tortorella V., Conte-Camerino D., Mambrini M., Ferrannini E., Bryant S. H. Stereospecificity of the chloride ion channel: the action of chiral clofibric acid analogues. J Med Chem. 1987 Aug;30(8):1267–1270. doi: 10.1021/jm00391a002. [DOI] [PubMed] [Google Scholar]
- Beynen A. C., Geelen M. J. Short-term inhibition of fatty acid biosynthesis in isolated hepatocytes by mono-aromatic compounds. Toxicology. 1982;24(3-4):183–197. doi: 10.1016/0300-483x(82)90001-4. [DOI] [PubMed] [Google Scholar]
- Burton J. D., Gronwald J. W., Somers D. A., Connelly J. A., Gengenbach B. G., Wyse D. L. Inhibition of plant acetyl-coenzyme A carboxylase by the herbicides sethoxydim and haloxyfop. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1039–1044. doi: 10.1016/s0006-291x(87)80236-x. [DOI] [PubMed] [Google Scholar]
- Granzer E., Nahm H. HCG 004, a new highly potent hypolipidaemic drug. Arzneimittelforschung. 1973 Sep;23(9):1353–1354. [PubMed] [Google Scholar]
- Häusler R. E., Holtum J. A., Powles S. B. Cross-Resistance to Herbicides in Annual Ryegrass (Lolium rigidum): IV. Correlation between Membrane Effects and Resistance to Graminicides. Plant Physiol. 1991 Nov;97(3):1035–1043. doi: 10.1104/pp.97.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. M., Holtum J. A., Liljegren D. R., Furness B., Powles S. B. Cross-Resistance to Herbicides in Annual Ryegrass (Lolium rigidum): I. Properties of the Herbicide Target Enzymes Acetyl-Coenzyme A Carboxylase and Acetolactate Synthase. Plant Physiol. 1990 Nov;94(3):1180–1186. doi: 10.1104/pp.94.3.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratterman D. M., Balke N. E. Diclofop-methyl increases the proton permeability of isolated oat-root tonoplast. Plant Physiol. 1989 Oct;91(2):756–765. doi: 10.1104/pp.91.2.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendina A. R., Felts J. M., Beaudoin J. D., Craig-Kennard A. C., Look L. L., Paraskos S. L., Hagenah J. A. Kinetic characterization, stereoselectivity, and species selectivity of the inhibition of plant acetyl-CoA carboxylase by the aryloxyphenoxypropionic acid grass herbicides. Arch Biochem Biophys. 1988 Aug 15;265(1):219–225. doi: 10.1016/0003-9861(88)90387-6. [DOI] [PubMed] [Google Scholar]
- Secor J., Cséke C. Inhibition of Acetyl-CoA Carboxylase Activity by Haloxyfop and Tralkoxydim. Plant Physiol. 1988 Jan;86(1):10–12. doi: 10.1104/pp.86.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro R. H., Walsh W. C., Hoerauf R. A. Metabolism and selectivity of diclofop-methyl in wild oat and wheat. J Agric Food Chem. 1979 May-Jun;27(3):615–623. doi: 10.1021/jf60223a008. [DOI] [PubMed] [Google Scholar]
- Wright J. P., Shimabukuro R. H. Effects of diclofop and diclofop-methyl on the membrane potentials of wheat and oat coleoptiles. Plant Physiol. 1987 Sep;85(1):188–193. doi: 10.1104/pp.85.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]


