Abstract
Japan has various death investigation systems; however, external examinations, postmortem computed tomography, macroscopic examinations, and microscopic examinations are performed regardless of the system used. These examinations can reveal morphological abnormalities, whereas the cause of death in cases with non-morphological abnormalities can be detected through additional examinations. Molecular autopsy and postmortem genetic analyses are important additional examinations. They are capable of detecting inherited arrhythmias or inherited metabolic diseases, which are representative non-morphological disorders that cause sudden death, especially in infants and young people. In this review, we introduce molecular autopsy reports from Japan and describe our experience with representative cases. The relationships between drug-related deaths and genetic variants are also reviewed. Based on the presented information, molecular autopsy is expected to be used as routine examinations in death investigations because they can provide information to save new lives.
Keywords: forensic autopsy, metabolic autopsy, molecular autopsy, postmortem genetic analysis, sudden death
Introduction
The Department of Forensic Medicine at Tokyo Imperial University was established in 1889, and the government of Japan introduced the crime procedure law and stipulated the provision of a judicial autopsy system in 18901, 2. Currently, both the criminal and civil justice system, as well as public health and social welfare programs are subjected to forensic medicine in Japan.
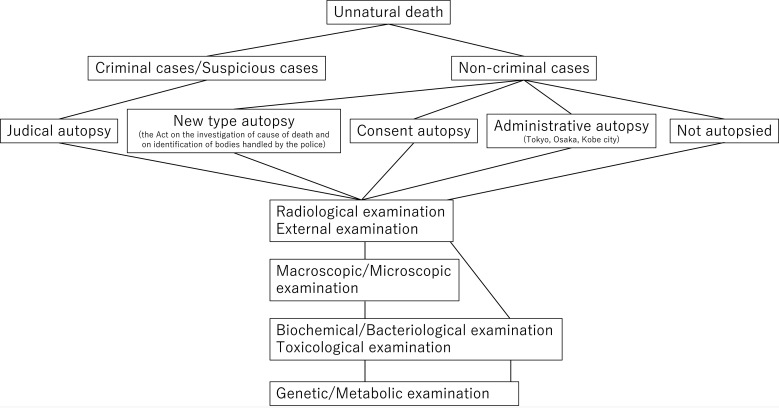
The current Japanese death investigation system varies across regions. All unnatural deaths must be reported to the police. Each case is then categorized as either criminal, suspicious, or non-criminal. Criminal cases and some suspicious cases are autopsied as part of a judicial autopsy, whereas non-criminal cases are not subjected to judicial autopsy. The medical examiner system, which covers the death investigation of non-criminal cases, is available mainly in Tokyo, Osaka, and Kobe city (administrative autopsy), while consent autopsy (consent of the bereaved family is necessary), which also targets non-criminal cases, is rarely performed except in a few regions1, 3. Recently, the Act on the Investigation of the Cause of Death and Identification of Bodies Handled by the Police was implemented, leading to the establishment of a new type of autopsy4 wherein some non-criminal cases are autopsied; however, this system remains imperfect. The autopsy rate is low, and in most non-criminal cases, a general clinician prepares a death certificate without an autopsy1.
Regardless of the applicable autopsy system, external examinations are performed first. Recently, postmortem computed tomography (CT) has been used for this purpose5. Macroscopic examination during forensic autopsy involves surveying the organs in the three main cavities (skull, thorax, and abdomen), as well as the subcutaneous soft tissue of the neck, back, and limbs. Microscopic examination is performed in almost all the cases (Fig. 1). This classical autopsy procedure helps to reveal morphological abnormalities, regardless of further macroscopic or microscopic examination; however, the cause of death in cases with non-morphological abnormalities is detected by additional examination. In this review, we introduced the examination of the causes of death in patients with non-morphological abnormalities, especially in the context of molecular autopsy.
Fig. 1.
Regardless of the autopsy system, external examination, postmortem radiological examination, and macroscopic examination are performed. Biochemical, bacteriological, and toxicological examinations are routinely performed in forensic autopsy. Genetic/Metabolic examinations are not routinely performed, but are recommended, even if an autopsy is not performed.
Toxicological Analysis
One of the most important non-morphological abnormalities in forensic science is drug-related death. Toxicological examinations, including those for alcohol, psychotropic drugs, and various other therapeutic drugs, are necessary for the diagnosis of this condition. Suicide, improper use of psychotropic drugs (overdose or criminal use), and illegal drug use have become major social problems. Death investigation systems and forensic autopsies can help uncover such cases because drug-related deaths have no specific findings and are difficult to diagnose without suspicion. Therefore, comprehensive drug screening is important, even if drug use is not suspected6, 7, 8. In a famous case of serial killing covered by the media, no drug involvement was suspected in the victim’s death; however, as another victim was later revealed to have been killed by some kind of drug, police investigations revealed that the first victim had also been administered drugs by the perpetrator. However, in addition to criminal cases, accidental or self-use cases also need to be investigated.
Caffeine is a commonly administered drug in daily use. It is found in coffee and tea, and its proper use is beneficial for humans. However, information on the risk of caffeine exposure has only been uncovered using forensic autopsies. Takayama et al. reported the case of a male in his early 20’s working a night shift, whose cause of death was diagnosed as caffeine intoxication9. He consumed many energy drinks to stay awake and alert, but this resulted in excessive caffeine intake. Energy drinks, unlike most medicines, can be consumed repeatedly; therefore, the authors emphasized the dangers of repeated and chronic consumption of caffeinated products such as energy drinks and caffeine-containing drugs. We also reported a case of caffeine-related death10. An 18-year-old female with depression was found dead at home. There was significant vomiting around her mouth, and her room contained a few hundred empty blister packs of sleepiness inhibitors, including caffeine and some vitamins. Toxicological tests revealed a high amount of caffeine in many samples, and caffeine intoxication was diagnosed. At that time, reports of fatal caffeine intoxication were rare in Japan. As caffeine is not an illegal drug and is very easy to obtain, information from death investigations and forensic autopsies notified society regarding the dangers of caffeine overdose. According to the Act on Promotion of Policy about Death Investigation established in 2019, “Knowledge obtained by death investigations should be widely used as information that contributes to the improvement and promotion of public health;”11 therefore, announcement of these cases is an important aspect of forensic science.
Sudden Natural Death and Molecular Autopsy
Sudden natural death is defined as death primarily attributed to an illness or an internal malfunction of the body and is not directly influenced by external forces12. Most cases of sudden death are attributed to cardiac arrest caused by morphological or non-morphological cardiac diseases. The latter is also an important non-morphological abnormality in forensic science. Although the details of histopathological examination for sudden natural death should be referred using text books and review articles13, a macroscopic autopsy alone often fails to detect the cause of death; in up to 40% of cases of sudden death, no definite cause of death is identifiable by autopsy14, 15. Molecular analysis is used to detect the cause of death in patients without morphological abnormalities.
Because deaths may occur suddenly, unexpectedly, and sometimes outside hospital settings, information regarding the health condition in these cases is not apparent to the relatives of the deceased or the medical staff. Owing to this, these cases are reported to the police as unnatural deaths but are mostly classified as non-criminal; therefore, autopsies are often not performed. However, in our region, the autopsy rate is rather high and cases of sudden death are likely to be autopsied, occasionally resulting in the diagnosis of rare diseases. Notably, the investigation of sudden death is important for medical pathophysiology and social issues. In this section, we focus on molecular autopsy in Japan from the perspective of postmortem genetic analysis.
Molecular autopsy involves postmortem genetic analyses16, 17. The sudden death of young individuals is associated with congenital channelopathy17, 18. Inherited arrhythmias are a cause of sudden death and include Brugada syndrome, long QT syndrome (LQTS), short QT syndrome, and catecholaminergic polymorphic ventricular tachycardia. Cardiomyopathies such as arrhythmogenic right ventricular cardiomyopathy (ARVC), dilated cardiomyopathy (DCM), and hypertrophic cardiomyopathy can also cause sudden death. Molecular autopsy revealed potentially pathogenic variants of the genes associated with these conditions in up to 25% of sudden death cases in a young population19.
Ackerman et al. reported the first molecular autopsy by identifying a novel LQTS pathogenic variant (KCNQ1) in a 19-year-old woman. They also performed postmortem SCN5A-variant analysis in 93 cases of sudden infant death and detected causative genetic variants in two sudden death cases20, 21. Initially, molecular autopsy targeted only one or a few genes22. However, similar to toxicological screening, genetic analysis is expected to be conducted in a comprehensive manner, because there are numerous candidate genes for these arrhythmias. Thus, next-generation sequencing (NGS) has been utilized for molecular autopsy since its development23, 24, 25. Currently, postmortem genetic analysis is performed in a comprehensive manner, including whole-genome and exome sequencing16, 26, 27.
Metabolic Autopsy
Inherited metabolic diseases are congenital diseases caused by genetic variants, sometimes resulting in sudden infant deaths. Metabolic autopsy is a death investigation method that focuses on metabolic diseases28. Metabolic autopsy was first reported in 1984 when an 18-month-old infant with non-specific malaise and mild infection of the upper respiratory tract developed a grand malconvulsion and died suddenly. The cause of death was initially considered sudden infant death syndrome (SIDS); however, diffuse fatty changes in the viscera and metabolic analysis revealed that the patient had medium-chain acyl-coenzyme A dehydrogenase (MCAD) deficiency29, 30. Metabolic autopsy includes the analysis of metabolic products in the blood, urine, and other fluids, enzyme analysis, and genetic analysis. Nagao reported that no 985A-to-G variant in the MCAD deficiency gene was detected in two Japanese patients who experienced SIDS31. Semba et al. reported an autopsy case of the neonatal form of carnitine palmitoyltransferase II (CPT II) deficiency in a 2-day-old Japanese boy32. We also performed metabolic autopsy in 30 cases of sudden infant death33. Although not a routine procedure, metabolic autopsy is advocated in pediatric guidelines34 and has been prevalent in Japan, not only in autopsy33, 35, 36, 37, 38, 39, 40, 41, but also in the emergency room42, 43, 44.
Molecular Autopsy in Japan
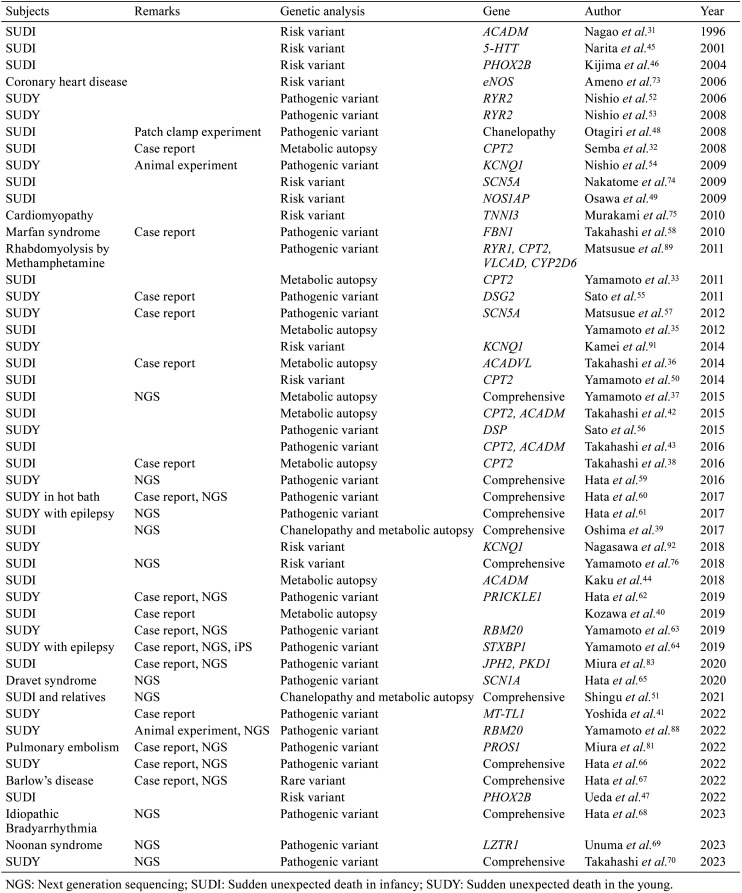
Molecular autopsy targets metabolic diseases, as well as other diseases. Table 1 presents the representative reports of postmortem genetic analyses conducted in Japan.
Table 1. Postmortem Genetic Analysis in Japan.
In 2001, Narita et al. reported for the first time a relationship between serotonin transporter gene variation and SIDS45. In 2004, Kijima et al. analyzed the PHOX2B gene in 23 cases of sudden infant death and did not find any variants, except for three polymorphic nucleotide substitutions46. The PHOX2B gene was recently analyzed from the perspective of a non-polyalanine repeat variant47. In 2008, Otagiri et al. analyzed arrhythmia related genes and revealed that close to 10% of patients who experienced SIDS carried variants of cardiac ion channel genes48. Osawa et al. showed that variations in the novel QT interval determinant NOS1AP may be involved in the occurrence of SIDS49. Similarly, we previously performed molecular autopsy to investigate sudden infant death33, 35, 37, 39, 50, 51.
For sudden deaths in young people, postmortem genetic analysis was introduced into the field of forensic science in the middle of the 2000’s. In studies conducted in 2006 and 2008, our group examined the RYR2 gene in several autopsy cases of sudden unexplained death and found that three cases carried the variant52, 53. They also found that one case among 17 sudden unexplained death autopsy cases carried a KCNQ1 variant, which was revealed to be pathogenic using knock-in mouse analysis54. Sato et al. reported four cases of ARVC-related variants55, 56 and Matsusue et al. reported a case of an SCN5A variant57. Takahashi et al. also reported a case of sudden death due to Marfan syndrome caused by an FBN1 gene variant58. These studies were performed using Sanger sequencing, and currently, NGS-based studies are being reported in Japan37, 39, 51, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70.
Although some global guidelines recommend molecular autopsy for sudden death, this has not yet become a routine procedure in most countries including Japan19. In some cases, inherited genetic disease testing is performed as part of medical practice, whereas postmortem genetic investigation is now performed as research. Therefore, the establishment of a suitable system is necessary in the future.
The relationship between genetic variants and sudden death has been studied extensively. As described above, variation in the serotonin transporter gene is famously considered a cause of sudden infant death45, 71, 72; ACADM, PHOX2B, eNOS, SCN5A, NOS1AP, TNNI3, CPT2, and LQTS-related gene variants have also been analyzed in this context31, 46, 47, 49, 50, 73, 74, 75, 76. In the following section, representative cases that we experienced are described. All cases have been reported elsewhere.
Case Presentations
Case 1: Metabolic autopsy for sudden unexpected death in infancy33
A 6-month-old boy with a fever for several days suddenly lost consciousness and died. External examination revealed no injury, and macroscopic examination revealed no distinctive abnormalities. Drug screening results were negative. Microscopic examination revealed liver steatosis, suggestive of Reye-like syndrome.
Reye-like syndrome sometimes occurs because of inherited metabolic disorders77, 78. Thus, postmortem biochemical analysis was performed to analyze the metabolic products, and the results revealed an increase in long-chain acylcarnitines, suggesting that the deceased had long-chain fatty acid oxidation defects, such as CPT II deficiency or carnitine-acylcarnitine translocase deficiency. Genetic analysis of these two metabolic diseases was performed using Sanger sequencing, and two substitutions in the CPT2 gene were detected. Finally, CPT II deficiency was diagnosed by metabolic autopsy; however, this was time consuming and costly because these two genes have many exons.
Case 2: Metabolic autopsy with NGS for the remaining relatives and a new life37
An 11-month-old girl with a fever and vomiting for several days suddenly lost consciousness and died. No distinctive abnormalities were detected on postmortem CT imaging and macroscopic examination; furthermore, drug screening was negative. Microscopic examination revealed fatty liver. However, morphological examination did not reveal a background pathophysiology. Unlike in Case 1, NGS was available at the time. A comprehensive postmortem genetic analysis was performed using a fatty acid oxidation-related gene panel. The results revealed two variants of CPT2 in only a single run within a few days.
In this case, pedigree analysis confirmed that each variant was inherited from the parents. Thus, we contacted the family doctor to prevent potential metabolic crises in any other children from future pregnancies, and newborn babies could then be subjected to medical follow-up during their asymptomatic period. Thus, forensic autopsies can help save new lives.
Case 3: Sudden arrhythmogenic death in sudden infant death51
A 3-month-old female infant died while sleeping. External examination revealed no injury and macroscopic and microscopic examinations revealed no abnormalities. SIDS was suspected to be the cause of death.
Postmortem genetic analysis revealed a D85N-KCNE1 variant, which was reported to be associated with LQTS79; it was thus suggested as a possible cause of sudden infant death at first glance. However, family screening was necessary to increase the diagnostic yield80. Trio analysis revealed that the infant’s healthy living mother who had no electrocardiogram abnormalities had the same variant. This suggested that this disease-associated genetic variant alone did not cause sudden infant death, as no symptoms were found in relatives, including parents. Thus, the genetic investigation of a single case may have misled the diagnosis.
Case 4: Molecular autopsy in a morphological abnormality case81
Molecular autopsy is not performed to diagnose the direct cause of death, but to reveal the pathophysiology of dying.
In this case, macroscopic autopsy revealed pulmonary embolism from an organizing thrombus in the inferior vena cava as the cause of death. If the aim of a forensic autopsy was only to determine the cause of death, this attempt might have been meaningless. However, the aim was not only to diagnose the cause of death but also to uncover its pathophysiology. The presence of an organizing thrombus with fibrosis and calcification and the absence of a history of trauma or chronic disease suggested that the deceased had subclinical congenital thrombophilia. Therefore, postmortem genetic analysis revealed that the deceased had the A139V-PROS1 variant. This variant has been reported to result in low Protein S activity and total Protein S antigen levels82. Therefore, we considered that subclinical congenital thrombophilia was related to the pathophysiology of pulmonary embolism in the deceased. It is an inherited disease, thus necessitating familial screening. To this end, information regarding the cause of death triggered an examination of family members.
Case 5: Molecular autopsy for death investigation without autopsy83
An autopsy is the most reliable method for detecting the cause of death11, 84. However, the autopsy rate in Japan is low (approximately 1.6%)1; therefore, all unnatural deaths are not subject to autopsy examinations. However, this does not imply that a death investigation is not conducted. Although postmortem radiological, needle pathological, toxicological, and biochemical examinations cannot be considered alternatives to autopsy, they may be used to suggest the cause of death.
An eight-month-old Japanese girl with polycystic kidney disease (PKD) died of acute cardiac failure after acute-onset dyspnea at the hospital. Autopsies are not performed in Japan for such non-criminal cases. The pediatrician proposed a postmortem molecular autopsy of the family and consulted our laboratory. Performing autopsies is the main task of forensic science; however, our laboratory also receives requests for postmortem molecular analysis. In this case, postmortem genetic analysis revealed JPH2 (p.T237A/p. I414L) as the causative variant of DCM and PKD1 (p.Q4193*) as the causative factor of PKD. PKD is reportedly associated with DCM (PKD cardiomyopathy)85, 86. Therefore, in this case, the PKD1 variant may have accelerated the onset and progression of DCM through impaired calcium cycling in cardiomyocytes.
Case 6: RNA sequencing and model animal experiments63
The deceased was a man in his 20’s with a diagnosis of sudden natural death. Postmortem genetic analysis revealed the I536T RBM20 variant. The RBM20 gene is known to be one of the causative genes for DCM via splicing abnormalities; however, the significance of the I536T variant is unknown87. To reveal the significance of this variant, RNA sequencing of cardiac samples from the deceased, splicing reporter assays, and knock-in mouse experiments were performed88. These investigations revealed that the I536T variant affects the splicing of cardiac structural proteins, resulting in the development of DCM.
As comprehensive genetic analyses have progressed, several variants of uncertain significance have been detected in sudden death cases. This underscores the importance of performing molecular autopsy and interpreting the significance of detected variants in diagnosing the cause of death.
Investigation of Drug-related Death Using Molecular Autopsy
There are two types of drug-related deaths. The first is death in which a fatal dose is detected and intoxication is the cause of death, as described above. The second involves a drug-induced secondary pathogenesis. Matsusue et al. focused on the genetic variants in methamphetamine users89. They examined the RYR1, CPT2, ACADVL, and CYP2D6 genes and found no obvious relationship between these genetic variants and rhabdomyolysis. Drug-induced arrhythmias are known to occur. LQTS is a life-threatening arrhythmic disease often caused by an inherited genetic variant, as described above; however, it is sometimes induced by drugs90. Kamei et al. performed a genetic analysis of KCNQ1 and KCNH2 in 10 cases of sudden death involving patients administered psychotropic medication, and concluded that administering psychotropic drug therapy to individuals carrying the G643S variant of KCNQ1 may increase the risk of prolonged QT intervals and life-threatening arrhythmias91. Nagasawa et al. also analyzed the KCNQ1 and KCNH2 genes in 20 cases (of which 10 died after taking methamphetamine and 10 died after using new psychoactive substances) and concluded that the use of new psychoactive substances, particularly synthetic cathinones, is associated with an elevated risk of serious cardiac arrhythmia and sudden death in individuals carrying G643S KCNQ192. Overall, genetic predisposition can provide important information for future mortality investigations.
Important Notes Regarding Genetic Variants
The importance of molecular autopsy has increased in both medical and legal fields. Of note are the incidents of scientific sleuths solving murder mysteries93. For instance, a mother whose child died after labored breathing, uncontrolled vomiting, and gastric distress was sent to prison for murder because the child was diagnosed with ethylene glycol poisoning. However, the diagnosis was later corrected to methylmalonic acidemia using metabolic autopsy28. Furthermore, in a case where a mother was convicted of the murder of three of her children and manslaughter of the fourth, Brohus et al. reported a genetic predisposition in the family, highlighting the importance of identifying genetic variants94, 95.
As described above, many genetic variants have been detected since the development of molecular autopsy using NGS, most of which would have remained unknown if it was not for molecular autopsy. Most of these variants show incomplete penetrance, and because the medical information of patients is lacking in cases of sudden death, it remains unclear whether a disorder is associated with a detected variant13. Further accumulation of knowledge and interpretation of such genetic analyses is thus necessary to prevent sudden death among bereaved family members96.
Some guidelines recommend saving samples in sudden death cases97. This facilitates genetic analyses during autopsy and later reinvestigation because toxicological examination and molecular autopsy, as described above, are not mandatory procedures.
Conclusion
In this review, we introduced a method for investigating the cause of death, especially in cases with non-morphological abnormalities, and described representative cases. Although toxicological examinations and molecular autopsy are not always performed, except during forensic autopsies, they can help detect potential crimes and accidents, as well as subclinical genetic abnormalities, providing important information to the surviving relatives. Thus, although classical autopsy (macroscopic and microscopic examinations) is the most important part of death investigations, other examinations, including molecular and metabolic autopsy, are also essential.
Disclosure of Potential Conflicts of Interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors would like to thank the Division of Legal Medicine, Department of Community Preventive Medicine, Niigata University Graduate School of Medicine and Dental Sciences, Niigata, Japan, and the Department of Pediatrics, Japanese Red Cross Kyoto Daiichi Hospital, Kyoto, Japan, for providing the patients.
References
- 1.Ishihara K, and Iwase H. Reform of the death investigation system in Japan. Med Sci Law. 60: 216–222. 2020. [DOI] [PubMed] [Google Scholar]
- 2.Zangpo D, Iino M, Nakatome M, Yoshimiya M, and Norbu N. Forensic medicine in South Asia: comparison to the developed countries. Yonago Acta Med. 65: 191–199. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujimiya T. Legal medicine and the death inquiry system in Japan: a comparative study. Leg Med (Tokyo). 11(Suppl 1): S6–S8. 2009. [DOI] [PubMed] [Google Scholar]
- 4.Act on investigation of cause of death and on identification of bodies handled by the police. https://elaws.e-gov.go.jp/document?lawid=424AC1000000034_20220617_504AC0000000068.
- 5.Iino M, and O’Donnell C. Postmortem computed tomography findings of upper airway obstruction by food. J Forensic Sci. 55: 1251–1258. 2010. [DOI] [PubMed] [Google Scholar]
- 6.Ishida T, Kudo K, Naka S, Toubou K, Noguchi T, and Ikeda N. Rapid diagnosis of drug intoxication using novel NAGINATA gas chromatography/mass spectrometry software. Rapid Commun Mass Spectrom. 21: 3129–3138. 2007. [DOI] [PubMed] [Google Scholar]
- 7.Kudo K, Nagamatsu K, Umehara T, Usumoto Y, Sameshima N, Tsuji A, and Ikeda N. Rapid and reliable screening method for detection of 70 pesticides in whole blood by gas chromatography-mass spectrometry using a constructed calibration-locking database. Leg Med (Tokyo). 14: 93–100. 2012. [DOI] [PubMed] [Google Scholar]
- 8.Tominaga M, Michiue T, Inamori-Kawamoto O, Hishmat AM, Oritani S, Takama M, Ishikawa T, and Maeda H. Efficacy of drug screening in forensic autopsy: retrospective investigation of routine toxicological findings. Leg Med (Tokyo). 17: 172–176. 2015. [DOI] [PubMed] [Google Scholar]
- 9.Takayama M, Waters B, Hara K, Kashwagi M, Matsusue A, Ikematsu N, and Kubo SI. An autopsy case of caffeine intoxication related by energy drink. Nihon Arukoru Yakubutsu Igakkai Zasshi. 51: 228–233. 2016. [PubMed] [Google Scholar]
- 10.Yamamoto T, Yoshizawa K, Kubo S, Emoto Y, Hara K, Waters B, Umehara T, Murase T, and Ikematsu K. Autopsy report for a caffeine intoxication case and review of the current literature. J Toxicol Pathol. 28: 33–36. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Act on promotion of policy about death investigation. https://elaws.e-gov.go.jp/document?lawid=501AC0100000033.
- 12.Sanchez O, Campuzano O, Fernández-Falgueras A, Sarquella-Brugada G, Cesar S, Mademont I, Mates J, Pérez-Serra A, Coll M, Pico F, Iglesias A, Tirón C, Allegue C, Carro E, Gallego MÁ, Ferrer-Costa C, Hospital A, Bardalet N, Borondo JC, Vingut A, Arbelo E, Brugada J, Castellà J, Medallo J, and Brugada R. Natural and undetermined sudden death: value of post-mortem genetic investigation. PLoS One. 11: e0167358. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markwerth P, Bajanowski T, Tzimas I, and Dettmeyer R. Sudden cardiac death-update. Int J Legal Med. 135: 483–495. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagnall RD, Weintraub RG, Ingles J, Duflou J, Yeates L, Lam L, Davis AM, Thompson T, Connell V, Wallace J, Naylor C, Crawford J, Love DR, Hallam L, White J, Lawrence C, Lynch M, Morgan N, James P, du Sart D, Puranik R, Langlois N, Vohra J, Winship I, Atherton J, McGaughran J, Skinner JR, and Semsarian C. A prospective study of sudden cardiac death among children and young adults. N Engl J Med. 374: 2441–2452. 2016. [DOI] [PubMed] [Google Scholar]
- 15.Castiglione V, Modena M, Aimo A, Chiti E, Botto N, Vittorini S, Guidi B, Vergaro G, Barison A, Rossi A, Passino C, Giannoni A, Di Paolo M, and Emdin M. Molecular autopsy of sudden cardiac death in the genomics era. Diagnostics (Basel). 11: 1378. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narula N, Tester DJ, Paulmichl A, Maleszewski JJ, and Ackerman MJ. Post-mortem whole exome sequencing with gene-specific analysis for autopsy-negative sudden unexplained death in the young: a case series. Pediatr Cardiol. 36: 768–778. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campuzano O, and Sarquella-Brugada G. Molecular autopsy in sudden cardiac death. Glob Cardiol Sci Pract. 2023: e202308. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiles MK, Wilde AAM, Abrams DJ, Ackerman MJ, Albert CM, Behr ER, Chugh SS, Cornel MC, Gardner K, Ingles J, James CA, Jimmy Juang JM, Kääb S, Kaufman ES, Krahn AD, Lubitz SA, MacLeod H, Morillo CA, Nademanee K, Probst V, Saarel EV, Sacilotto L, Semsarian C, Sheppard MN, Shimizu W, Skinner JR, Tfelt-Hansen J, and Wang DW. 2020 APHRS/HRS expert consensus statement on the investigation of decedents with sudden unexplained death and patients with sudden cardiac arrest, and of their families. Heart Rhythm. 18: e1–e50. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-Barrios E, Grassi S, Brión M, Toro R, Cesar S, Cruzalegui J, Coll M, Alcalde M, Brugada R, Greco A, Ortega-Sánchez ML, Barberia E, Oliva A, Sarquella-Brugada G, and Campuzano O. Molecular autopsy: twenty years of post-mortem diagnosis in sudden cardiac death. Front Med (Lausanne). 10: 1118585. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ackerman MJ, Tester DJ, Porter CJ, and Edwards WD. Molecular diagnosis of the inherited long-QT syndrome in a woman who died after near-drowning. N Engl J Med. 341: 1121–1125. 1999. [DOI] [PubMed] [Google Scholar]
- 21.Ackerman MJ, Siu BL, Sturner WQ, Tester DJ, Valdivia CR, Makielski JC, and Towbin JA. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. JAMA. 286: 2264–2269. 2001. [DOI] [PubMed] [Google Scholar]
- 22.Tester DJ, and Ackerman MJ. The role of molecular autopsy in unexplained sudden cardiac death. Curr Opin Cardiol. 21: 166–172. 2006. [DOI] [PubMed] [Google Scholar]
- 23.Bagnall RD, Das K J, Duflou J, and Semsarian C. Exome analysis-based molecular autopsy in cases of sudden unexplained death in the young. Heart Rhythm. 11: 655–662. 2014. [DOI] [PubMed] [Google Scholar]
- 24.Campuzano O, Sanchez-Molero O, Allegue C, Coll M, Mademont-Soler I, Selga E, Ferrer-Costa C, Mates J, Iglesias A, Sarquella-Brugada G, Cesar S, Brugada J, Castellà J, Medallo J, and Brugada R. Post-mortem genetic analysis in juvenile cases of sudden cardiac death. Forensic Sci Int. 245: 30–37. 2014. [DOI] [PubMed] [Google Scholar]
- 25.Loporcaro CG, Tester DJ, Maleszewski JJ, Kruisselbrink T, and Ackerman MJ. Confirmation of cause and manner of death via a comprehensive cardiac autopsy including whole exome next-generation sequencing. Arch Pathol Lab Med. 138: 1083–1089. 2014. [DOI] [PubMed] [Google Scholar]
- 26.Anderson JH, Tester DJ, Will ML, and Ackerman MJ. Whole-exome molecular autopsy after exertion-related sudden unexplained death in the young. Circ Cardiovasc Genet. 9: 259–265. 2016. [DOI] [PubMed] [Google Scholar]
- 27.Andersen JD, Jacobsen SB, Trudsø LC, Kampmann ML, Banner J, and Morling N. Whole genome and transcriptome sequencing of post-mortem cardiac tissues from sudden cardiac death victims identifies a gene regulatory variant in NEXN. Int J Legal Med. 133: 1699–1709. 2019. [DOI] [PubMed] [Google Scholar]
- 28.Bennett MJ, and Rinaldo P. The metabolic autopsy comes of age. Clin Chem. 47: 1145–1146. 2001. [PubMed] [Google Scholar]
- 29.Howat AJ, Bennett MJ, Variend S, and Shaw L. Deficiency of medium chain fatty acylcoenzyme A dehydrogenase presenting as the sudden infant death syndrome. Br Med J (Clin Res Ed). 288: 976–976. 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howat AJ, Bennett MJ, Variend S, Shaw L, and Engel PC. Defects of metabolism of fatty acids in the sudden infant death syndrome. Br Med J (Clin Res Ed). 290: 1771–1773. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagao M. Frequency of 985A-to-G mutation in medium-chain acyl-CoA dehydrogenase gene among patients with sudden infant death syndrome, Reye syndrome, severe motor and intellectual disabilities and healthy newborns in Japan. Acta Paediatr Jpn. 38: 304–307. 1996. [DOI] [PubMed] [Google Scholar]
- 32.Semba S, Yasujima H, Takano T, and Yokozaki H. Autopsy case of the neonatal form of carnitine palmitoyltransferase-II deficiency triggered by a novel disease-causing mutation del1737C. Pathol Int. 58: 436–441. 2008. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto T, Tanaka H, Kobayashi H, Okamura K, Tanaka T, Emoto Y, Sugimoto K, Nakatome M, Sakai N, Kuroki H, Yamaguchi S, and Matoba R. Retrospective review of Japanese sudden unexpected death in infancy: the importance of metabolic autopsy and expanded newborn screening. Mol Genet Metab. 102: 399–406. 2011. [DOI] [PubMed] [Google Scholar]
- 34.Japanese Society for Inherited Metabolic Diseases. https://jsimd.net/pdf/newborn-mass-screening-disease-practice-guideline2019.pdf.
- 35.Yamamoto T, Emoto Y, Murayama K, Tanaka H, Kuriu Y, Ohtake A, and Matoba R. Metabolic autopsy with postmortem cultured fibroblasts in sudden unexpected death in infancy: diagnosis of mitochondrial respiratory chain disorders. Mol Genet Metab. 106: 474–477. 2012. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi Y, Sano R, Nakajima T, Kominato Y, Kubo R, Takahashi K, Ohshima N, Hirano T, Kobayashi S, Shimada T, Tokue H, Awata S, Hirasawa S, and Ishige T. Combination of postmortem mass spectrometry imaging and genetic analysis reveals very long-chain acyl-CoA dehydrogenase deficiency in a case of infant death with liver steatosis. Forensic Sci Int. 244: e34–e37. 2014. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto T, Mishima H, Mizukami H, Fukahori Y, Umehara T, Murase T, Kobayashi M, Mori S, Nagai T, Fukunaga T, Yamaguchi S, Yoshiura KI, and Ikematsu K. Metabolic autopsy with next generation sequencing in sudden unexpected death in infancy: Postmortem diagnosis of fatty acid oxidation disorders. Mol Genet Metab Rep. 5: 26–32. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi Y, Sano R, Kominato Y, Kubo R, Takahashi K, Nakajima T, Takeshita H, and Ishige T. A case of sudden unexpected infant death involving a homozygotic twin with the thermolabile CPT2 variant, accompanied by rotavirus infection and treatment with an antibiotic containing pivalic acid. Leg Med (Tokyo). 22: 13–17. 2016. [DOI] [PubMed] [Google Scholar]
- 39.Oshima Y, Yamamoto T, Ishikawa T, Mishima H, Matsusue A, Umehara T, Murase T, Abe Y, Kubo SI, Yoshiura KI, Makita N, and Ikematsu K. Postmortem genetic analysis of sudden unexpected death in infancy: neonatal genetic screening may enable the prevention of sudden infant death. J Hum Genet. 62: 989–995. 2017. [DOI] [PubMed] [Google Scholar]
- 40.Kozawa S, Yamamoto T, Ikematsu K, and Nata M. An autopsy case of sudden death suspected by mitochondrial disorder and Pearson’s marrow pancreas syndrome. Forensic Sci Int. 1: 100026. 2019. [Google Scholar]
- 41.Yoshida K, Sato H, Kimura S, Tanaka T, and Kasai K. A case of sudden cardiac death due to mitochondrial disease. Leg Med (Tokyo). 55: 102026. 2022. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T, Yamada K, Kobayashi H, Hasegawa Y, Taketani T, Fukuda S, and Yamaguchi S. Metabolic disease in 10 patients with sudden unexpected death in infancy or acute life-threatening events. Pediatr Int. 57: 348–353. 2015. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi T, Hasegawa Y, Yamada K, Bo R, Kobayashi H, Taketani T, Fukuda S, and Yamaguchi S. Metabolic survey of hidden inherited metabolic diseases in children with Apparent Life-Threatening Event (ALTE) or Sudden Unexpected Death in Infancy (SUDI) by analyses of organic acids and acylcarnitines using mass spectrometries. Shimane J Med Sci. 32: 61–68. 2016. [Google Scholar]
- 44.Kaku N, Ihara K, Hirata Y, Yamada K, Lee S, Kanemasa H, Motomura Y, Baba H, Tanaka T, Sakai Y, Maehara Y, and Ohga S. Diagnostic potential of stored dried blood spots for inborn errors of metabolism: a metabolic autopsy of medium-chain acyl-CoA dehydrogenase deficiency. J Clin Pathol. 71: 885–889. 2018. [DOI] [PubMed] [Google Scholar]
- 45.Narita N, Narita M, Takashima S, Nakayama M, Nagai T, and Okado N. Serotonin transporter gene variation is a risk factor for sudden infant death syndrome in the Japanese population. Pediatrics. 107: 690–692. 2001. [DOI] [PubMed] [Google Scholar]
- 46.Kijima K, Sasaki A, Niki T, Umetsu K, Osawa M, Matoba R, and Hayasaka K. Sudden infant death syndrome is not associated with the mutation of PHOX2B gene, a major causative gene of congenital central hypoventilation syndrome. Tohoku J Exp Med. 203: 65–68. 2004. [DOI] [PubMed] [Google Scholar]
- 47.Ueda A, Osawa M, Naito H, Ochiai E, and Kakimoto Y. Non-polyalanine repeat mutation in PHOX2B is detected in autopsy cases of sudden unexpected infant death. PLoS One. 17: e0267751. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otagiri T, Kijima K, Osawa M, Ishii K, Makita N, Matoba R, Umetsu K, and Hayasaka K. Cardiac ion channel gene mutations in sudden infant death syndrome. Pediatr Res. 64: 482–487. 2008. [DOI] [PubMed] [Google Scholar]
- 49.Osawa M, Kimura R, Hasegawa I, Mukasa N, and Satoh F. SNP association and sequence analysis of the NOS1AP gene in SIDS. Leg Med (Tokyo). 11(Suppl 1): S307–S308. 2009. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto T, Tanaka H, Emoto Y, Umehara T, Fukahori Y, Kuriu Y, Matoba R, and Ikematsu K. Carnitine palmitoyltransferase 2 gene polymorphism is a genetic risk factor for sudden unexpected death in infancy. Brain Dev. 36: 479–483. 2014. [DOI] [PubMed] [Google Scholar]
- 51.Shingu K, Murase T, Yamamoto T, Abe Y, Shinba Y, Mitsuma M, Umehara T, Yamashita H, and Ikematsu K. Accurate interpretation of genetic variants in sudden unexpected death in infancy by trio-targeted gene-sequencing panel analysis. Sci Rep. 11: 21532. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nishio H, Iwata M, and Suzuki K. Postmortem molecular screening for cardiac ryanodine receptor type 2 mutations in sudden unexplained death: R420W mutated case with characteristics of status thymico-lymphatics. Circ J. 70: 1402–1406. 2006. [DOI] [PubMed] [Google Scholar]
- 53.Nishio H, Iwata M, Tamura A, Miyazaki T, Tsuboi K, and Suzuki K. Identification of a novel mutation V2321M of the cardiac ryanodine receptor gene of sudden unexplained death and a phenotypic study of the gene mutations. Leg Med (Tokyo). 10: 196–200. 2008. [DOI] [PubMed] [Google Scholar]
- 54.Nishio H, Kuwahara M, Tsubone H, Koda Y, Sato T, Fukunishi S, Tamura A, and Suzuki K. Identification of an ethnic-specific variant (V207M) of the KCNQ1 cardiac potassium channel gene in sudden unexplained death and implications from a knock-in mouse model. Int J Legal Med. 123: 253–257. 2009. [DOI] [PubMed] [Google Scholar]
- 55.Sato T, Nishio H, and Suzuki K. Sudden death during exercise in a juvenile with arrhythmogenic right ventricular cardiomyopathy and desmoglein-2 gene substitution: a case report. Leg Med (Tokyo). 13: 298–300. 2011. [DOI] [PubMed] [Google Scholar]
- 56.Sato T, Nishio H, and Suzuki K. Identification of arrhythmogenic right ventricular cardiomyopathy-causing gene mutations in young sudden unexpected death autopsy cases. J Forensic Sci. 60: 457–461. 2015. [DOI] [PubMed] [Google Scholar]
- 57.Matsusue A, Kashiwagi M, Hara K, Waters B, Sugimura T, and Kubo S. An autopsy case of sudden unexpected nocturnal death syndrome with R1193Q polymorphism in the SCN5A gene. Leg Med (Tokyo). 14: 317–319. 2012. [DOI] [PubMed] [Google Scholar]
- 58.Takahashi M, Sato T, Nishiguchi M, Suzuki K, and Nishio H. Postmortem genetic analysis for a sudden death case complicated with Marfan syndrome. Leg Med (Tokyo). 12: 305–307. 2010. [DOI] [PubMed] [Google Scholar]
- 59.Hata Y, Kinoshita K, Mizumaki K, Yamaguchi Y, Hirono K, Ichida F, Takasaki A, Mori H, and Nishida N. Postmortem genetic analysis of sudden unexplained death syndrome under 50 years of age: a next-generation sequencing study. Heart Rhythm. 13: 1544–1551. 2016. [DOI] [PubMed] [Google Scholar]
- 60.Hata Y, Kinoshita K, and Nishida N. An autopsy case of sudden unexpected death of a young adult in a hot bath: molecular analysis using next-generation DNA sequencing. Clin Med Insights Case Rep. 10: 1179547617702884. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hata Y, Yoshida K, Kinoshita K, and Nishida N. Epilepsy-related sudden unexpected death: targeted molecular analysis of inherited heart disease genes using next-generation DNA sequencing. Brain Pathol. 27: 292–304. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hata Y, Yoshida K, and Nishida N. Sudden unexpected death with rare compound heterozygous variants in PRICKLE1. Neurogenetics. 20: 39–43. 2019. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto T, Miura A, Itoh K, Takeshima Y, and Nishio H. RNA sequencing reveals abnormal LDB3 splicing in sudden cardiac death. Forensic Sci Int. 302: 109906. 2019. [DOI] [PubMed] [Google Scholar]
- 64.Yamamoto T, Otsu M, Okumura T, Horie Y, Ueno Y, Taniguchi H, Ohtaka M, Nakanishi M, Abe Y, Murase T, Umehara T, and Ikematsu K. Generation of three induced pluripotent stem cell lines from postmortem tissue derived following sudden death of a young patient with STXBP1 mutation. Stem Cell Res (Amst). 39: 101485. 2019. [DOI] [PubMed] [Google Scholar]
- 65.Hata Y, Oku Y, Taneichi H, Tanaka T, Igarashi N, Niida Y, and Nishida N. Two autopsy cases of sudden unexpected death from Dravet syndrome with novel de novo SCN1A variants. Brain Dev. 42: 171–178. 2020. [DOI] [PubMed] [Google Scholar]
- 66.Hata Y, Hachiwaka R, Ichimata S, Yamaguchi Y, and Nishida N. An autopsy case of sudden unexpected death of a young adult with progressive intraventricular conduction delay. Pathol Res Pract. 240: 154226. 2022. [DOI] [PubMed] [Google Scholar]
- 67.Hata Y, Tomita N, Shibata A, Yokoyama S, Fukahara K, and Nishida N. An autopsy case of sudden unexpected death with Barlow’s disease. Cardiovasc Pathol. 61: 107462. 2022. [DOI] [PubMed] [Google Scholar]
- 68.Hata Y, Ichimata S, Hirono K, Yamaguchi Y, Oku Y, Ichida F, and Nishida N. Pathological and comprehensive genetic investigation of autopsy cases of idiopathic bradyarrhythmia. Circ J. 87: 111–119. 2022. [DOI] [PubMed] [Google Scholar]
- 69.Unuma K, Tomomasa D, Noma K, Yamamoto K, Matsuyama TA, Makino Y, Hijikata A, Wen S, Ogata T, Okamoto N, Okada S, Ohashi K, Uemura K, and Kanegane H. Case report: molecular autopsy underlie COVID-19-associated sudden, unexplained child mortality. Front Immunol. 14: 1121059. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi Y, Fukuda H, Hayakawa A, Sano R, Kubo R, Kawabata-Iwakawa R, Nakajima T, Ishige T, Tokue H, Asano K, Seki T, Hsiao YY, Ishizawa F, Takei H, and Kominato Y. Postmortem genetic analysis of 17 sudden cardiac deaths identified nonsense and frameshift variants in two cases of arrhythmogenic cardiomyopathy. Int J Legal Med. 137: 1927–1937. 2023. [DOI] [PubMed] [Google Scholar]
- 71.Van Norstrand DW, and Ackerman MJ. Genomic risk factors in sudden infant death syndrome. Genome Med. 2: 86. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pfisterer N, Meyer-Bockenkamp F, Qu D, Preuss V, Rothämel T, Geisenberger D, Läer K, Vennemann B, Albers A, Engelmann TA, Frieling H, Rhein M, and Klintschar M. Sudden infant death syndrome revisited: serotonin transporter gene, polymorphisms and promoter methylation. Pediatr Res. 92: 694–699. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ameno K, Ameno S, Kinoshita H, Jamal M, Wang W, Kumihashi M, Uekita I, and Ijiri I. Autopsy and postmortem examination case study on genetic risk factors for cardiac death: polymorphisms of endothelial nitric oxide synthase gene Glu298Asp variant and T-786C mutation, human paraoxonase 1 (PON1) gene and alpha2beta-adrenergic receptor gene. Vojnosanit Pregl. 63: 357–361, discussion 362–363. 2006. [DOI] [PubMed] [Google Scholar]
- 74.Nakatome M, Yamamoto T, Isobe I, and Matoba R. Diplotype analysis of the human cardiac sodium channel regulatory region in Japanese cases of sudden death by unknown causes. Leg Med (Tokyo). 11: 298–301. 2009. [DOI] [PubMed] [Google Scholar]
- 75.Murakami C, Nakamura S, Kobayashi M, Maeda K, Irie W, Wada B, Hayashi M, Sasaki C, Nakamaru N, Furukawa M, and Kurihara K. Analysis of the sarcomere protein gene mutation on cardiomyopathy—mutations in the cardiac troponin I gene. Leg Med (Tokyo). 12: 280–283. 2010. [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto T, Matsusue A, Umehara T, Kubo SI, and Ikematsu K. No association between cardiac ion channel variants and sudden infant death. Pediatr Int. 60: 483–484. 2018. [DOI] [PubMed] [Google Scholar]
- 77.Reye RD, Morgan G, and Baral J. Encephalopathy and fatty degeneration of the viscera. A disease entity in childhood. Lancet. 2: 749–752. 1963. [DOI] [PubMed] [Google Scholar]
- 78.Pugliese A, Beltramo T, and Torre D. Reye’s and Reye’s-like syndromes. Cell Biochem Funct. 26: 741–746. 2008. [DOI] [PubMed] [Google Scholar]
- 79.Nishio Y, Makiyama T, Itoh H, Sakaguchi T, Ohno S, Gong YZ, Yamamoto S, Ozawa T, Ding WG, Toyoda F, Kawamura M, Akao M, Matsuura H, Kimura T, Kita T, and Horie M. D85N, a KCNE1 polymorphism, is a disease-causing gene variant in long QT syndrome. J Am Coll Cardiol. 54: 812–819. 2009. [DOI] [PubMed] [Google Scholar]
- 80.Harris SL, and Lubitz SA. Clinical and genetic evaluation after sudden cardiac arrest. J Cardiovasc Electrophysiol. 31: 570–578. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miura A, Funayama K, Nyuzuki H, Takahashi N, Yamamoto T, Koyama A, Ikeuchi T, Takatsuka H, and Nishio H. PROS1 variant in sudden death case of pulmonary embolism caused by calcification in the inferior vena cava: the importance of postmortem genetic analysis. Leg Med (Tokyo). 55: 102029. 2022. [DOI] [PubMed] [Google Scholar]
- 82.Taniguchi F, Morishita E, Sekiya A, Nomoto H, Katsu S, Kaneko S, Asakura H, and Ohtake S. Gene analysis of six cases of congenital protein S deficiency and functional analysis of protein S mutations (A139V, C449F, R451Q, C475F, A525V and D599TfsTer13). Thromb Res. 151: 8–16. 2017. [DOI] [PubMed] [Google Scholar]
- 83.Miura A, Kondo H, Yamamoto T, Okumura Y, and Nishio H. Sudden unexpected death of infantile dilated cardiomyopathy with JPH2 and PKD1 gene variants. Int Heart J. 61: 1079–1083. 2020. [DOI] [PubMed] [Google Scholar]
- 84.Ministry of Health, Labour and Welfare. https://www.mhlw.go.jp/content/10800000/shiin_keikaku.pdf.
- 85.Morita H. Secondary cardiomyopathy in polycystic kidney disease ssyndrome. Int Heart J. 60: 10–11. 2019. [DOI] [PubMed] [Google Scholar]
- 86.Suwa Y, Higo S, Nakamoto K, Sera F, Kunimatsu S, Masumura Y, Kanzaki M, Mizote I, Mizuno H, Fujio Y, Hikoso S, and Sakata Y. Old-age onset progressive cardiac contractile dysfunction in a patient with polycystic kidney disease harboring a PKD1 frameshift mutation. Int Heart J. 60: 220–225. 2019. [DOI] [PubMed] [Google Scholar]
- 87.Guo W, Schafer S, Greaser ML, Radke MH, Liss M, Govindarajan T, Maatz H, Schulz H, Li S, Parrish AM, Dauksaite V, Vakeel P, Klaassen S, Gerull B, Thierfelder L, Regitz-Zagrosek V, Hacker TA, Saupe KW, Dec GW, Ellinor PT, MacRae CA, Spallek B, Fischer R, Perrot A, Özcelik C, Saar K, Hubner N, and Gotthardt M. RBM20, a gene for hereditary cardiomyopathy, regulates titin splicing. Nat Med. 18: 766–773. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamamoto T, Sano R, Miura A, Imasaka M, Naito Y, Nishiguchi M, Ihara K, Otani N, Kominato Y, Ohmuraya M, Kuroyanagi H, and Nishio H. I536T variant of RBM20 affects splicing of cardiac structural proteins that are causative for developing dilated cardiomyopathy. J Mol Med (Berl). 100: 1741–1754. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsusue A, Hara K, Kashiwagi M, Kageura M, Sugimura T, and Kubo S. Genetic analysis of the rhabdomyolysis-associated genes in forensic autopsy cases of methamphetamine abusers. Leg Med (Tokyo). 13: 7–11. 2011. [DOI] [PubMed] [Google Scholar]
- 90.Itoh H, Sakaguchi T, Ding WG, Watanabe E, Watanabe I, Nishio Y, Makiyama T, Ohno S, Akao M, Higashi Y, Zenda N, Kubota T, Mori C, Okajima K, Haruna T, Miyamoto A, Kawamura M, Ishida K, Nagaoka I, Oka Y, Nakazawa Y, Yao T, Jo H, Sugimoto Y, Ashihara T, Hayashi H, Ito M, Imoto K, Matsuura H, and Horie M. Latent genetic backgrounds and molecular pathogenesis in drug-induced long-QT syndrome. Circ Arrhythm Electrophysiol. 2: 511–523. 2009. [DOI] [PubMed] [Google Scholar]
- 91.Kamei S, Sato N, Harayama Y, Nunotani M, Takatsu K, Shiozaki T, Hayashi T, and Asamura H. Molecular analysis of potassium ion channel genes in sudden death cases among patients administered psychotropic drug therapy: are polymorphisms in LQT genes a potential risk factor? J Hum Genet. 59: 95–99. 2014. [DOI] [PubMed] [Google Scholar]
- 92.Nagasawa S, Saitoh H, Kasahara S, Chiba F, Torimitsu S, Abe H, Yajima D, and Iwase H. Relationship between KCNQ1 (LQT1) and KCNH2 (LQT2) gene mutations and sudden death during illegal drug use. Sci Rep. 8: 8443. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hoffman M. Scientific sleuths solve a murder mystery. Science. 254: 931. 1991. [DOI] [PubMed] [Google Scholar]
- 94.Brohus M, Arsov T, Wallace DA, Jensen HH, Nyegaard M, Crotti L, Adamski M, Zhang Y, Field MA, Athanasopoulos V, Baró I, Ribeiro de Oliveira-Mendes BB, Redon R, Charpentier F, Raju H, DiSilvestre D, Wei J, Wang R, Rafehi H, Kaspi A, Bahlo M, Dick IE, Chen SRW, Cook MC, Vinuesa CG, Overgaard MT, and Schwartz PJ. Infanticide vs. inherited cardiac arrhythmias. Europace. 23: 441–450. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arnadottir GA, and Arnar DO. Unexplained sudden death: next-generation sequencing to the rescue? Europace. 23: 327–328. 2021. [DOI] [PubMed] [Google Scholar]
- 96.Ko T, and Morita H. Molecular genomic autopsy—clues to preventing further tragedy? Circ J. 87: 120–122. 2022. [DOI] [PubMed] [Google Scholar]
- 97.Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, and Zipes DP. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Heart Rhythm. 8: 1308–1339. 2011. [DOI] [PubMed] [Google Scholar]