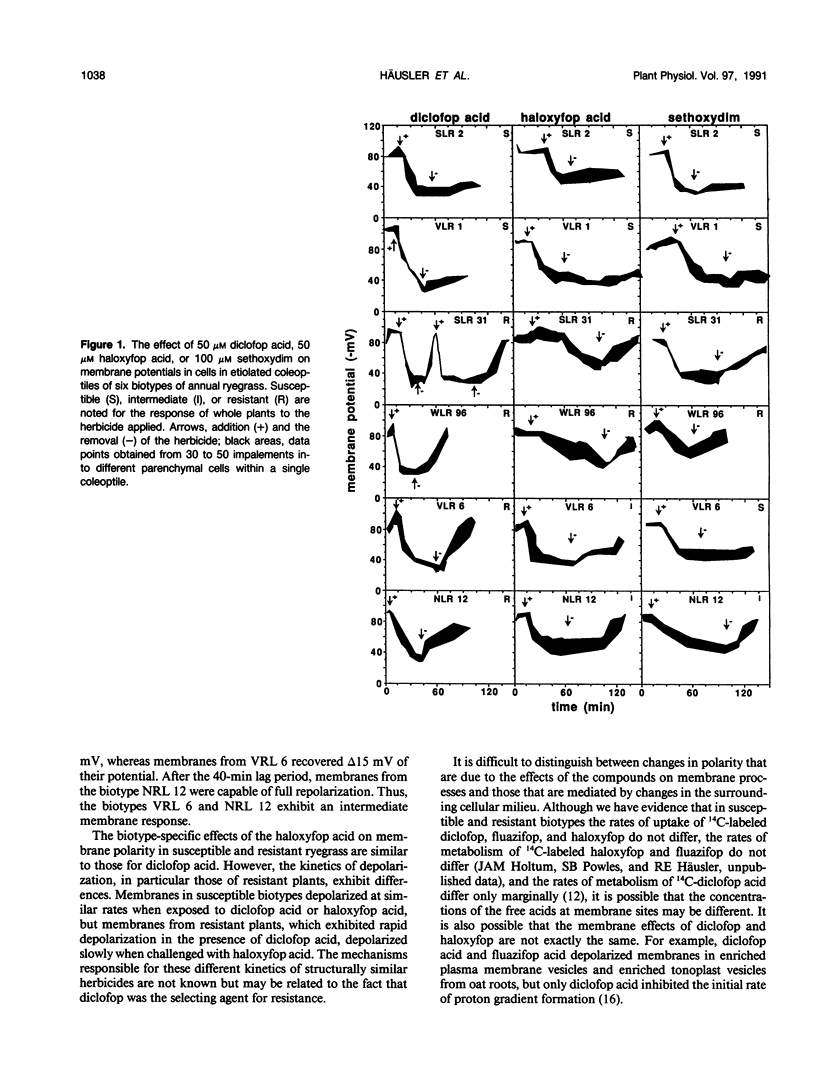
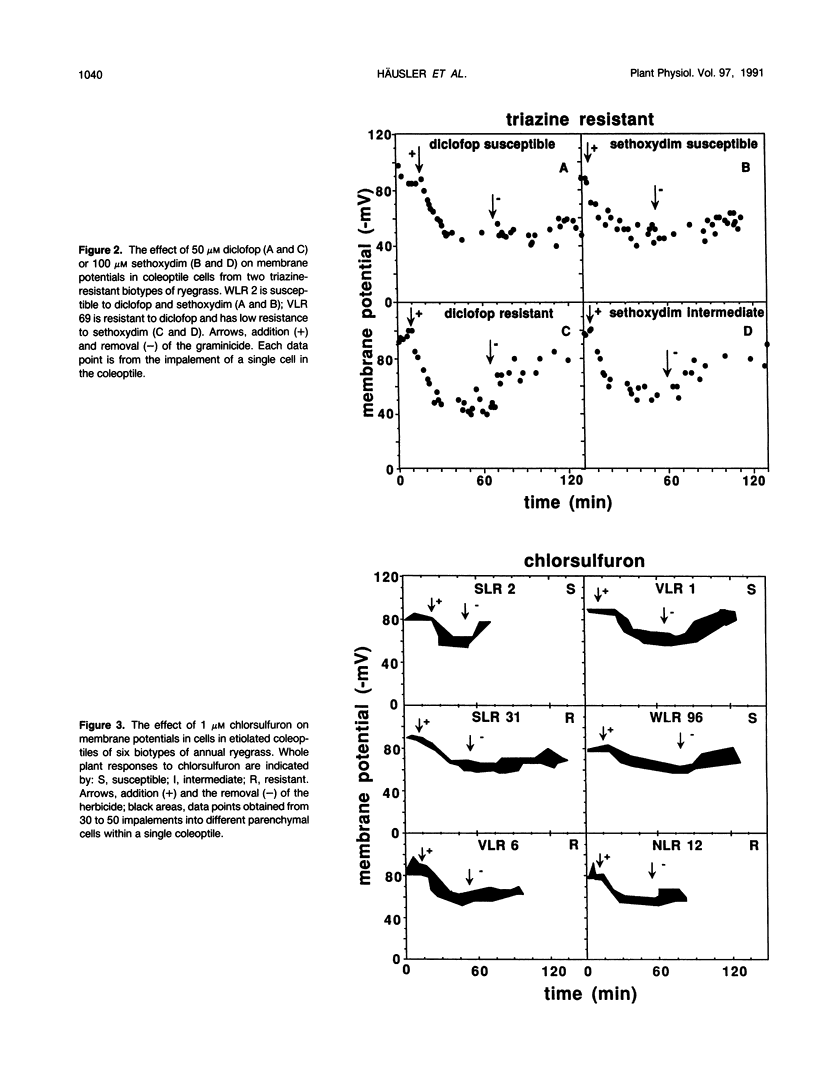
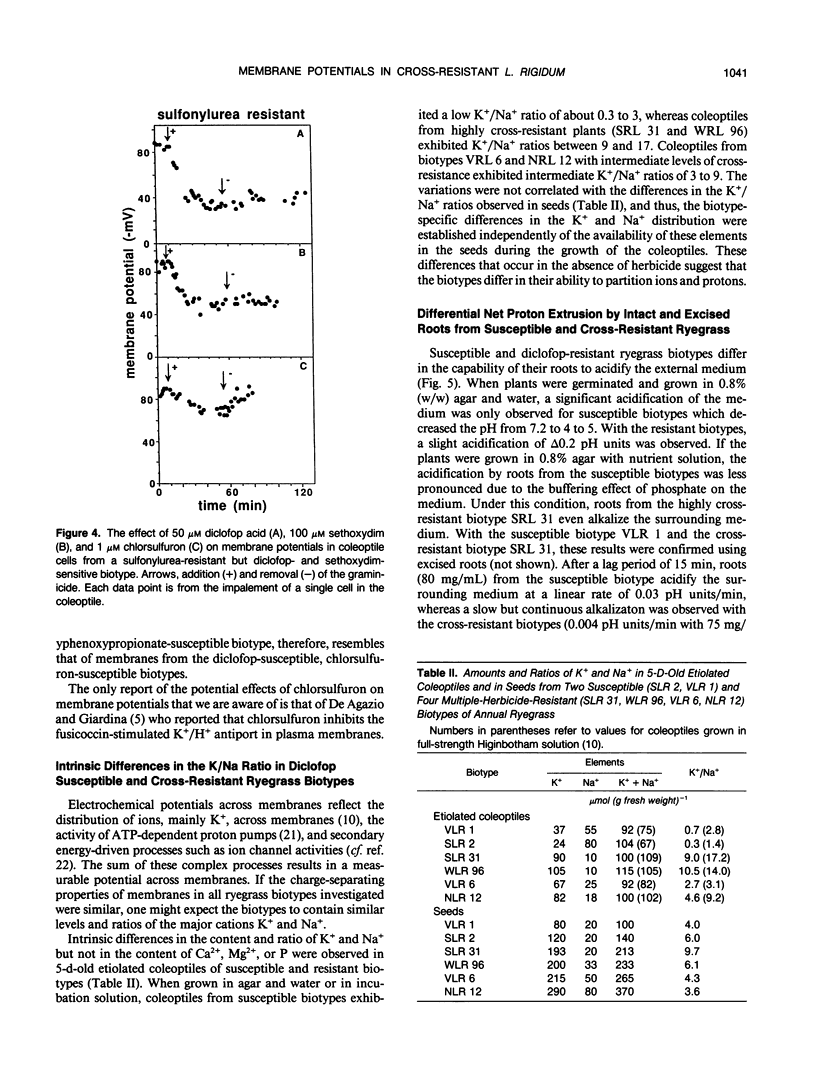
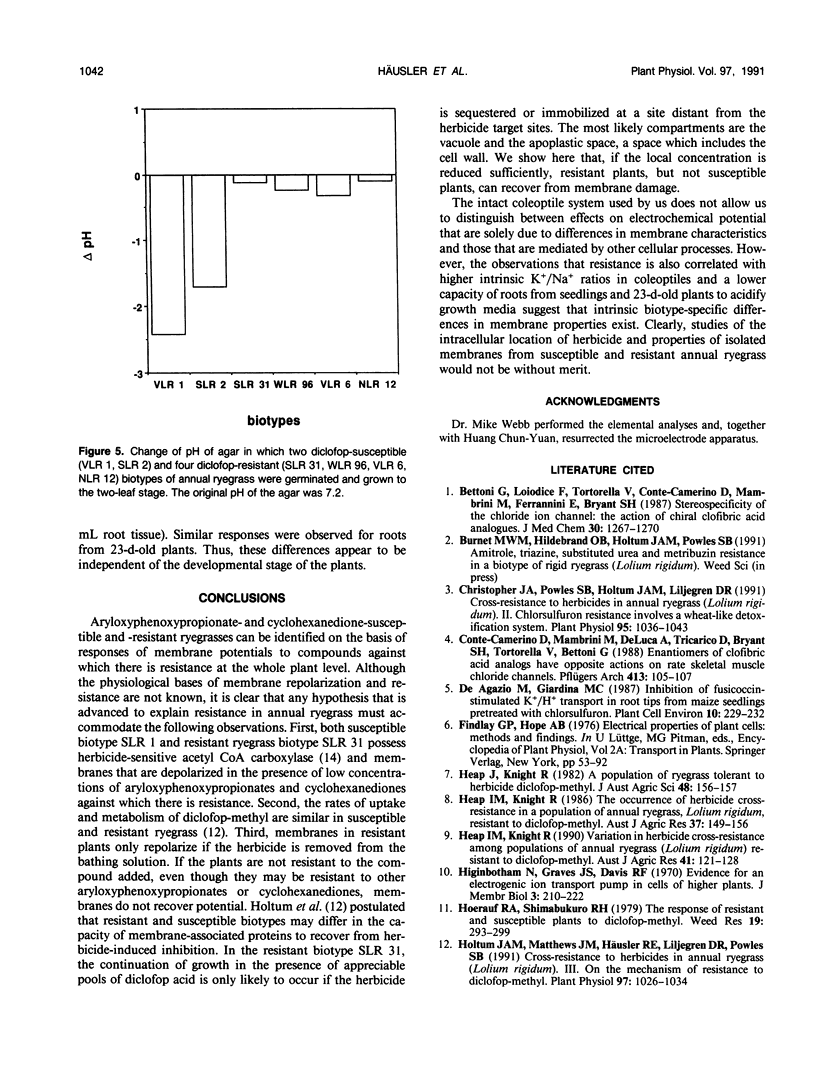
Abstract
The herbicidally active aryloxyphenoxypropionates diclofop acid, haloxyfop acid, and fluazifop acid and the cyclohexanedione sethoxydim depolarized membranes in coleoptiles of eight biotypes of herbicide-susceptible and herbicide-resistant annual ryegrass (Lolium rigidum). Membrane polarity was reduced from −100 millivolts to −30 to −50 millivolts. Membranes repolarized after removal of the compounds only in biotypes with resistance to the compound added. Repolarization was not observed in herbicide-susceptible L. rigidum, nor was it observed in biotypes resistant to triazine, triazole, triazinone, phenylurea, or sulfonylurea herbicides but not resistant to aryloxyphenoxypropionates and cyclohexanediones. Chlorsulfuron, a sulfonylurea herbicide, at a saturating concentration of 1 micromolar, reduced membrane polarity in all biotypes studied by only 15 millivolts. The recovery of membrane potential following the removal of chlorsulfuron was restricted to chlorsulfuron-susceptible and -resistant biotypes that did not exhibit diclofop resistance. These differences in membrane responses are correlated with resistance to dicloflop rather than with resistance to chlorsulfuron. It is suggested that the differences may reflect altered membrane properties of diclofop-resistant biotypes. Further circumstantial evidence for dissimilarity of properties of membranes from diclofop-resistant and diclofop-susceptible ryegrass is provided by observations that K+/Na+ ratios were significantly higher in coleoptiles from diclofop-resistant biotypes than in coleoptiles from susceptible plants. Intact and excised roots from susceptible biotypes were capable of acidifying the external medium, whereas roots from resistant biotypes were unable to do so. The ineluctable conclusion is that in L. rigidum the phenomena of membrane repolarization and resistance to aryloxyphenoxypropionate and cyclohexanedione herbicides are correlated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bettoni G., Loiodice F., Tortorella V., Conte-Camerino D., Mambrini M., Ferrannini E., Bryant S. H. Stereospecificity of the chloride ion channel: the action of chiral clofibric acid analogues. J Med Chem. 1987 Aug;30(8):1267–1270. doi: 10.1021/jm00391a002. [DOI] [PubMed] [Google Scholar]
- Conte-Camerino D., Mambrini M., DeLuca A., Tricarico D., Bryant S. H., Tortorella V., Bettoni G. Enantiomers of clofibric acid analogs have opposite actions on rat skeletal muscle chloride channels. Pflugers Arch. 1988 Nov;413(1):105–107. doi: 10.1007/BF00581238. [DOI] [PubMed] [Google Scholar]
- Holtum J. A., Matthews J. M., Häusler R. E., Liljegren D. R., Powles S. B. Cross-Resistance to Herbicides in Annual Ryegrass (Lolium rigidum) : III. On the Mechanism of Resistance to Diclofop-Methyl. Plant Physiol. 1991 Nov;97(3):1026–1034. doi: 10.1104/pp.97.3.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J., Wilson C., Wright J. P. Perturbation of Chara Plasmalemma Transport Function by 2[4(2',4'-Dichlorophenoxy)phenoxy]propionic Acid. Plant Physiol. 1984 Jan;74(1):61–66. doi: 10.1104/pp.74.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. M., Holtum J. A., Liljegren D. R., Furness B., Powles S. B. Cross-Resistance to Herbicides in Annual Ryegrass (Lolium rigidum): I. Properties of the Herbicide Target Enzymes Acetyl-Coenzyme A Carboxylase and Acetolactate Synthase. Plant Physiol. 1990 Nov;94(3):1180–1186. doi: 10.1104/pp.94.3.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. B. Site of action of chlorsulfuron: inhibition of valine and isoleucine biosynthesis in plants. Plant Physiol. 1984 Jul;75(3):827–831. doi: 10.1104/pp.75.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. P., Shimabukuro R. H. Effects of diclofop and diclofop-methyl on the membrane potentials of wheat and oat coleoptiles. Plant Physiol. 1987 Sep;85(1):188–193. doi: 10.1104/pp.85.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]


