Abstract
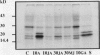
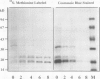
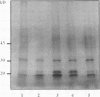
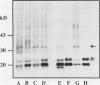
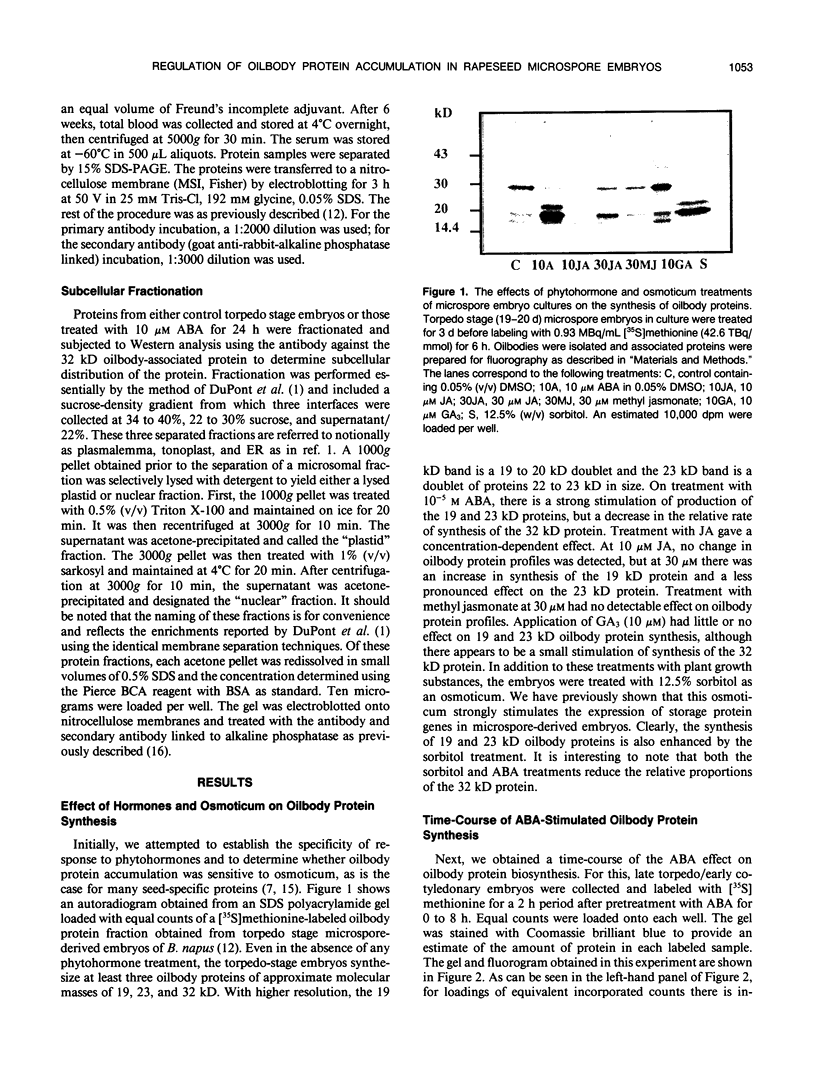
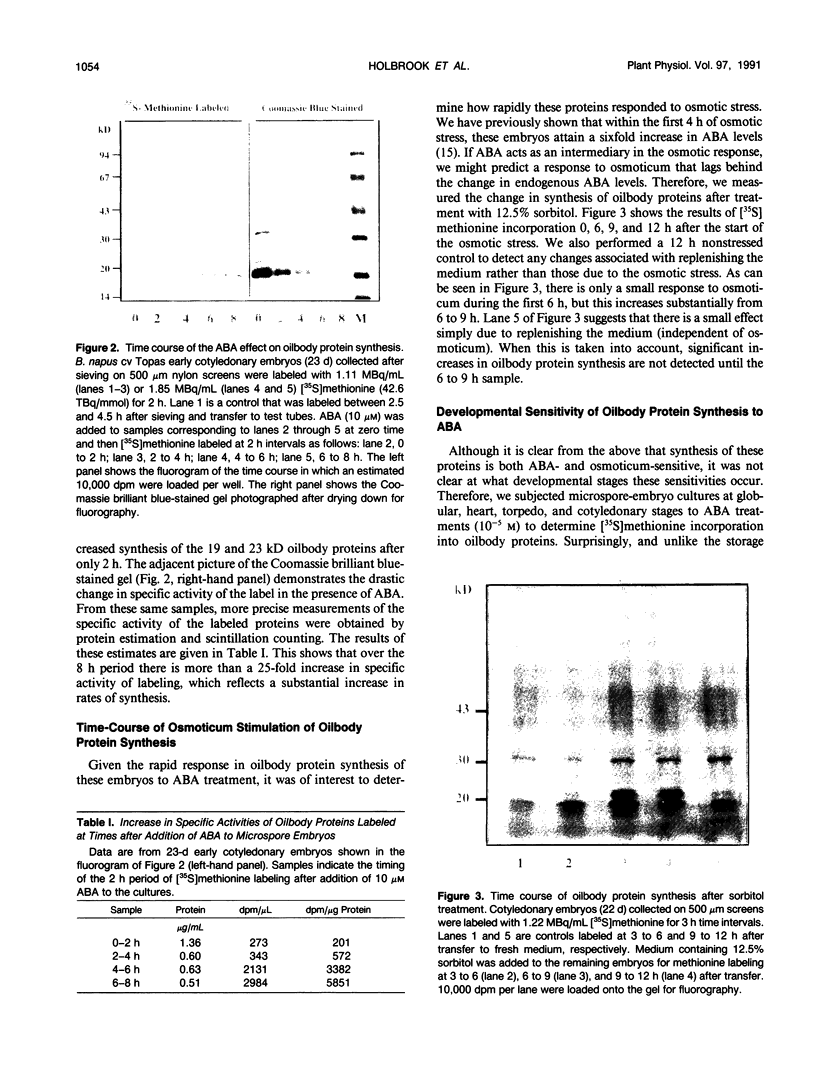
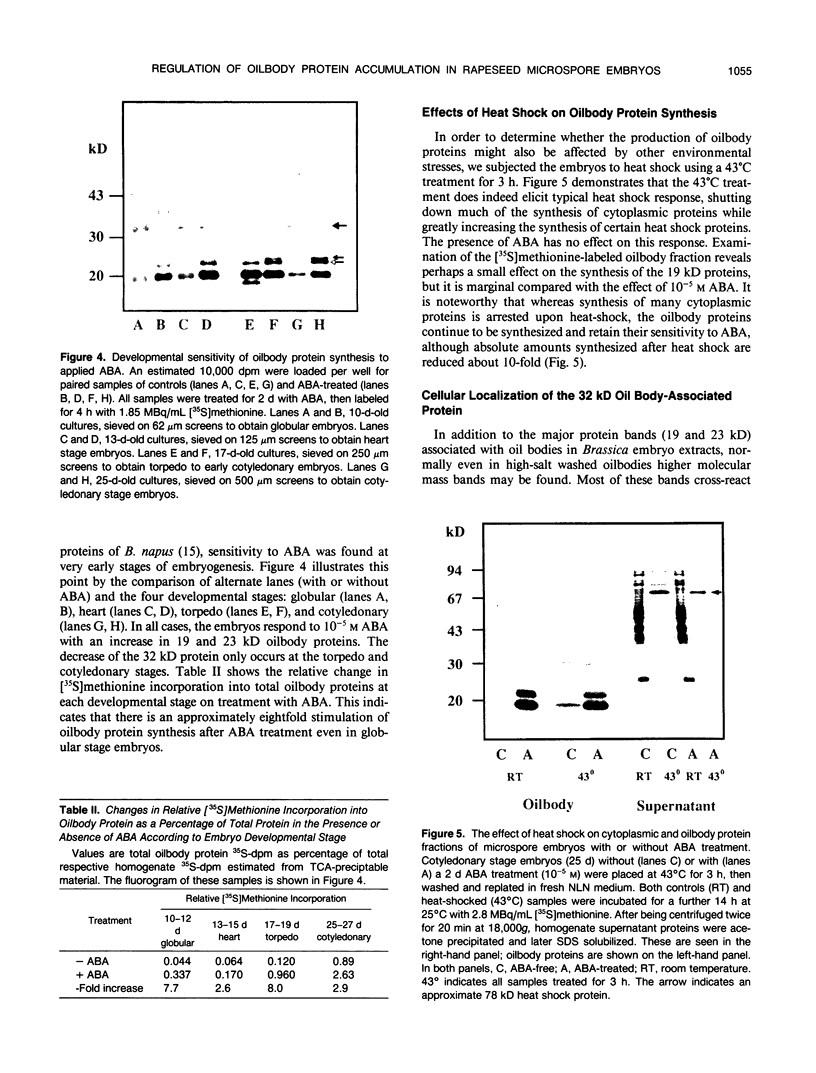
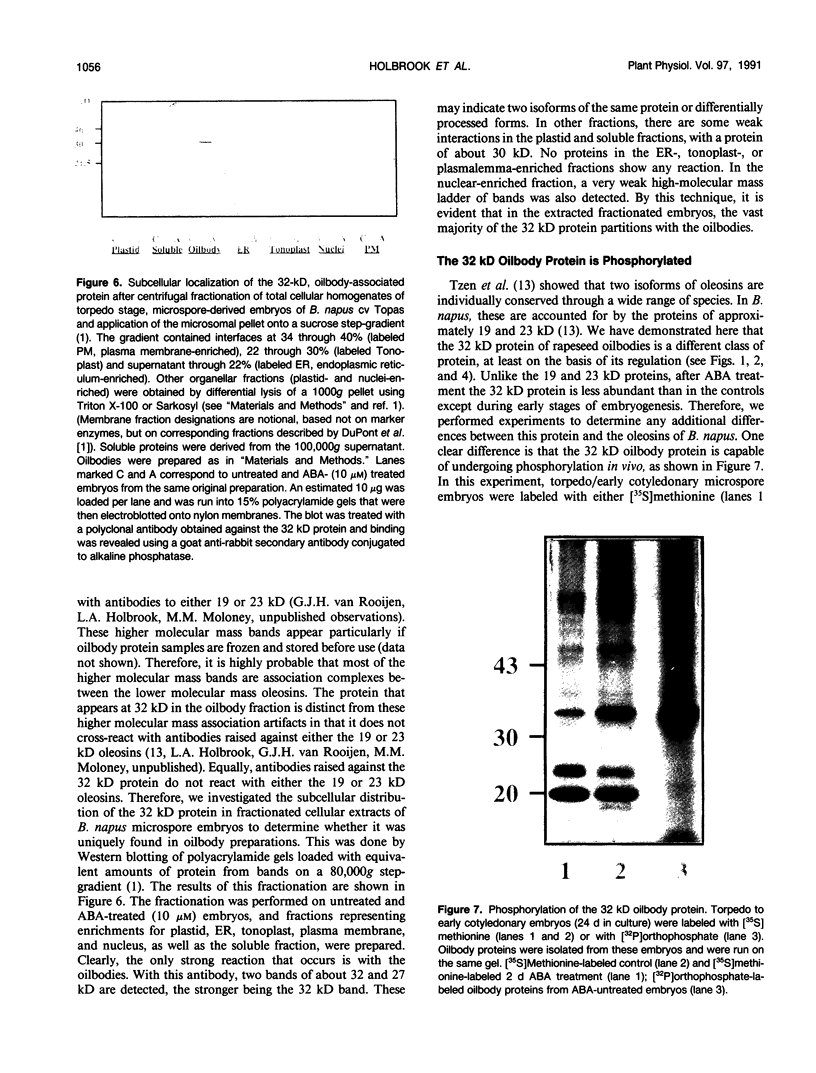
A number of treatments were tested for their ability to affect the synthesis of oilbody proteins in microspore-derived embryos of rapeseed (Brassica napus). Synthesis of the oilbody proteins was determined by [35S]methionine incorporation in vivo and sodium dodecyl sulfate-polyacrylamide gel electrophoresis of washed oilbody fractions. Oilbody proteins of approximately 19, 23, and 32 kilodaltons were found to be prominent. These proteins showed differential patterns of regulation. The 19 and 23 kilodalton proteins (oleosins) were greatly enhanced by treatments with abscisic acid, jasmonic acid, and osmotic stress imposed using sorbitol (12.5%). Synthesis of the 32 kilodalton protein was inhibited by abscisic acid and by sorbitol (12.5%), but unaffected by jasmonates. The strong promotion of synthesis of the 19 and 23 kilodalton oilbody proteins appeared to be specific as they are not seen with gibberellic acid treatment or with a stress such as heat shock. Time course experiments revealed that the abscisic acid stimulation of oleosin synthesis is quite rapid (less than 2 hours), reaching a maximum at 6 to 8 hours. The response of the oleosins to abscisic acid is found in all stages of embryogenesis, with a major increase in synthetic rates even in globular embryos on abscisic acid treatment. This suggests that these proteins may accumulate much earlier in embryogenesis than has previously been believed. The 32 kilodalton oilbody-associated protein appears different from the oleosins in several ways, including its distinct pattern of regulation and its unique property, among the oilbody proteins, of undergoing phosphorylation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dupont F. M., Tanaka C. K., Hurkman W. J. separation and Immunological Characterization of Membrane Fractions from Barley Roots. Plant Physiol. 1988 Mar;86(3):717–724. doi: 10.1104/pp.86.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzopoulos P., Franz G., Choy L., Sung R. Z. Interaction of nuclear factors with upstream sequences of a lipid body membrane protein gene from carrot. Plant Cell. 1990 May;2(5):457–467. doi: 10.1105/tpc.2.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski A., Weisemann J. M., Matthews B. F., Herman E. M. Molecular cloning of a protein associated with soybean seed oil bodies that is similar to thiol proteases of the papain family. J Biol Chem. 1990 Aug 15;265(23):13843–13848. [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. J., Cummins I., Kang A. S. Synthesis of the major oil-body membrane protein in developing rapeseed (Brassica napus) embryos. Integration with storage-lipid and storage-protein synthesis and implications for the mechanism of oil-body formation. Biochem J. 1989 Feb 15;258(1):285–293. doi: 10.1042/bj2580285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. J., Keen J. N., O'Sullivan J. N., Au D. M., Edwards E. W., Jackson P. J., Cummins I., Gibbons T., Shaw C. H., Ryan A. J. A class of amphipathic proteins associated with lipid storage bodies in plants. Possible similarities with animal serum apolipoproteins. Biochim Biophys Acta. 1991 Jan 17;1088(1):86–94. doi: 10.1016/0167-4781(91)90156-g. [DOI] [PubMed] [Google Scholar]
- Qu R. D., Huang A. H. Oleosin KD 18 on the surface of oil bodies in maize. Genomic and cDNA sequences and the deduced protein structure. J Biol Chem. 1990 Feb 5;265(4):2238–2243. [PubMed] [Google Scholar]
- Tzen J. T., Lai Y. K., Chan K. L., Huang A. H. Oleosin isoforms of high and low molecular weights are present in the oil bodies of diverse seed species. Plant Physiol. 1990 Nov;94(3):1282–1289. doi: 10.1104/pp.94.3.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance V. B., Huang A. H. The major protein from lipid bodies of maize. Characterization and structure based on cDNA cloning. J Biol Chem. 1987 Aug 15;262(23):11275–11279. [PubMed] [Google Scholar]
- Wilen R. W., Mandel R. M., Pharis R. P., Holbrook L. A., Moloney M. M. Effects of Abscisic Acid and High Osmoticum on Storage Protein Gene Expression in Microspore Embryos of Brassica napus. Plant Physiol. 1990 Nov;94(3):875–881. doi: 10.1104/pp.94.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilen R. W., van Rooijen G. J., Pearce D. W., Pharis R. P., Holbrook L. A., Moloney M. M. Effects of jasmonic Acid on embryo-specific processes in brassica and linum oilseeds. Plant Physiol. 1991 Feb;95(2):399–405. doi: 10.1104/pp.95.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsu L. Y., Jacks T. J. Spherosome membranes: half unit-membranes. Plant Physiol. 1972 Jun;49(6):937–943. doi: 10.1104/pp.49.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]