Abstract
Although tissues become exposed to both exogenous and endogenous cell-activating mediators during infection, there is little appreciation of the effects of subjecting cells to multiple mediators. We examined the hypothesis that the response of neutrophils to bacterial lipopolysaccharide (LPS) is significantly altered in the presence of the endogenous mediator tumor necrosis factor alpha (TNF). The data showed that human neutrophils pretreated with TNF for 10 to 30 min, displayed significantly enhanced superoxide production in response to LPS (from either Escherichia coli K-235 or E. coli 0127:B8), measured as lucigenin-dependent chemiluminescence (CL), seen as an increase in the initial peak rate as well as the total CL accumulated over the incubation period. TNF amplified the response to LPS at 1 to 100 U of TNF/106 neutrophils and was able to enhance the response to a wide range of concentrations of LPS (0.01 to 1,000 ng/ml). The TNF-induced increase in the LPS response was paralleled by an increase in LPS binding to the neutrophils, which could be abrogated by an anti-CD14 monoclonal antibody. The results demonstrate that TNF significantly increases the LPS-induced release of oxygen radicals in neutrophils through the upregulation of cell surface CD14.
Neutrophils, while playing a key role in microbial killing in infections, may become harmful to host tissues as a result of their nonspecific stimulation by both endogenous and exogenous mediators (1, 5, 16). Bacterial lipopolysaccharide (LPS), a glycolipid from the outer leaflet of the cell wall of gram-negative bacteria, is released into body fluids during infection. Because of its marked biological activity, LPS induces severe pathological changes in host tissues (20). Tumor necrosis factor alpha (TNF) is readily produced by a range of local tissue cells and inflammatory leukocytes during bacterial infections as a result of stimulation by microbial products such as LPS (4, 11, 20). TNF has been shown to have a broad spectrum of biological activities which collectively give rise to the pathophysiological processes seen in a wide range of diseases and disorders (1, 10, 25). The cytokine has previously been shown to upregulate neutrophil responses to some surface acting agonists (5, 6, 9, 17, 21). Although LPS is known to trigger release of O · 2− and H2O2 (2, 21), the relationship between cytokines and microbial components such as LPS in terms of the neutrophil oxidative respiratory burst has not been defined. It was therefore of interest to determine if the response of neutrophils to LPS was increased by the presence of TNF in an attempt to better understand the relationship between these two agents.
Neutrophils with >96% purity and >99% viability (by trypan blue exclusion test) were prepared by centrifugation of blood from healthy volunteers on Hypaque-Ficoll medium with a density of 1.114 (7). Superoxide production was measured by the bis-N-methylacridinium nitrate (lucigenin)-dependent chemiluminescence (CL) assay (14). Lucigenin was purchased from Sigma Chemical Co., St. Louis, Mo. This response is specific for superoxide and can be totally inhibited by superoxide dismutase (12, 15). Briefly, 106 neutrophils in 100 μl of Hanks balanced salt solution, pH 7.3, were transferred into luminometer tubes, and then various reagents were added in an assay volume of 500 μl. The neutrophils were pretreated for 20 min (unless specified otherwise) with the concentrations of TNF indicated in the figure legends. Human recombinant TNF (6 × 107 U/mg, 500 μg/ml, >99% pure) was produced by Genentech, Inc. (San Francisco, Calif.) and was kindly provided by G. R. Adolf (Ernst Boehringer Institute, Vienna, Austria). The endotoxin contamination was less than 0.125 endotoxin U/ml as assessed by the Limulus lysate assay. During the pretreatment times, the tubes were incubated at 37°C in an atmosphere of 95% air–5% CO2 and high humidity. The neutrophils were then treated with LPS (from Escherichia coli K-235 [Sigma; chromatographically purified by gel filtration]) in the presence of 1% heat-inactivated (56°C, 30 min) autologous donor serum and then transferred into a luminometer (water jacketed, 37°C, model 1251 [Bioorbit Oy, Turku, Finland] with MultiUse software, version 1.08). A 500-μl volume of lucigenin was automatically added at a final concentration of 127.5 μg/ml per tube to bring the final assay volume to 1 ml. The resulting CL (in millivolts) in all tubes was measured at the same time in the luminometer chamber. The results were recorded as the peak initial rate of superoxide production and also as the total amount of superoxide produced over a fixed time period by integration of the area under the curve (in millivolts). Some data are expressed as stimulation indices. To obtain these, the means of the treatments were divided by the means of the baseline values.
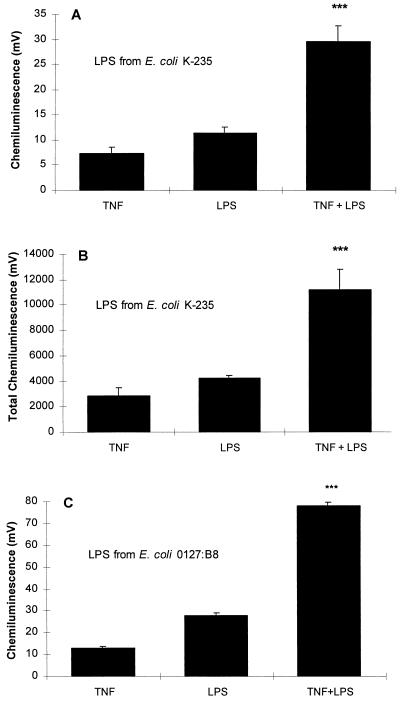
Preliminary studies revealed that pretreatment of neutrophils for 20 min with 20 U of TNF per 106 cells was optimal for priming of these cells for subsequent responses to other agonists (6). Examination of the lucigenin-dependent CL induced by LPS showed that TNF caused significant enhancement of this response (P < 0.001). The effect of TNF was seen as an increase in both the initial peak rate of CL and the total CL produced over the incubation period of 50 min (Fig. 1A and B). To ensure that the effects observed were not restricted to a specific E. coli serotype, the experiments were repeated with LPS from a different serotype, E. coli 0127:B8 (Sigma; chromatographically purified by gel filtration). The results were similar to those obtained with serotype K-235 (Fig. 1C).
FIG. 1.
(A) Effect of TNF on the LPS-induced neutrophil CL response. Neutrophils (106) were preincubated with 20 U of TNF for 20 min and then challenged with 100-ng/ml LPS (E. coli K-235), and CL was measured over 50 min of incubation. The values shown are the peak initial rate of CL and represent the means ± the standard error of the mean of eight experiments, each conducted in duplicate with neutrophils from eight different donors. The basal CL of 6.1 mV was deducted from each of the experimental values. Neutrophils treated with TNF and LPS showed a significantly increased response compared to responses induced by either of the agents alone (∗∗∗, P < 0.001 for TNF versus TNF plus LPS and LPS versus TNF plus LPS; P < 0.015 for the sum of values for the individual TNF and LPS treatments versus cotreatment with TNF plus LPS, [analysis of variance]). (B) Data expressed as a function of the total accumulated CL generated over the incubation period. The baseline value of 2528 mV was subtracted from each column. (∗∗∗, P < 0.001 for TNF versus TNF plus LPS and LPS versus TNF plus LPS; P < 0.015 for the sum of values for the individual TNF and LPS treatments versus cotreatment with TNF plus LPS [analysis of variance]). (C) Effect of LPS (E. coli 0127:B8) under the same conditions as in A. The basal CL of 13.3 mV was deducted from each of the experimental values. The increased response to stimulation with both TNF and LPS was highly significant (∗∗∗, P < 0.001 [analysis of variance]).
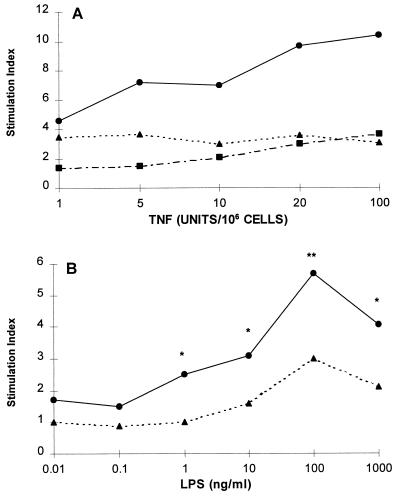
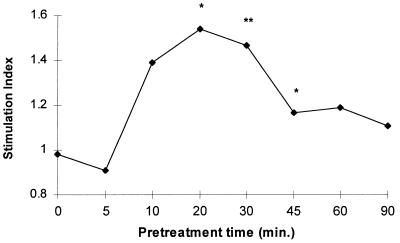
Studies of the effects of different doses of TNF (1, 5, 10, 20, and 100 U/106 cells) showed that TNF pretreatment was effective in significantly enhancing the LPS-induced CL over the entire dose range (Fig. 2A). A wide range of LPS concentrations, from 0.01 to 1,000 ng/ml, was examined, and significant priming was observed from 1 to 1,000 ng/ml, with an optimal effect at 100 ng/ml (Fig. 2B). The priming effects of TNF for the LPS-induced CL response were evident within 10 min of TNF pretreatment and maximal at 20 min. The priming effect had decreased by 45 min but was still above the baseline at 90 min (Fig. 3).
FIG. 2.
(A) Effects of varying TNF amounts on the LPS-induced CL response. Neutrophils were pretreated with the indicated concentrations of TNF or diluent for 20 min and then challenged with 1-μg/ml of LPS (E. coli K-235) or diluent for another 50 min. Neutrophils were treated with TNF only (▪), LPS only (▴), or TNF plus LPS (•). Results are the means of four duplicate experiments, expressed as stimulation indices. These were obtained by dividing the means of the treatments by the means of the baseline values. At all TNF doses, the effects of the combined action of TNF and LPS were significantly different from those of either agonist alone (P values of <0.0001 to <0.05 [analysis of variance]). (B) Response of TNF-primed neutrophils to varying concentrations of LPS (E. coli K-235). The cells were pretreated with 20 U of TNF/106 cells (•) or diluent (▴) for 20 min and then stimulated with the indicated concentrations of LPS. Values are stimulation indices. The data are the means of four experiments conducted in duplicate (∗, P < 0.05; ∗∗, P < 0.01 [analysis of variance]).
FIG. 3.
Effect of varying TNF pretreatment times on the response of neutrophils to LPS (E. coli K-235). Cells were pretreated with 20 U of TNF/106 cells for 20 min and then with LPS (100 ng/ml) for 50 min. The results are derived from eight experiments with different donor cells. The results are expressed as stimulation indices (∗, P, <0.035; ∗∗, P < 0.015 [analysis of variance]).
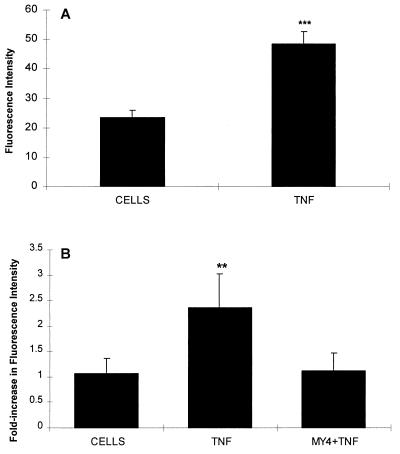
To elucidate the mechanism by which TNF alters the response to LPS, the effects of TNF on the expression of CD14 and on the surface binding of LPS were investigated. The changes in CD14 expression were examined first. Neutrophils (106/100 μl) were treated with either TNF at 20 U/100 μl or diluent at 37°C for 20 min, the tubes were centrifuged at 4°C, and the cells were resuspended in buffer containing murine anti-human CD14 monoclonal antibody MY4 (Coulter Corporation) at a dilution of 1:50 and left on ice for 30 min. Cells in an additional tube were incubated with a murine immunoglobulin G2b antibody in place of the anti-CD14 monoclonal antibody to serve as an isotype control. After two washes with ISOTONE II solution (Coulter Electronics), the cells were treated with a secondary antibody (affinity purified goat anti-mouse phycoerythrin diluted 1:50; Jackson Immuno-Research Laboratories, West Grove, Pa.) for 30 min on ice. The cells were washed three times at 4°C and then fixed in formaldehyde at 1:33 in ISOTONE II solution. The fluorescence intensity of the cell population was analyzed by flow cytometry on a FACScan (Becton Dickinson). Ten thousand cells were counted, and the data were processed with LYSYS II software. TNF pretreatment led to a 2.1-fold increase in CD14 surface expression (Fig. 4A).
FIG. 4.
(A) Effect of TNF pretreatment on surface CD14 on neutrophils. Neutrophils 106 were incubated with either buffer or 20 U of TNF for 20 min. At 4°C the cells were incubated with anti-CD14 and then washed and incubated with goat anti-mouse phycoerythrin. The intensity of fluorescence was determined as described in the text. The data shown are the means and standard errors of five experiments. The increase in fluorescence was 2.1-fold (∗∗∗, P < 0.001 [Student t test]). (B) Effect of TNF on LPS binding. Neutrophils were stimulated as described above. The cells were washed and resuspended in serum (5%, autologous, heat inactivated for 30 min at 56°C) and incubated with FITC-labelled LPS (E. coli 0127:B8) at a final concentration of 16 μg/ml for 30 min on ice. After three washes, flow cytometry was performed as described in the text. The increase was 2.2-fold and could be abrogated by incubating the cells with MY4 (dilution, 1:10) for 30 min prior to adding serum and FITC-LPS. The data shown are the means and standard errors of five experiments (∗∗, P < 0.01 [analysis of variance]).
Assessment of LPS binding to neutrophils (106/100 μl) was done by pretreating cells with either TNF at 20 U/100 μl or diluent for 20 min at 37°C. The tubes were centrifuged at 4°C and the cells were resuspended in 5% heat-inactivated serum. Fluorescein isothiocyanate (FITC)-labelled LPS (E. coli serotype 0127:B8 [Sigma; chromatographically purified by gel filtration]) was added at a final concentration of 16 μg/ml. Unlabelled LPS at the same concentration served as a background control. After 30 min of incubation on ice, the cells were washed three times at 4°C and then fixed in formaldehyde at 1:33 in ISOTONE II solution. The fluorescence intensity of the cell population was analyzed by flow cytometry, and 10,000 cells were counted. Flow cytometry analysis showed a 2.2-fold increase in LPS binding by neutrophils pretreated with TNF compared to the buffer solution. The increase in fluorescence was prevented by incubating TNF-treated neutrophils for 30 min on ice with MY4 at a dilution of 1:10 prior to adding serum and FITC-LPS (Fig. 4B).
TNF is a cytokine with multiple biological activities. These include cytotoxicity, growth modulation, cellular differentiation, and anti-infective properties (1, 5, 8, 11, 20, 25). It has the ability to act locally (paracrine) and systemically (endocrine). While its role in infection as a mediator of the pathological effects of LPS in sepsis is well understood, little is known about how TNF modulates the interaction between neutrophils and LPS. Our data demonstrate that TNF alters neutrophil responses to LPS in vitro. Neutrophils pre-exposed to human recombinant TNF showed significantly increased oxidative respiratory activity and release of oxygen-derived reactive species in response to LPS. Thus, while TNF alone is an incomplete secretagogue and a weak stimulator of the neutrophil respiratory burst (17, 21), it is likely to substantially increase the release of oxygen radicals at foci of infection in the presence of a microbial product such as LPS. This finding may, in part, explain the skewing of the balance toward pathophysiological reactions as a result of cytokine production in the presence of microbial components. LPS, a potent stimulator of TNF production by macrophages, may hence, set up a vicious cycle of neutrophil priming for an increased release of oxygen radicals in response to LPS. In support of this concept was our finding that in the presence of TNF only 1 ng of LPS per ml was required to achieve levels of oxygen-derived radical species production by neutrophils similar to those that LPS at 100 ng/ml achieved in the absence of the cytokine (Fig. 2B). It is evident from these studies that an LPS concentration of 10 ng/ml had to be reached to stimulate neutrophils. However, an LPS concentration as low as 0.1 ng/ml has been shown to stimulate TNF production by macrophages. It is therefore likely that at initial stages of bacterial infections, the production of TNF precedes neutrophil stimulation by LPS. When neutrophils were pretreated with very low doses of LPS (0.01 to 1 ng/ml), they were still able to respond to the combined effects of subsequent TNF and LPS additions (data not presented).
The time course of the neutrophil response strongly implies a mechanism of either intracellular enzyme activation or receptor upregulation. It has been shown that the expression of the LPS receptor CD14, a 55-kDa glycoprotein on the cell surface, doubled after treatment with TNF for 20 min, suggesting mobilization of the molecule to the surface from an intracellular pool (27). While it had been suggested previously that the reservoir of CD14 in neutrophils was the specific granules, current evidence suggests that these molecules are stored primarily in the azurophilic granules (23). We confirmed that under our conditions, TNF treatment caused a significant increase in CD14 expression on neutrophils and an associated increase in LPS binding to the cells. Furthermore, this increase in binding could be abrogated by a monoclonal antibody to CD14. This provides evidence that an increase in CD14 receptor expression is the major basis for the TNF effect.
The mechanisms by which LPS induces intracellular signalling are not clear. While binding of the lipoprotein binding protein-LPS complex to CD14 seems essential (27–29), the receptor is attached to the cell surface via a phosphatidylinositol anchor only. Many investigators believe that molecules anchored in this manner are unlikely to produce signals per se because of the absence of an intracellular domain, while others consider it possible (22, 24). Several investigators have postulated the existence of a membrane protein facilitating signal transduction (26). This putative LPS receptor or LPS receptor cofactor was initially described as a 73-kDa protein in murine splenocytes (18, 19, 30) and human leukocytes (13). However, this protein has since been identified as albumin (3). Nevertheless, the existence of a transmembrane molecule facilitating signalling by LPS is still a likely model to explain CD14 independent activation of cells (26). If such a molecule exists, it may also undergo changes subsequent to TNF stimulation. Irrespective of the existence of additional LPS receptors, our data demonstrated a significant increase in LPS binding by TNF-primed neutrophils. The magnitude of this increase correlated with an increase in surface CD14 and also an increase in the CL response. Based on these observations, we propose that TNF primes neutrophils for an enhanced CL response to LPS, most likely via upregulation of surface CD14, leading to increased LPS binding.
Acknowledgments
This work was supported by funds from the National Health and Medical Research Council of Australia. Hubertus P. A. Jersmann is a recipient of a Reginald Walker scholarship, a scholarship of the University of Adelaide for Medical Graduates.
REFERENCES
- 1.Beutler B, editor. Tumor necrosis factors, the molecules and their emerging role in medicine. New York, N.Y: Raven Press; 1992. [Google Scholar]
- 2.Dahinden C A, Fehr J, Hugli T E. Role of cell surface contact in the kinetics of superoxide production by granulocytes. J Clin Invest. 1983;72:113–121. doi: 10.1172/JCI110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dziarski R. Cell-bound albumin is the 70-kDa peptidoglycan-, lipopolysaccharide-, and lipoteichoic acid-binding protein on lymphocytes and macrophages. J Biol Chem. 1994;269:20431–20436. [PubMed] [Google Scholar]
- 4.Fast D J, Schlievert P M, Nelson R D. Toxic shock syndrome-associated staphylococcal and streptococcal pyrogenic toxins are potent inducers of tumor necrosis factor production. Infect Immun. 1989;57:291–294. doi: 10.1128/iai.57.1.291-294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrante A, Kowanko I, Bates E J. Mechanisms of host tissue damage by cytokine-activated neutrophils. In: Coffey R G, editor. Granulocyte responses to cytokines: basic and clinical research. New York, N.Y: Marcel Dekker, Inc.; 1992. pp. 499–521. [PubMed] [Google Scholar]
- 6.Ferrante A, Martin A J, Bates E J, Goh D H B, Harvey D P, Parsons D, Rathjen D, Russ G, Dayer J-M. Killing of Staphylococcus aureus by tumor necrosis factor α-activated neutrophils: the role of serum opsonins, integrin receptors, respiratory burst and degranulation. J Immunol. 1993;151:4821–4828. [PubMed] [Google Scholar]
- 7.Ferrante A, Thong Y H. A rapid one-step procedure for purification of mononuclear and polymorphonuclear leukocytes from human blood using a modification of the Hypaque-Ficoll technique. J Immunol Methods. 1978;24:389–393. doi: 10.1016/0022-1759(78)90143-6. [DOI] [PubMed] [Google Scholar]
- 8.Ferrante A, Hii C S T, Rathjen D A. Positive perspectives on TNFs: promise versus popular purpose. Today’s Life Sci. 1995;7:40–47. [Google Scholar]
- 9.Figari I S, Mori N A, Palladino M A., Jr Regulation of neutrophil migration and superoxide production by recombinant tumour necrosis factors α and β: comparison to recombinant interferon and interleukin-1. Blood. 1987;70:979–984. [PubMed] [Google Scholar]
- 10.Girardin E, Grau G E, Dayer J-M, Roux-Lombard P, Lambert P-H. Tumor necrosis factor and interleukin-1 in the serum of children with severe infectious purpura. N Engl J Med. 1988;319:397–400. doi: 10.1056/NEJM198808183190703. [DOI] [PubMed] [Google Scholar]
- 11.Guthrie L A, McPhail L C, Henson P M, Johnston R B., Jr Priming or neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. J Exp Med. 1984;160:1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gyllenhammar H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide production. J Immunol Methods. 1986;97:209–213. doi: 10.1016/0022-1759(87)90461-3. [DOI] [PubMed] [Google Scholar]
- 13.Halling J L, Hamill D R, Lei M-G, Morrison D C. Identification and characterization of lipopolysaccharide-binding proteins on human peripheral blood cell populations. Infect Immun. 1992;60:845–852. doi: 10.1128/iai.60.3.845-852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy S J, Ferrante A, Poulos A, Robinson B S, Johnson D W, Murray A W. Effect of exogenous fatty acids with greater than 22 carbon atoms (very long chain fatty acids) on superoxide production by human neutrophils. J Immunol. 1994;153:1754–1761. [PubMed] [Google Scholar]
- 15.Hennet T, Richter C, Peterhans E. Tumour necrosis factor-α induces superoxide anion generation in mitochondria of L929 cells. Biochem J. 1993;289:587–592. doi: 10.1042/bj2890587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henson P M, Henson J E, Fittschen C, Kimani G, Bratton D L, Riches D W H. Phagocytic cells: degranulation and secretion. In: Gallin J A, Goldstein I M, Snyderman R, editors. Inflammation, basic principles and clinical correlates. New York, N.Y: Raven Press; 1988. pp. 363–390. [Google Scholar]
- 17.Klebanoff S J, Vadas M A, Harlan J M, Sparks L H, Gamble J R, Agosti J M, Waltersdorph A M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986;136:4220–4225. [PubMed] [Google Scholar]
- 18.Lei M-G, Morrison D C. Specific endotoxic lipopolysaccharide-binding proteins on murine splenocytes. II. Membrane localization and binding characteristics. J Immunol. 1988;141:1006–1011. [PubMed] [Google Scholar]
- 19.Lei M-G, Stimpson S A, Morrison D C. Specific endotoxic lipopolysaccharide-binding receptors on murine splenocytes. III. Binding specificity and characterization. J Immunol. 1991;147:1925–1932. [PubMed] [Google Scholar]
- 20.Morrison D C, Ryan J L. Endotoxin and disease mechanisms. Annu Rev Med. 1987;38:417–432. doi: 10.1146/annurev.me.38.020187.002221. [DOI] [PubMed] [Google Scholar]
- 21.Nathan C F. Neutrophil activation on biological surfaces: massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987;80:1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson P J. Phosphatidyl inositol membrane anchors and T-cell activation. Immunol Today. 1991;12:35–41. doi: 10.1016/0167-5699(91)90110-F. [DOI] [PubMed] [Google Scholar]
- 23.Rodeberg D A, Morris R E, Babcock G F. Azurophilic granules of human neutrophils contain CD14. Infect Immun. 1997;65:4747–4753. doi: 10.1128/iai.65.11.4747-4753.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schumann R R. Function of lipopolysaccharide (LPS)-binding protein (LBP) and CD14, the receptor for LPS/LBP complexes: a short review. Res Immunol. 1992;143:11–15. doi: 10.1016/0923-2494(92)80074-u. [DOI] [PubMed] [Google Scholar]
- 25.Tracey K J, Cerami A. Tumour necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- 26.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 27.Wright S D, Ramos R A, Hermanowski-Vosatka A, Rockwell P, Detmers P A. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J Exp Med. 1991;173:1281–1286. doi: 10.1084/jem.173.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 29.Wright S D, Ramos R A, Patel M, Miller D S. Septin: a factor in plasma that opsonises lipopolysaccharide-bearing particles for recognition by CD14 on phagocytes. J Exp Med. 1992;176:719–722. doi: 10.1084/jem.176.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright S D. Multiple receptors for endotoxin. Curr Opin Immunol. 1991;3:83–90. doi: 10.1016/0952-7915(91)90082-c. [DOI] [PubMed] [Google Scholar]