Abstract
Psilocybin is a classical psychedelic which has been utilised for healing purposes for millenia. However, with its classification as a Schedule I substance, research into this compound is scarce with few FDA-approved clinical studies. Despite this, profound findings into its antidepressant effects (largely through its action on 5-HT1a receptors) in mental illnesses such as major depressive disorder have rapidly increased interest back into their potential therapeutic benefits. This systematic review provides an analysis of the studies examining the clinical use of psilocybin for major depressive disorder. In total 6 studies were selected, including 319 participants, with half being randomised controlled trials and half open label trials. In every study psychological support was included as an integral part of the treatment. It was found that every study significantly favoured the use of psilocybin in reducing depressive symptoms, with few side effects. This gives psilocybin an advantage over commonly prescribed selective serotonin reuptake inhibitors (SSRIs), which carry more risk and cause more adverse effects. This drug therefore shows promise for being used as a clinical treatment for major depressive disorder, however future research should develop a paradigm for its use, with the timing of sessions and type of psychological support specified to allow for more precise analysis of the clinical effects of the drug. Additionally, more studies into its clinical efficacy are needed for appropriate detection of any publication bias. With this, psilocybin could prove to be revolutionary in treating depression and become an alternative medication to SSRIs.
Keywords: Psilocybin, Depression
INTRODUCTION
Psilocybin
Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine), is a naturally occurring compound found in over 200 species of fungi, with many belonging to the genus Psilocybe such as P.azurescens, P.semilanceata and P.cyanescens [1]. This classical psychedelic has been used for religious, shamanic as well as spiritual ceremonies for millenia, with the South American Aztec Indians referring to them as “Gods Flesh” [2]. The first official medical report of the consumption of this compound was in 1799, and by the late 1950s psilocybin was identified and synthesised by Albert Hofmann, thus promoting clinical studies investigating its potential use [3,4]. This however was short lived with escalated nonmedical/recreational use of the drug, associating it with counterculture. This resulted in huge political backlash and the Controlled Substances Act classifying it as a Schedule I substance, ending its use and funding into human psychedelic research [5]. Modern research is now reinitiating interest back into psychedelics, with US Food and Drug Administration (FDA)-approved clinical studies indicating strong potential for psilocybin-assisted psychotherapy in treating a range of mental health disorders but this research is scarce with strict regulatory constraints [5].
Synthesis and Mechanism of Action
Upon oral ingestion of these fungi, the major chemical constituent psilocybin functions as a prodrug which rapidly dephosphorylates to yield the psychotropic agent psilocin as seen in Figure 1 [6]. This in vivo dephosphorylation by alkaline phosphatase takes place in the intestinal mucosa to activate this active metabolite, which subsequently acts on the central nervous system [6]. As a substituted indolealkylamine it is based upon the structure of tryptamine, illustrated in Figure 2, which acts as a predominant agonist in the serotonergic system at 5-hydroxytryptamine (5-HT) 1a and 2a/c subtype receptors [7]. These receptors (5-HT2a and 5-HT1a) are bound with different affinities. The former is responsible for the hallucinogenic effects, derealization, depersonalization and changes in perception, while the 1a receptor subtype activation provides the antidepressant, anxiolytic and antipsychotic effects [8].
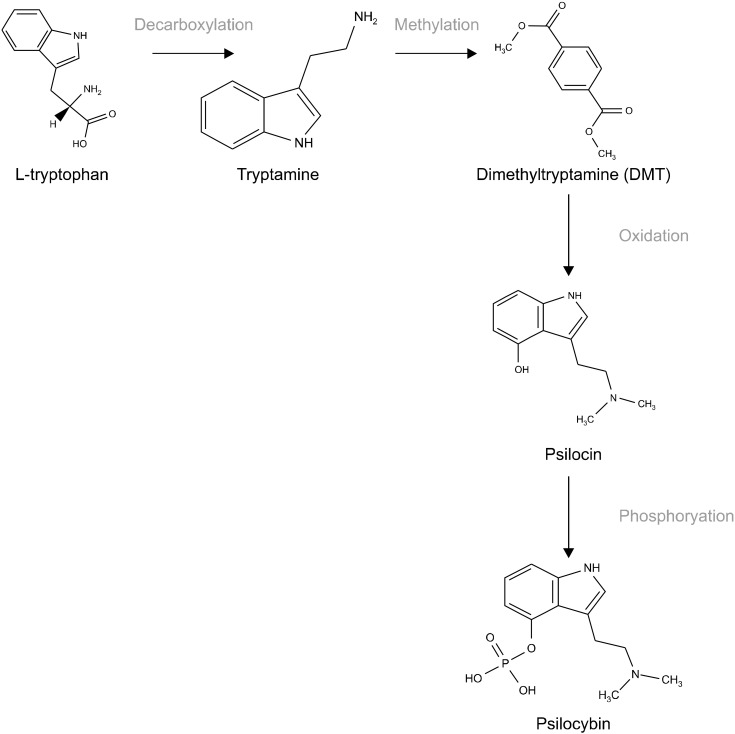
Fig. 1.
Synthesis of psilocybin [38]. L-Tryptophan is decarboxylated into tryptamine, to become methylated to form dimethyltryptamine (DMT). DMT is then oxidised forming psi-locin, which with the addition of a phosphate group forms psilocybin.
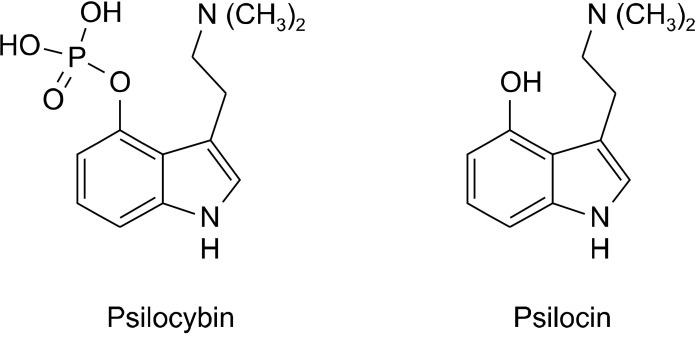
Fig. 2.
Structures of psilocybin and psilocin [2].
Major Depressive Disorder
In recent years psilocybin has reinstated interest because of these antidepressant effects, with the use of this drug as a therapeutic agent the basis of a plethora of re-search. With direct action on 5-HT1a receptors, some of the most profound findings into this drug’s clinical use have been in the treatment of major depressive disorder (MDD). MDD is a mood disorder characterised by a persistent depressive mood, anhedonia, impaired cognitive functions and physical symptoms such as sleep disturb-ances. With MDD being a highly prevalent mental health disorder and its incidence on the rise since the COVID-19 pandemic, one in six adults will now experience depression within their lifetime [9]. The need to adequately treat MDD in its early stages is crucial to prevent morphological and functional abnormalities, like reward system defects [10,11].
There are cognitive, behavioural and pharmacological therapies for MDD, with selective serotonin reuptake inhibitors (SSRIs) being the most popular and successful drug treatment, usually in combination with psychologi-cal therapy. Similarly to psilocybin, this class of drugs acts on the serotonergic system on 5-HT1A receptors where the reuptake of serotonin is inhibited, subsequently increasing serotonin levels at the synaptic cleft and improving mood [12]. Evidence suggests that they induce neuroplastic changes in the brain such as decreasing activation in limbic areas and reducing negative thought rumination, reducing activation of the amygdala and negative emotional processing, and strengthening functional connectivity [13,14].
SSRIs are the best performing antidepressant drugs to date, but despite this only have a 60% response rate [13]. Many individuals on SSRIs discontinue their treatment due to the non-negligible side effects they can induce, or choose to only undergo psychological therapies. One such long term side effect is motor impairments, with a connection found between extrapyramidal SSRI side effects and the onset of parkinsons, dyskinesia and related disorders [15,16]. Most of these cases occurred in the first month of using SSRIs, but past this timescale other serious adverse side effects can occur [17]. Long term use can lead to social side effects such as emotional blunting and sexual dysfunction, with patients in Garland and Baerg’s study [18] found to no longer care about social interaction or behaving in acceptable manners.
The risk of serotonin syndrome also increases with raising dosages as tolerance for the drug builds. Serotonin syndrome/toxicity is potentially life threatening, as overaccumulation of serotonin in synapses can lead to neuromuscular and autonomic dysfunctions including tachycardia and hyperthermia, as well as negative mental symp-toms such as delirium and suicidality [19]. Worsening of suicidal ideation is also not uncommon in SSRI users, which adds further risk to the development of serotonin syndrome from attempted overdosing [20]. This suggests the urgent need for a different type of antidepressant which is safer for use in individuals with MDD which can combat these mortality risks, unpleasant side effects and high discontinuation rates.
Psilocybin for Treating MDD
Conversely, psilocybin has a very low physiological toxicity and the administration of moderate doses to well prepared subjects in a monitored environment is “remark-ably safe” and associated with an acceptable level of risk [21]. Acute side effects occur only during the 6−8 hours “trip” and commonly include transient anxiety, nausea and headaches. These have not been found to extend beyond this dosing period, and longitudinal studies have not found lasting evidence for any persisting abuse of the drug, psychosis or impairment of functioning [8]. This demonstrates low potential for harm and addiction, with the 8-factor of the Controlled Substances Act analysis stating “there is no clear evidence of physical dependence and withdrawal in preclinical or clinical studies.” [22].
The clinical use for psilocybin is centred around its antidepressant effects. These are induced by changes in cerebral blood flow and oxygenation to brain areas rich in 5-HT1a and 5-HT2a/c receptor types such as the prefrontal cortex (PFC). The amygdala and posterior cingulate cortex are hyperactive in MDD, but after administration of psilocybin, cerebral blood flow to these areas reduce impacting beneficially upon mood, memory and the perception of self [23]. The default mode network (DMN) is also implicated, with its hyperconnectivity a hallmarker for MDD which produces depressive rumination symptoms [24]. Part of the DMN is the medial PFC, which through fMRI studies shows hyperactivity in MDD. Dosing with psilocybin decreases cerebral blood flow and oxygenation to this area and returns activity to a more normal state [25]. The hypoconnectivity of the DMN with the salience and executive networks also contributes to depressive symptoms. Functional cartography has shown psilocybin to reduce DMN recruitment and increase between-network integration with the executive and salience networks to counteract this depressive mechanism [26]. This proposes that psilocybin can increase communication in more segregated brain regions in the areas of cortex which resemble conjunction maps of these three networks, through the binding of 5HT2a receptors [27]. These 5HT2a receptor rich networks become more functionally interconnected and flexible as a result leading to long term changes and potentially long lasting antide-pressant effects [26].
Altering the integration of different signals increases cognitive flexibility, but also temporarily diminishes the DMNs generation of a ‘sense of self’, accounting for the ego-dissolution experienced by individuals who have taken moderate to higher doses of psilocybin [28]. The experience of ego-dissolution contributes to long term changes and increased cortical entropy [26,29]. Brain entropy there-fore can be said to grow with intake of psilocybin, from the number of significant resting-state functional connect-ions throughout the brain increasing which suggests that this compound can increase neuroplasticity and create long lasting changes, potentially treating MDD [30,31].
Rationale for Research
The current standard pharmacological treatments for depression are not advised for long term use, and although symptomatic remission can be seen, most indi-viduals do not fully recover from SSRIs alone or become resistant to them [32]. This leaves an alarmingly high amount of individuals suffering from MDD, and a substantial portion of those who do respond enduring residual side effects. Psilocybin is a potential alternative treatment to SSRIs, with recent studies showing that psilocybin in conjunction with psychotherapy is a highly pro-mising treatment for MDD and even treatment resistant depression [23,33-37].
Previous systematic reviews have shown the promise psilocybin holds through analysing the overall effects this drug has as a treatment for several different mental health issues and comorbidities [30,38,39]. What these reviews lack is specific findings for this compound’s effects in different psychological disorders, which can determine whether the use of this drug is appropriate or not. Research into the efficacy of psilocybin is accelerating rapidly, meaning previous systematic reviews which have focused on its clinical use for MDD are lacking recent studies in their analysis [40]. Therefore, this review aims to: 1) provide an up to date evaluation of the progression of this pharmacological agent, 2) conclude if it is a viable possible clinical treatment for MDD, and 3) evaluate whether this treatment could, or should replace modern antidepressants for this disorder.
METHODS
The aim of this work is to review the potential use of psilocybin in the treatment of MDD. Based on the Primary Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) guidelines, the Web of Science and PubMed/MEDLINE databases were interrogated to identify clinical studies for analysis [41]. This ensures the identification of a broad coverage of literature as well as the inclusion of medicinal and biomedical literature. The defined inclusion criteria for this search were: primary data only, free text available, only studies in humans, the use of the drug psilocybin, participants > 18 years with an established diagnosis of depressive disorder, and not currently taking any other psychotropic medications (or have done for two weeks minimum prior to the study to allow for the drugs full metabolism out of the body).
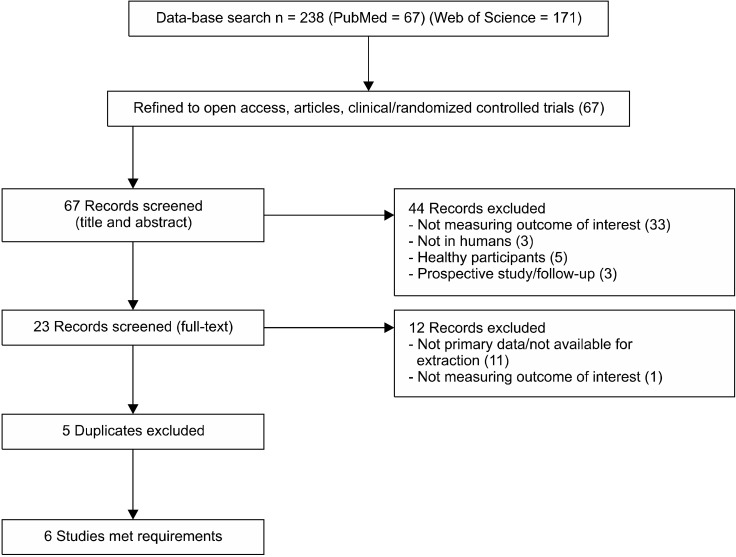
The literature search was conducted during November of 2022. The concepts had controlled vocabulary (psilocybin and depressive disorder), and associated terms (treatment- resistant depression and depressive disorder, treatment resistant). Using the Boolean operator tools, combining the terms (depressive disorder) OR (depressive disorder, treatment resistant) on PubMed, combining (treatment-resistant depression) OR (depressive disorder) on Web of Science, and combining these AND (psilocybin) 238 results were found, 67 on PubMed and 171 on Web of Science (Supplementary Material; available online). When the search was refined to open access publications, articles and clinical/randomised controlled trials there were 67 results obtained. The titles and abstracts of each were initially screened, leading to the exclusion of 33 records due to a different outcome to the one interested in being measured, 3 due to the study being in rodents, 5 as they used healthy participants, and 3 for being prospective studies or follow ups of previous ones. The full text of 23 records was therefore analysed in detail, with a further 11 records being excluded due to not using primary data or data available for extraction, and a final 1 study excluded for measuring a different outcome of interest. Six studies remained for analysis after the 5 duplicates were removed (see PRISMA flow diagram in Figure 3 and Table 1 for the characteristics of each study).
Fig. 3.
PRISMA flow diagram of the database search, selection of studies and articles to include in the sys-tematic review.
PRISMA, Primary Reporting Items for Systematic Reviews and Meta-Analyses Statement.
Table 1.
Characteristics of each study included
| ID | Study/year | Study design | Sample size | Sample mean age (years ± SD) | Sex (F/M) |
|---|---|---|---|---|---|
| A1 | Carhart-Harris et al., 2021 [34] | Randomised | n = 30 treatment group | 43.3 ± 11.7 | 11/19 |
| A2 | Davis et al., 2021 [35] | Randomised | n = 13 treatment group | 43.6 ± 13.0 | 9/4 |
| A3 | Carhart-Harris et al., 2016 [33] | Open-label trial | n = 12 | 42.7 ± 10.2 | 6/6 |
| A4 | Lyons et al., 2018 [37] | Open-label trial | n = 15 treatment group | 45.4 ± 2.9 | 4/11 |
| A5 | Carhart-Harris et al., 2017 [23] | Open-label trial | n = 16 | 42.8 ± 10.1 | 4/12 |
| A6.a | Goodwin et al., 2022 [36] | Randomised | n = 79 (group A) | 40.2 ± 12.2 | 44/79 |
| A6.b | n = 75 (group B) | 40.6 ± 12.8 | 41/75 | ||
| A6.c | n = 79 (group C) | 38.7 ± 11.7 | 36/79 |
SD, standard deviation; F, female; M, male.
Data extracted from the remaining studies included the authors, article title, publication date, study design, sample size and characteristics (age, sex, depression severity), the intervention used (dose/s of psilocybin) and the outcome/s. These outcomes were measured using psychometric instruments such as the Quick Inventory of Depressive Symptomatology (QIDS), Hamilton Depres-sion Rating Scale (HAM-D), Beck Depression Inventory (BDI) and the Montgomery–Åsberg Depression Rating Scale (MADRS). These are all self-reported questionnaire-like tests which assess depression severity and consist of questions/statements for the client to select their answer out of multiple choices. They’re designed to assess the severity of depressive symptoms in those with an already established diagnosis and are administered by clinicians. The HAM-D sees scores of 0−9 indicating no depression, and over 17 indicating moderate to severe symptoms. The QIDS-16 test sees scores from 0−5 indicating no depression, and scores over 21 indicating very severe depres-sion. The BDI sees scores of 30−63 indicating no depression, whereas 0−18 indicating severe depression. The MADRS sees scores ranging from 0−6 as indicating no depression, and scores of over 35 indicating severe depression.
RESULTS
Included Studies and Trials Characteristics
The 6 included studies were published between the years of 2016 and 2022 with the doses of psilocybin used ranging from 1 mg to 25 mg (and one study using patient weight to determine dosage) with either one or two intervention sessions. To standardise the data for the analysis as best as possible, only the studies including a medium to high dosage (17.5 mg to 30 mg) were included in the main analysis for each of the diagnostic tools. Every study administered the drug in a therapeutic and supportive setting with at least one psychologist or psychotherapist present at all times. Each participant had an established diagnosis of major depression or treatment-resistant depression and adequate scoring on diagnostic tools to confirm moderate-severe depressive symptoms. The effects of psilocybin were all quantitatively measured through these diagnostic tools (QIDS, BDI, HAM-D, MADRS) through comparing the scores before and after the intervention. Positive outcomes occurred quickly, from 1 week post treatment and clinical improvements reported to last at least 3 months after treatment. In 4 of the studies, mild to moderate transient adverse reactions were reported and are shown in Table 2.
Table 2.
Findings of each study included
| ID | QIDS mean change (SE) | HAM-D mean change (SE) | BDI mean change (SE) | MADRS mean change (SE) | Side effects recorded |
|---|---|---|---|---|---|
| A1 | Baseline to 6 weeks post = −8.0 (1.0) | Baseline to 6 weeks post = −10.5 (1.0) | Baseline to 6 weeks = −14 (1.7) | Headache (n = 12), nausea (n = 4) | |
| A2 | Baseline to 5 weeks post = −11.0 (1.62) Baseline to 8 weeks post = −10.7 (1.41) |
Baseline to 5 weeks post = −14.9 (2.2) Baseline to 8 weeks post = −14.4 (1.9) |
(BDI-II) Baseline to 5 weeks post = −23.7 (3.14) Baseline to 8 weeks post = −23.7 (2.75) |
N/A | Mild to moderate headache |
| A3 | Baseline to: 1 week = −11.8 (1.53), 2 weeks = −12.9 (1.45), 3 weeks = −12.8 (1.58), 5 weeks = −11.0 (1.66), 3 months = −9.2 (1.83) |
Baseline to 1 week post = −14.0 (2.4) | Baseline to 1 week post = −25.0 (3.18) Baseline to 3 months post = −18.5 (3.78) |
N/A | Mild anxiety (n = 12), confusion (n = 9), mild nausea (n = 4), transient headache (n = 4), mild transient paranoia (n = 1) |
| A4 | N/A | N/A | Baseline to 1 week post = −22.2 (3.18) | N/A | None recorded |
| A5 | Baseline to 5 weeks = −8.0 (1.42) | N/A | N/A | N/A | None recorded |
| A6.a | N/A | N/A | N/A | Baseline to 3 weeks post = −12.0 (1.3) | Headache, nausea, and/ or dizziness (n = 179) |
| A6.b | N/A | N/A | N/A | Baseline to 3 weeks post = −7.9 (1.4) | See A6.a |
| A6.c | N/A | N/A | N/A | Baseline to 3 weeks post = −5.4 (1.4) | See A6.a |
QIDS, Quick Inventory of Depressive Symptomatology; HAM-D, Hamilton Depression Rating Scale; BDI, Beck Depression Inventory; MADRS, Montgomery–Åsberg Depression Rating Scale; SE, standard error; N/A, not available.
Primary Outcomes
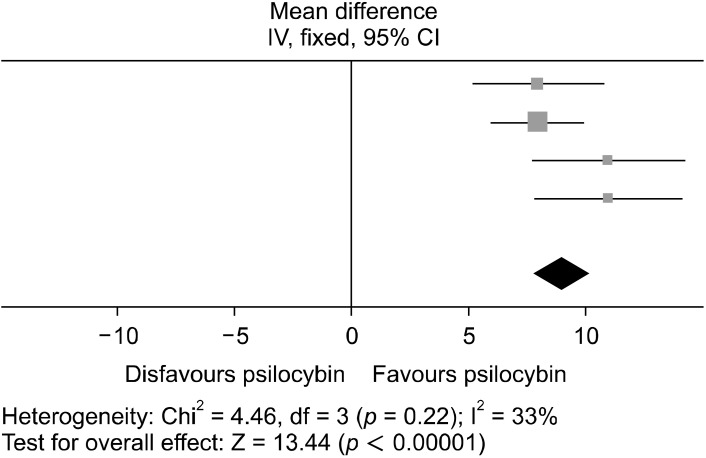
For studies A1, A2, A3, and A5 using the QIDS scale, the most measured outcome was at 5−6 weeks post intervention. The meta-analysis for this is graphically reported in Figures 4 and 5. In total, 71 patients were included in this meta analysis, with a fixed effects model being adopted as heterogeneity was moderate (I2 = 33%). The data for the post dosing QIDS scores were signi-ficantly favored (weighted mean difference [WMD] = 9.00; 95% confidence interval [CI] = [7.69, 10.31]; p < 0.00001). This means that participant depression symptoms were significantly improved after treatment with psilocybin, with every study in this analysis showing a positive clinical response to the intervention (see Table 3 for the overall effect of the treatment).
Fig. 4.
Forest plot conducted using RevMan showing the MD between baseline and 5/6 weeks post intervention scores on QIDS. This illustrates how each study significantly favors psilocybin for reducing depressive symptoms. The squares represent the individual studies with the size representative of the weight of the study in the analysis. The diamond represents the overall/summary effect, and the lines represent the confidence intervals.
MD, mean difference; CI, confidence interval; QIDS, Quick Inventory of Depressive Symptomatology.
Fig. 5.
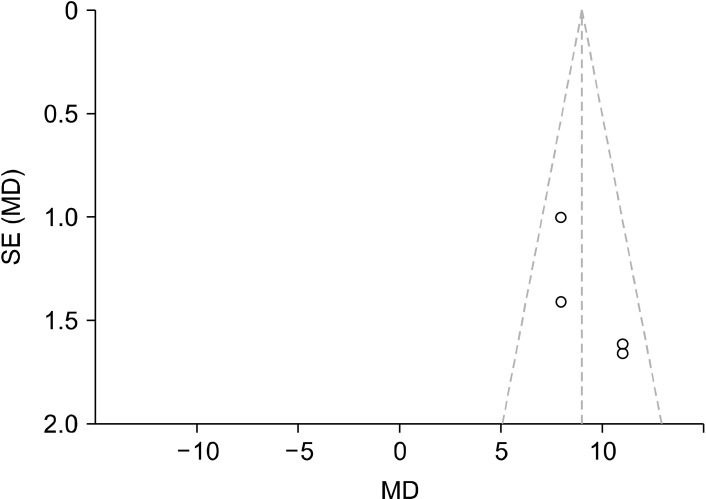
Funnel plot conducted using RevMan to analyse publication bias, of which shows that there is a possibility of publication bias due to the asymmetry of the data points for the QIDS baseline and 5/6 weeks post intervention scores. The dotted lines represent the 95% confidence intervals while the middle vertical line is the overall effect. Each study is represented by a dot, with the standardised MD result plotted on the x-axis, and their precision/standard error (SE) on the y-axis.
MD, mean difference; QIDS, Quick Inventory of Depressive Symptomatology.
Table 3.
Overall effects of psilocybin on depression using the QIDS, BDI, and HAM-D tools
| Outcome | WMD (95% CI) | I2 (%) | pvalue | Model used |
|---|---|---|---|---|
| QIDS | 9.00 (7.69, 10.31) | 33 | < 0.00001* | Fixed |
| BDI | 23.63 (20.05, 27.21) | 0 | < 0.00001* | Fixed |
| HAM-D | 11.22 (10.15, 12.29) | 57 | < 0.00001* | Fixed |
QIDS, Quick Inventory of Depressive Symptomatology; HAM-D, Hamilton Depression Rating Scale; BDI, Beck Depression Inventory; WMD, weighted mean difference; CI, confidence interval.
*Indicates a significant result.
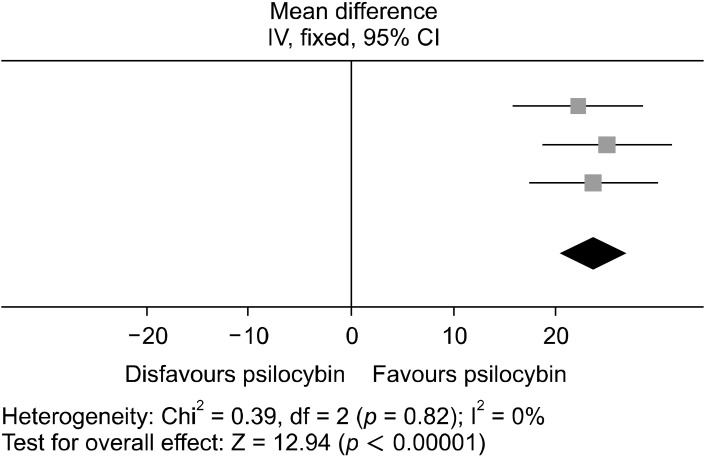
Studies A1, A2, A3, and A4 adopted the BDI as the measurement for depressive symptomatology. A fixed model design was again used as heterogeneity was low (I2 = 0%), and 40 patients were included in the analysis, of which Figures 6 and 7 illustrates. The data for the post dosing BDI scores were significantly favored (WMD = 23.63; 95% CI = [20.05, 27.21]; p < 0.00001). This denotes that for every study in this analysis psilocybin could significantly reduce depressive symptoms in participants as measured through BDI and produce a positive clinical response.
Fig. 6.
Forest plot conducted using RevMan, showing the MD between baseline and up to 6 weeks post intervention BDI scores. Each study significantly favors psilocybin for reducing depressive symptoms. The squares represent the individual studies with the size representative of the weight of the study in the analysis. The diamond represents the overall/summary effect, and the lines represent the confidence intervals.
MD, mean difference; CI, confidence interval; BDI, Beck Depression Inventory.
Fig. 7.
Funnel plot conducted using RevMan showing that pub-lication bias is unlikely in the BDI baseline and up to 6 weeks post intervention scores due to the symmetry of the plotted points shown. The dotted lines represent the 95% confidence intervals while the middle vertical line is the overall effect. Each study is represented by a dot, with the standardised MD result plotted on the x-axis, and their precision/standard error (SE) on the y-axis.
MD, mean difference; BDI, Beck Depression Inventory.
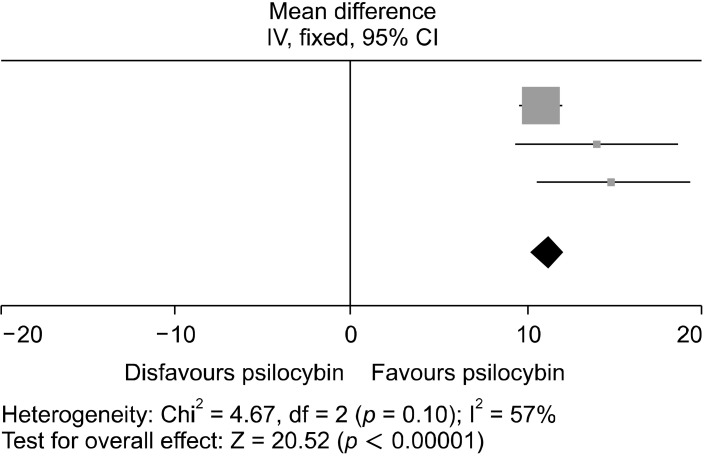
The HAM-D was the last diagnostic tool used in the analysis, including studies A1, A2, and A3. The homogeneity was highest for this test, with I2 = 57%. The data for the post dosing HAM-D scores were significantly favored (WMD = 11.22; 95% CI = [10.15, 12.29]; p < 0.00001) and are shown in Figures 8 and 9. Psilocybin therefore significantly reduced depressive symptoms in every study used in this analysis and produced a positive clinical response.
Fig. 8.
Forest plot conducted using RevMan, showing the MD be-tween baseline and up to 6 weeks post intervention HAM-D scores. Each study in this analysis significantly favors psilocybin for reducing depressive symptoms at up to 6 weeks post intervention with the drug. The squares represent the individual studies with the size repre-sentative of the weight of the study in the analysis. The diamond represents the overall/summary effect, and the lines represent the confidence intervals.
MD, mean difference; CI, confidence interval; HAM-D, Hamilton Depression Rating Scale.
Fig. 9.
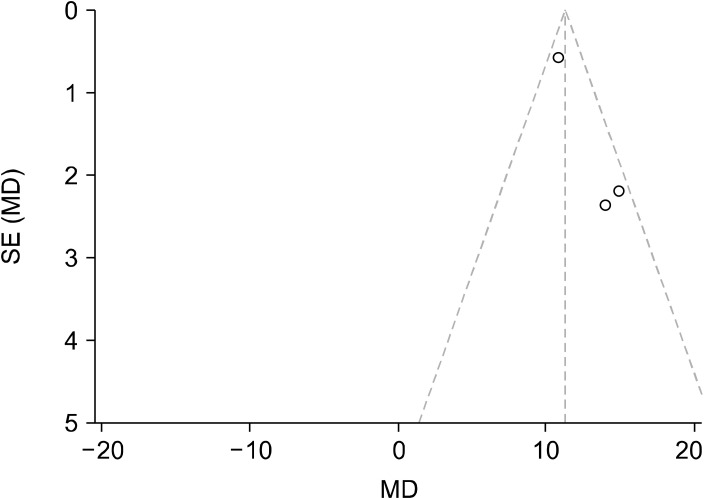
Funnel plot conducted using RevMan showing asymmetry for the HAM-D baseline and up to 6 weeks post intervention scores meaning there is possibility of publication bias. The dotted lines represent the 95% confidence intervals while the middle vertical line is the overall effect. Each study is represented by a dot, with the standardised MD result plotted on the x-axis, and their precision/ standard error (SE) on the y-axis.
MD, mean difference; HAM-D, Hamilton Depression Rating Scale.
The results in A6 did not include any standard deviations of the data, thus the MADRS tool was not tested for bias or common effect. However, the dose dependent effects were examined within this study given the three different doses used (A6a: 1 mg, A6b: 10 mg and A6c: 25 mg). An independent ttest found a statistically significant difference in scores between the 1 mg and 25 mg dosages (A6a and A6c), t(156) = 3.4546, 95% CI (2.826, 10.374), p = 0.0007. The difference between the 10 mg and 25 mg dosages (A6b and A6c) were also statistically significant, with t(152) = 2.1487, 95% CI (−7.870, −0.330), p = 0.0332. There was no significant difference however between the 1 mg and 10 mg groups (A6a and A6b), with t(152) = 1.2618, 95% CI (−1.414, 6.414), p = 0.2089. This shows that the 25 mg dose has a substantially larger effect on reducing depressive symptomology in comparison to lower 1 mg and 10 mg doses, of which both produce similarly non-significant clinical responses. These lower doses on their own therefore are not suitable as a treatment for MDD as they do not reduce depressive symptoms effectively.
Time effects were also measured, through comparing studies A1 and A2 as the former administered the second dosage with almost double the time in between. An independent t-test was conducted showing no significant difference between the mean difference in QIDS score, t(41) = 1.6174, 95% CI (−6.7460, 0.7460), p = 0.1135. This shows that the timings used in these studies between dosing sessions do not have a substantial effect on reducing depressive symptomatology, inferring that this is not a contributing factor to the drug’s clinical effects.
DISCUSSION
Using a systematic search 6 studies were reviewed and analysed to view the progression of the clinical efficacy of psilocybin using up to date studies for treating MDD. Every study demonstrated a positive clinical response, with depressive symptoms significantly lessening in all experiments and shown to last weeks after the final intervention session. Psilocybin is a classical psychedelic which acts as a predominant agonist in the serotonergic system, and is based upon the structure of tryptamine. As a schedule I drug, research into this compound has previously been prohibited, but interest has recently been initiated with FDA approved studies suggesting its huge potential for treating psychological disorders such as MDD [23, 33-37]. The mechanism of action of this pharmaceutical agent is largely within the DMN, with particular focus on the alteration of activity within the PFC and between the DMN salience and executive networks [25,26]. These alterations have proven to reduce depressive rumination and as a result depressive symptomatology. The present review supports this, similarly to previous systematic reviews which have assessed this drug’s use for several mental health disorders [38,39], as well as individually on MDD [40]. The included up to date studies in the present review therefore are in accordance with the notion of psilocybin being a potential therapeutic agent for MDD.
Every analysis conducted showed significant improvements in depression rating on the tested diagnostic tools (QIDS, HAM-D, BDI), thus an alleviation of depressive symptoms. However for the HAM-D and QIDS analyses there is possibility of publication bias as illustrated in the funnel plots (Figs. 5 and 9). There is visible asymmetry in these, however this is likely due to the small number of studies used in the analyses rather than from publication bias. The proper detection of publication bias is underpowered with small study sizes and the resultant funnel plots do not have sufficient power. Each of the studies used in these analyses are published within reputable journals with rigorous peer-review processes such as the New England Journal of Medicine, so this risk of publication bias is not outstanding. With this the validity of the present meta-analysis is not reduced, but highlights something to be tested for again when there are sufficient numbers of studies. Nonetheless, the funnel plots show the overall effect of the studies and the degree of precision of these.
Other research into this substance has wholly supported its therapeutic use, with the only criticisms being the transient side effects experienced during the treatment period [8]. These side effects lasting the length of the sessions commonly include mild anxiety, confusion, nausea, dizziness, headaches and sometimes mild transient paranoia, but relative to the side effects experienced from common MDD medications like SSRIs and the period of time these are experienced for are minor. Side effects of psilocybin are all manageable with the psychological support provided in the sessions, and no persisting side effects have been found - contrary to SSRIs which can have serious long-term adverse effects [17]. With treatments using psilocybin continuing for commonly 2 weeks in total, this is significantly shorter than treatment with SSRIs which can take two months alone to begin taking beneficial effect [17]. This shorter treatment period would be more attractive to individuals suffering with MDD, especially considering the length of time many suffer with the disorder. However, this compounds administration needs to be in a specific therapeutic setting alongside mental health professionals which is less accessible than being prescribed SSRIs which are taken by the patient at home.
Another limitation of this study is the differing times between dosing sessions and post-intervention assessments. Accurately analysing each study according to their time of assessment was difficult, and with this it is possible that the results of the post-intervention tests could have differed had they been assessed a week later or before. For the QIDS analysis, the scores used were rounded to 5 or 6 weeks post intervention, and for the BDI and HAM-D analyses included data up to 6 weeks post intervention. The latter two analyses mean that studies A3 and A4 providing data for one week post intervention were included in the analysis amongst data collected 6 weeks post intervention. The nature of this drug’s action however promotes neuroplasticity, inducing long lasting effects which are unlikely to significantly reduce within a week [31]. These effects can be seen in Table 2, with the mean difference in self-reported depressive symptoms persisting for at least 3 months post-intervention [33]. This indicates that the time of assessment would not significantly change scores on the diagnostic tools or significantly change the results found in the analyses. Other follow up studies also report these persisting changes, with Agin-Liebes et al.’s study [30] finding that reductions in depression were sustained 4.5 years post-treatment in up to 80% of partici-pants.
The timing between the dosing sessions and therefore the intensity of the intervention also could have influenced the results of each analysis, but in the independent ttest conducted comparing time effects in studies A1 and A2 no significant difference in results were found. This infers that the time between dosing sessions does not impact upon the treatment outcomes which suggests that this is not a confounding variable. To further confirm this, research should compare this drug’s effects with different time periods between dosing sessions, for example using less than a week between, making treatment more intense, or with 1 month between reducing intensity. This would consequently contribute towards the development of a paradigm for the clinical use of psilocybin for MDD. Li et al. [42] reviewed dose dependent effects, suggesting that psilocybin is most effective at 30−35 mg/70 kg for producing antidepressant effects. This should therefore be added to the proposed paradigm, again allowing for more accurate meta-analyses across research papers. Lastly, with the psychological support being noted as an integral part of treatment further investigation into the type of support provided would be useful in determining the optimal conditions for this intervention as well as the training required for professionals to undergo should this treatment be approved. This is both feasible to determine and imperative for establishing the most efficient manner to administrate psilocybin for maximum efficacy.
A final limitation to be noted of this review is the use of open-label experiments with a lack of adequate blinding, or a control group. The nature of this design type means that experimenter bias and subject bias could have influenced results, severely impacting the internal validity of these studies. However, experiments using randomised con-trolled trials have similar results to those without blinding, indicating that the significant results shown are still valid and the potential strength of this influence is low. Con-sidering the intense and powerful effects of psilocybin too, both participants and researchers (if present for the intervention) almost certainly would know if they are in the treat-ment or placebo group, defeating the purpose of blinding.
CONCLUSION
This systematic review into the clinical efficacy of psilocybin in treating MDD shows that the most recently conducted trials examining this also display significant clinical improvements in depressive symptoms to previous ones, thus contributing to the evidence for psilocybin being a potential viable treatment for MDD. This could be an alternative treatment to SSRIs, and is likely to appear more attractive to MDD sufferers with its minor and transient side effects, shorter treatment time and low assessed risk. This fufils all aims of the review, however, further studies with larger sample sizes are required to test for any elements of bias in the studies conducted, and the most effective intervention conditions are still to be determined. The best type of accompanying psychological support and the timing/intensity of dosing sessions are yet to be distinguished and applied into a paradigm, which would aid the analysis of clinical trials and subsequent development of this drug into an approved treatment for MDD. This drug holds tremendous promise and without doubt could prove to be a revolutionary psychedelic medicine.
Supplemental Materials
ACKNOWLEDGEMENTS
Authors thank Keele University’s library to facilitate this research through access to numerous journals.
Funding Statement
Funding None.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Designed the study, performed research and data analyses, wrote the initial draft: Tessa Watford. Supervised the study, edited the initial draft: Naqash Masood.
References
- 1.Stamets P, Weil A. Psilocybin mushrooms of the world: An identification guide. Ten Speed Press; 1996. [Google Scholar]
- 2.Nichols DE. Psilocybin: From ancient magic to modern medicine. J Antibiot (Tokyo) 2020;73:679–686. doi: 10.1038/s41429-020-0311-8. [DOI] [PubMed] [Google Scholar]
- 3.Hofmann A, Heim R, Brack A, Kobel H. [Psilocybin, a psychotropic substance from the Mexican mushroom Psilicybe mexicana Heim] Experientia. 1958;14:107–109. doi: 10.1007/BF02159243. German. [DOI] [PubMed] [Google Scholar]
- 4.Mahmood ZA. Natural Products, editor. Bioactive alkaloids from fungi: Psilocybin. In: Ramawat K, Mérillon JM, editors. Springer; 2013. pp. 523–552. German. [DOI] [Google Scholar]
- 5.Johnson MW, Griffiths RR, Hendricks PS, Henningfield JE. The abuse potential of medical psilocybin according to the 8 factors of the controlled substances act. Neuropharmacology. 2018;142:143–166. doi: 10.1016/j.neuropharm.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tylš F, Páleníček T, Horáček J. Psilocybin--summary of knowledge and new perspectives. Eur Neuropsychopharmacol. 2014;24:342–256. doi: 10.1016/j.euroneuro.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Dinis-Oliveira RJ. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab Rev. 2017;49:84–91. doi: 10.1080/03602532.2016.1278228. [DOI] [PubMed] [Google Scholar]
- 8.Studerus E, Kometer M, Hasler F, Vollenweider FX. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. J Psychopharmacol. 2011;25:1434–1452. doi: 10.1177/0269881110382466. [DOI] [PubMed] [Google Scholar]
- 9.Tang F, Liang J, Zhang H, Kelifa MM, He Q, Wang P. COVID- 19 related depression and anxiety among quarantined re-spondents. Psychol Health. 2021;36:164–178. doi: 10.1080/08870446.2020.1782410. [DOI] [PubMed] [Google Scholar]
- 10.Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 11.Duval F, Lebowitz BD, Macher JP. Treatments in depression. Dialogues Clin Neurosci. 2006;8:191–206. doi: 10.31887/DCNS.2006.8.2/fduval. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritter J, Flower RJ, Henderson G, Loke YK, Rang HP. Rang and Dale's pharmacology. 9th ed 2020. [Google Scholar]
- 13.Celada P, Puig M, Amargós-Bosch M, Adell A, Artigas F. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. J Psychiatry Neurosci. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- 14.Rădulescu I, Drăgoi AM, Trifu SC, Cristea MB. Neuroplasticity and depression: Rewiring the brain's networks through pharmacological therapy (Review) Exp Ther Med. 2021;22:1131. doi: 10.3892/etm.2021.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mörkl S, Seltenreich D, Letmaier M, Bengesser S, Wurm W, Grohmann R, et al. Extrapyramidal reactions following treatment with antidepressants: Results of the AMSP multinational drug surveillance programme. World J Biol Psychiatry. 2020;21:308–316. doi: 10.1080/15622975.2019.1648871. [DOI] [PubMed] [Google Scholar]
- 16.Guo MY, Etminan M, Procyshyn RM, Kim DD, Samii A, Kezouh A, et al. Association of antidepressant use with drug-related extrapyramidal symptoms: A pharmacoepidemiological study. J Clin Psychopharmacol. 2018;38:349–356. doi: 10.1097/JCP.0000000000000911. [DOI] [PubMed] [Google Scholar]
- 17.Moret C, Isaac M, Briley M. Problems associated with long-term treatment with selective serotonin reuptake inhibitors. J Psychopharmacol. 2009;23:967–974. doi: 10.1177/0269881108093582. [DOI] [PubMed] [Google Scholar]
- 18.Garland EJ, Baerg EA. Amotivational syndrome associated with selective serotonin reuptake inhibitors in children and adolescents. J Child Adolesc Psychopharmacol. 2001;11:181–186. doi: 10.1089/104454601750284090. [DOI] [PubMed] [Google Scholar]
- 19.Talton CW. Serotonin syndrome/serotonin toxicity. Fed Pract. 2020;37:452–459. doi: 10.12788/fp.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edinoff AN, Akuly HA, Hanna TA, Ochoa CO, Patti SJ, Ghaffar YA, et al. Selective serotonin reuptake inhibitors and adverse effects: A narrative review. Neurol Int. 2021;13:387–401. doi: 10.3390/neurolint13030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sloshower J, Guss J, Krause R, Wallace RM, Williams MT, Reed S, et al. Psilocybin-assisted therapy of major depressive disorder using acceptance and commitment therapy as a therapeutic frame. J Contextual Behav Sci. 2020;15:12–19. doi: 10.1016/j.jcbs.2019.11.002. [DOI] [Google Scholar]
- 22.Dos Santos RG, Bouso JC, Rocha JM, Rossi GN, Hallak JE. The use of classic hallucinogens/psychedelics in a therapeutic context: Healthcare policy opportunities and challenges. Risk Manag Healthc Policy. 2021;14:901–910. doi: 10.2147/RMHP.S300656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carhart-Harris RL, Roseman L, Bolstridge M, Demetriou L, Pannekoek JN, Wall MB, et al. Psilocybin for treatment-resistant depression: fMRI-measured brain mechanisms. Sci Rep. 2017;7:13187. doi: 10.1038/s41598-017-13282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: Implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–333. doi: 10.1016/j.biopsych.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling S, Ceban F, Lui LMW, Lee Y, Teopiz KM, Rodrigues NB, et al. Molecular mechanisms of psilocybin and implications for the treatment of depression. CNS Drugs. 2022;36:17–30. doi: 10.1007/s40263-021-00877-y. [DOI] [PubMed] [Google Scholar]
- 26.Daws RE, Timmermann C, Giribaldi B, Sexton JD, Wall MB, Erritzoe D, et al. Increased global integration in the brain after psilocybin therapy for depression. Nat Med. 2022;28:844–851. doi: 10.1038/s41591-022-01744-z. [DOI] [PubMed] [Google Scholar]
- 27.Beliveau V, Ozenne B, Strother S, Greve DN, Svarer C, Knudsen GM, et al. The structure of the serotonin system: A PET imaging study. Neuroimage. 2020;205:116240. doi: 10.1016/j.neuroimage.2019.116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smigielski L, Scheidegger M, Kometer M, Vollenweider FX. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. Neuroimage. 2019;196:207–215. doi: 10.1016/j.neuroimage.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Roseman L, Nutt DJ, Carhart-Harris RL. Quality of acute psychedelic experience predicts therapeutic efficacy of psilocybin for treatment-resistant depression. Front Pharmacol. 2018;8:974. doi: 10.3389/fphar.2017.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agin-Liebes GI, Malone T, Yalch MM, Mennenga SE, Ponté KL, Guss J, et al. Long-term follow-up of psilocybin-assisted psychotherapy for psychiatric and existential distress in patients with life-threatening cancer. J Psychopharmacol. 2020;34:155–166. doi: 10.1177/0269881119897615. [DOI] [PubMed] [Google Scholar]
- 31.Barrett FS, Doss MK, Sepeda ND, Pekar JJ, Griffiths RR. Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci Rep. 2020;10:2214. doi: 10.1038/s41598-020-59282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson JC. Augmentation and combination strategies in resistant depression. J Clin Psychiatry. 2009;70:e20. doi: 10.4088/JCP.BM8017br4c. [DOI] [PubMed] [Google Scholar]
- 33.Carhart-Harris RL, Bolstridge M, Rucker J, Day CM, Erritzoe D, Kaelen M, et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry. 2016;3:619–627. doi: 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
- 34.Carhart-Harris R, Giribaldi B, Watts R, Baker-Jones M, Murphy-Beiner A, Murphy R, et al. Trial of psilocybin versus escitalopram for depression. N Engl J Med. 2021;384:1402–1411. doi: 10.1056/NEJMoa2032994. [DOI] [PubMed] [Google Scholar]
- 35.Davis AK, Barrett FS, May DG, Cosimano MP, Sepeda ND, Johnson MW, et al. Effects of psilocybin-assisted therapy on major depressive disorder: A randomized clinical trial. JAMA Psychiatry. 2021;78:481–489. doi: 10.1001/jamapsychiatry.2020.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodwin GM, Aaronson ST, Alvarez O, Arden PC, Baker A, Bennett JC, et al. Single-dose psilocybin for a treatment-resistant episode of major depression. N Engl J Med. 2022;387:1637–1648. doi: 10.1056/NEJMoa2206443. [DOI] [PubMed] [Google Scholar]
- 37.Lyons T, Carhart-Harris RL. More realistic forecasting of future life events after psilocybin for treatment-resistant depression. Front Psychol. 2018;9:1721. doi: 10.3389/fpsyg.2018.01721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goel DB, Zilate S. Potential therapeutic effects of psilocybin: A systematic review. Cureus. 2022;14:e30214. doi: 10.7759/cureus.30214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Amsterdam J, van den Brink W. The therapeutic potential of psilocybin: A systematic review. Expert Opin Drug Saf. 2022;21:833–840. doi: 10.1080/14740338.2022.2047929. [DOI] [PubMed] [Google Scholar]
- 40.Vargas AS, Luís Â, Barroso M, Gallardo E, Pereira L. Psilocybin as a new approach to treat depression and anxiety in the context of life-threatening diseases-a systematic review and meta-analysis of clinical trials. Biomedicines. 2020;8:331. doi: 10.3390/biomedicines8090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group, author. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 42.Li NX, Hu YR, Chen WN, Zhang B. Dose effect of psilocybin on primary and secondary depression: A preliminary systematic review and meta-analysis. J Affect Disord. 2022;296:26–34. doi: 10.1016/j.jad.2021.09.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.