Abstract
Vagus nerve stimulation (VNS) has been approved as an adjunctive treatment for epilepsy and depression. As the progress of VNS treatment for these neuropsychiatric disorders continues, its applications have expanded to a wide range of conditions, including inflammatory diseases to cognitive dysfunctions. The branches of the vagal nerves directly or indirectly innervate the anatomical structures implicated in these neuropsychiatric conditions, which has led to promising results regarding the effectiveness of VNS. Previous studies investigating the effectiveness of VNS have mostly utilized invasive forms of stimulation. However, current preclinical and clinical research indicates that non-invasive forms of VNS, such as transcutaneous vagus nerve stimulation, hold the promise for treating various neuropsychiatric conditions. This review aims to delve into relevant clinical studies of VNS in various illness states, different methods of VNS, and the potential mechanisms underlying the therapeutic effects in these neuropsychiatric conditions.
Keywords: Vagus nerve stimulation, Vagus nerves, Neuropsychiatric disorder, Depression
INTRODUCTION
Vagus nerve stimulation (VNS) refers to any therapeutic technique applying intermittent stimulation on vagus nerve, which plays a central role in maintaining homeostasis by connecting the body with the brain through 80% of afferent and 20% of efferent pathways. VNS was approved as a treatment for refractory epilepsy and treatment-resistant depression (TRD) by the US Food and Drug Administration (FDA) in 1997 and 2005, respectively. Since then, numerous studies have identified and expanded the application of VNS through clinical trials and small pilot studies. We focused the current review primarily on the clinical implication and psychiatric treatment of VNS on diverse clinical states from neuropsychiatric illnesses (e.g., epilepsy and depression) to relatively less severe health problems (e.g., pain and cognitive problems). That is, we briefly overview the anatomy of vagus nerve, methods of VNS, clinical application of VNS in various illness states, and rationale for applying VNS in each clinical condition along with its effectiveness.
ANATOMY AND FUNCTION OF VAGUS NERVE
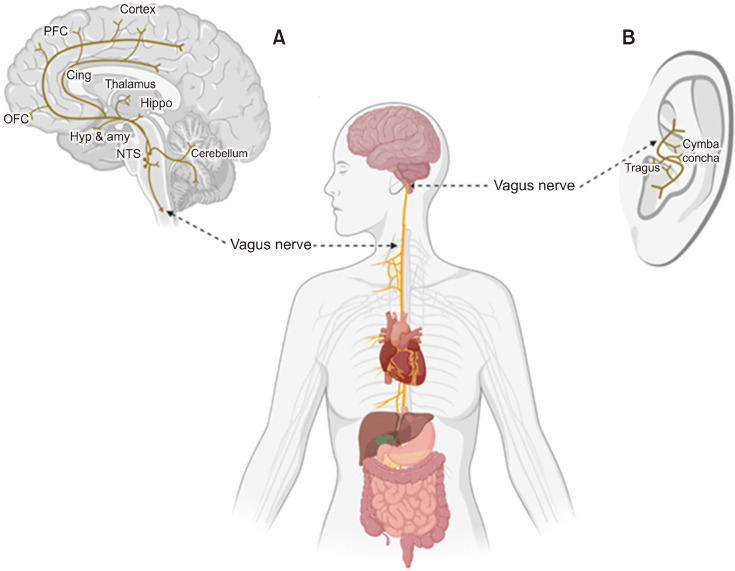
Vagus nerve is a mixed parasympathetic nerve with 20% of motor efferent fibers responsible for automatic regulation of cardiorespiratory and gastric tone [1-3], and with 80% of sensory afferent fibers carrying bodily information (i.e., gustatory, visceral sensory, and other peripheral information) to the brain (Fig. 1A) [4]. Each vagus nerve consisting of efferent and afferent fibers originates at the medulla oblongata, exits the brainstem through the jugular foramina, and courses along the neck within the carotid sheath bilaterally (i.e., right and left vagus nerves). They diffuse into numerable branches to innervate the body structures including bronchi, lungs, heart, esophagus, stomach, liver, and pancreas [5,6]. Most of the efferent fibers are parasympathetic projections to these regions working as a component of autonomic nervous system, but another important function of vagus nerve is on afferent fibers transmitting the sensory vagal inputs throughout the different brain regions [7]. Most of the incoming sensory information of vagal afferents are initially integrated in the nucleus of the tractus solitarius (NTS) in the medulla, and the NTS relays them directly (e.g., by projecting to the reticular formation in the medulla) or indirectly (e.g., by projecting through parabrachial nucleus and the locus coeruleus [LC]) to the rest of the brain regions including the amygdala, hypothalamus, and orbitofrontal cortex [8]. Considering the important role of these brain regions in numerous neuropsychiatric disorders where the afferents of vagus nerve are projected, stimulation on these circuits has been suggested and approved as a therapeutic technique, which is the VNS.
Fig. 1.
The anatomy of vagus nerve. (A) Area of the brains to which afferent vagus nerve is connected. (B) Illustration of areas of application in transcutaneous auricular vagus nerve stimulation. Adapted from the article of Zhu et al. (Front Endocrinol (Lausanne) 2022;13:1000758) [3]. PFC, prefrontal cortex; Cing, cingulate cortex; OFC, orbital frontal cortex; Hyp, hypothalamus; amy, amygdala; Hippo, hippocampus; NTS, nucleus tractus solitarius.
METHODS AND AREAS OF APPLICATION OF VAGUS NERVE STIMULATION
The methods of VNS can be roughly classified depending on whether they are invasive or non-invasive, and which of the left or right vagus nerve related areas they are applied to (Table 1). The most common clinical application of VNS follows an invasive method of implanting a pulse generator (e.g., NCP system; LivaNova PLCⓒ[formerly Cyberonics]) on the left upper chest or left axillary border, which transmits stimulation through the electrode lead wire attached to the mid-cervical left vagus nerve [9]. The stimulation variables such as signal frequency (20−30 Hz), intensity (~mA), pulse width (~us), signal on time (30−90 seconds), and signal off time are manipulated using the programing wand [10]. Because the parasympathetic fibers composing the right vagus nerve are more actively engaged in innervating the cardiac atria [11], left vagus stimulation is more favored as an area for stimulation to avoid any cardiac risk. Thus, less is known about the effect of right vagus stimulation in treating patients with depres-sion. Instead, prior studies suggest the effectiveness of right cervical VNS in treating heart failure [12,13] with devices such as CardioFit System (BioControl Medicalⓒ) and FitNeS System (BioControl Medicalⓒ).
Table 1.
Methods, application region, and common devices used for vagus nerve stimulation (VNS)
| Invasiveness | Device | Region | |||
|---|---|---|---|---|---|
|
| |||||
| Left | Right | ||||
| Invasive: surgery to implant generator | Neurocybernetic prothesis (NCP system) | Treatment for epilepsy and depression Generator implanted on the left upper chest or left axillary border The electrode lead wire attached to the left mid-cervical vagus nerve |
Risk of cardiac effect Relatively less evidence for treatment of epilepsy Less evidence for treatment of depression |
||
| CardioFit System FitNeS System |
Less evidence for treatment of heart failure | Treatment for heart failure Generator implanted on the right chest wall The electrode lead wire attached to the right mid-cervical vagus nerve |
|||
| Non- invasive | Transcutaneous forms of VNS (t-VNS) | ta-VNS | NEMOS | Left cymba conchae | |
| TENS | Unilaterally or bilaterally on cymba conchae | ||||
| n-VNS | Gamma Core | Unilaterally or bilaterally on the neck | |||
ta-VNS, transcutaneous auricular VNS; n-VNS, non-invasive VNS.
NCP System (Cyberonics Inc.); CardioFit System (BioControl Medical Inc.); FitNeS System (BioControl Medical Inc.); NEMOS (Cerbomed GmbH); TENS (Auri-Stim Medical Inc.); Gamma Core (electro Core Inc.).
Transcutaneous electrical stimulation (t-VNS) is a non- invasive method of VNS involving transcutaneous auricular vagus nerve stimulation (ta-VNS) where the electrical stimulation is applied to the left cymba conchae of the outer ear (Fig. 1B), and non-invasive vagus nerve stimulation (n-VNS) where the electrical stimulation is applied on the neck. The area of cymba conchae is known to receive the auricular branch of the vagus nerve [14], and prior studies targeting the area have revealed the consistent effect on the brain with that of traditional VNS applied on the left vagus nerve, suggesting the feasibility and beneficial effects of using t-VNS to treat various neuropsychiatric conditions in a non-invasive way [15,16]. Another non-invasive form of t-VNS is conducted using the transcutaneous electrical nerve stimulator (TENS; Auri- Stim Medicalⓒ) devices applied unilaterally or bilaterally on cymba conchae. Device for n-VNS (e.g., gamma Core; electroCoreⓒ) targets the cervical path of vagus nerve, and is applied unilaterally or bilaterally on the neck. t- VNS is useful in that it is a non-invasive and low risk form of stimulation selectively targeting the area of vagus nerve afferents without any surgery [14], but it lacks established administration protocol compared to the traditional invasive form of VNS [17].
CLINICAL APPLICATION OF VNS
Treatment of VNS for Epilepsy
When the sensory information of afferents is integrated in the NTS, it projects to the numerous regions of the brain. Among the wide range of projection pathways, LC is a major source of norepinephrine in connection with the amygdala, hypothalamus, orbitofrontal cortex and limbic regions [18]. Because the norepinephrine and serotonin responses are known to exert anticonvulsant effects [19,20]. VNS effects on the LC via NTS may be potentially relevant to the mechanisms of seizure reduction in that LC functions as a noradrenergic and serotonergic modulation system of the brain [8]. In addition, further connection between LC and other limbic regions of the brain may contribute to the antiseizure effect of VNS by changing the levels of gamma-aminobutyric acid (GABA) and glutamate concentrations in the NTS, thus enhancing the resistance for motor limbic seizure [21]. The rationale for VNS in epilepsy is based on such prior studies in animal and human samples strongly supporting the altered activities of the limbic system and increased activities of the LC in moderating the downstream release of noradrenergic neurotransmitter [19-23] as modalities of anticonvulsant effect.
Animal studies revealed mainly three distinct temporal patterns of antiseizure effect of VNS: (a) acute abortive effect where the ongoing seizure is attenuated after the acute application of VNS, (b) acute prophylactic effect where the application of VNS weakens the effect of seizure-inducing stimulus, and (c) chronic progressive prophylactic effects where the total numbers of seizures gradually decrease during the longer duration application of VNS than the short term stimulation [8]. Studies conducted on humans where pulse generator-implanted patients were randomly assigned to either high-stimulation or low-stimulation conditions as a 14-week treatment paradigm, also demonstrated the efficacy of VNS with more patients in high-stimulation condition (i.e., 38.7%) achieving at least 50% reduction in seizure than those in low-stimulation condition (i.e., 19.4%) [24].
Studies examining the relatively long-term effect of VNS showed a similar or greater seizure reduction than acute studies [25,26]. Morris and Mueller [26] assessed the seizure frequencies of 454 patients who had implanted a pulse generator at 6-month intervals until 3 years, and the result indicated continual effect of VNS in seizure reduction compared with the baseline (i.e., 1 year: 35%; 2 years: 44.3%; 3 years: 44.1%). Another study by Labar [25] compared the seizure rates of 269 patients at 3 and 12 months of VNS without changing any types or doses of antiepileptic drugs (AEDs). He concluded that median seizure rates reduction (i.e., median percent reduction in seizure frequency) was significant at both 3 (45%) and 12 months (58%) of VNS, supporting the long-term effectiveness of VNS controlling for the effect of AEDs. Such results along with other studies led to a suggestion that the longer duration of VNS might increase the seizure control [27-31]. However, the relationship between the duration of VNS and antiseizure effect is elusive at present, because it is likely to be under methodological bias or errors associated with retrospective design, regression to the mean, and the confounding impact of any changes in AEDs, thereby needs careful interpretation [32].
US FDA has expanded the age restriction of VNS as a treatment for drug-resistant epilepsy from age over 12 to over 4 years in 2017, based on the fact that the prevalence of epilepsy in the pediatric population is 0.5−1% [33] and about 33% of children with epilepsy are resistant to AED [34], demanding application of other effective treatments in children and young adults with drug resistant epilepsy. Studies conducted on children with epilepsy have corroborated the similar antiseizure effect of VNS previously reported in the adult population. In a study by Helmers et al. [35], 125 patients with the mean age of 12 years and onset age of 2 years showed the average seizure reduction of 36.1% and 44.7%, respectively, at 3 and 6 months of VNS. The use of VNS was also effective in the children under the age of 12 [32], and in children with developmental delays along with epilepsy [36]. The non-invasive application of t-VNS has been utilized as a safe and effective treatment in suppressing the seizure both in adult [37-40] and children [41], yielding a similar effect with the traditional VNS applied on the left vagus nerve. Specifically, a systematic review was conducted to assess the anti-epileptic effects of t-VNS in patients with epilepsy [42]. The review included 10 studies, which measured mean reduction in seizure frequency and improvement in quality of life in patients with epilepsy. Results reported a range of 30% to 65% in mean seizure reduction across studies, suggesting a possible benefit of utilizing non-invasive VNS for treating patients with epilepsy. Further-more, three studies demonstrated a significant improvement in patient’s quality of life (p < 0.05). However, research on the children was mostly restricted to the pilot study with only one ongoing randomized controlled trial [43]. Thus further research is needed to confirm the efficacy of t-VNS in children with refractory epilepsy.
Treatment of VNS for Depression
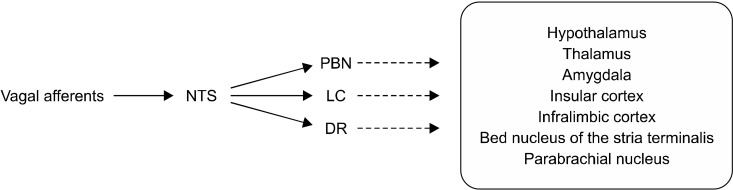
As in epilepsy, the mechanism of VNS in patients with TRD is closely related to the neurochemical change in limbic and major cortical structures that are actively engaged in mood regulation via secondary projections from NTS (Fig. 2). For example, parabrachial nucleus, dorsal raphe (DR), and LC secondarily relay information from NTS to the limbic-cortical regions such as thalamus, hypothalamus, nucleus of the stria terminalis, and amygdala [44]. LC and DR are known to contain the noradrenergic and serotonergic projection pathways, respectively, thus the application of VNS may result in changing the levels of norepinephrine [23], serotonin [45], GABA, and glutamate [21] implicated as the main neurotransmitters underlying the pathogenesis of depression [18]. Neuroimaging studies where the patients with invasive VNS therapy reported (a) metabolic changes in the limbic system similar to the effects of antidepressants through positron emission tomography scans [46], and (b) increased blood oxygenation level-dependent (BOLD) response in mood-related brain regions measured through functional magnetic resonance imaging (fMRI) acquired by synchronizing the signal of VNS stimulator [47], support the hypothesis that VNS induces a change in the brain comparable to that of antidepressants. Aside from evidence of neuroimaging studies, clinical observations also provide a rationale for VNS in depression in that (a) VNS substantially reduces mood symptoms even in patients with epilepsy [24,48, 49], and (b) anticonvulsants which share a mechanism of action with VNS, also have a mood-stabilizing effects [50,51].
Fig. 2.
Multiple direct/indirect pro-jection pathways of vagal afferents to the brain regions involved in mood regulation. Dash lines indicate se-condary projection from PBN, LC, and DR to other brain regions.
NTS, nucleus tractus solitarius; PBN, parabrachial nucleus; LC, locus coeruleus; DR, dorsal raphe.
In 2005, invasive VNS was approved by the US FDA as a treatment for depression, especially for those who are treatment refractory and had multiple records of failed medication trials. Short-term efficacy of VNS for TRD has been demonstrated in a 10-week pilot study [52], in which about 40% of adult outpatients reported at least 50% reduction, and 17% of them showed a complete remission in baseline 28-item Hamilton Depression Rating Scale (HDRS28, remission: HDRS28 ≤ 10). However, such short-term efficacy of VNS was not revealed in a randomized and controlled trial [53], where a total of 235 TRD patients with 10-weeks of VNS were compared to the sham treatment group, with both groups showing a marked response in HDRS (active VNS group: 15.2% reduction; sham group: 10% reduction). Rush et al. [54] further analyzed the long-term 12-month effect of VNS on the same TRD patients as a naturalistic follow-up study, where the previously active and sham group both received additional VNS along with treatment as usual (TAU). When compared to the baseline score of 24-item Hamilton Rating Scale for Depression (HRSD24), patients showed a significant reduction of 45 ± 0.05 points per month on average, suggesting a potential long-term benefit of VNS that is not noticeable with only short-term use of VNS. Cumulative effects of VNS for TRD was also revealed in a 5-year prospective, nonrandomized study with a large sample of 795 TRD patients [55], where a 5-year cumulative response rate of adjunctive VNS group (67.6%) was significantly higher than that of TAU group (40.9%). Bottomley et al. [56] conducted a meta-analysis with 22 eligible studies which described clinical outcomes of adjunctive VNS (VNS + TAU) or TAU alone in TRD. Poolable efficacy outcomes were measured with Montgomery- Asberg Depression Rating Scale, Clinical Global Impression- Improvement, and HRSD, and meta-analysis showed a consistent superior benefit of adjunctive VNS over TAU alone in TRD measures. In case of invasive application of VNS, ta-VNS has become an appealing adjunctive treatment for TRD as it is portable and easy to self-administer. Studies found that (a) the application of ta-VNS on depressive patients significantly reduced depressive symptoms compared to the sham ta-VNS group [57,58], and (b) decreased BOLD-signal in limbic regions of the brain, similar to the effect of antidepressants observed in healthy subjects under ta-VNS [16]. However, because less is known about the optimal parameter and antidepressant dosing recommendation [59], further exploration is needed regarding the efficacy of non-invasive forms of VNS in treating patients with depression.
In addition to invasive VNS, transcranial direct current stimulation (tDCS) has emerged as one of the promising neuromodulation options for treating depression [60,61]. tDCS offers benefits such as high tolerability and less side effects [62] due to its application of low-intensity direct currents non-invasively. However, it has shown only a moderate reduction of depression [63] with relatively short term effects [64]. On the other hands, a 5-year observational study revealed that VNS demonstrated sustained antidepressants effects [55], and its effectiveness in reducing depressive symptoms has been examined in multiple clinical trials [65]. However, since FDA-approved VNS requires the risk of surgical implantation, the choice of adjunctive treatments should be based on each patient’s symptoms.
Treatment of VNS for Inflammatory Diseases
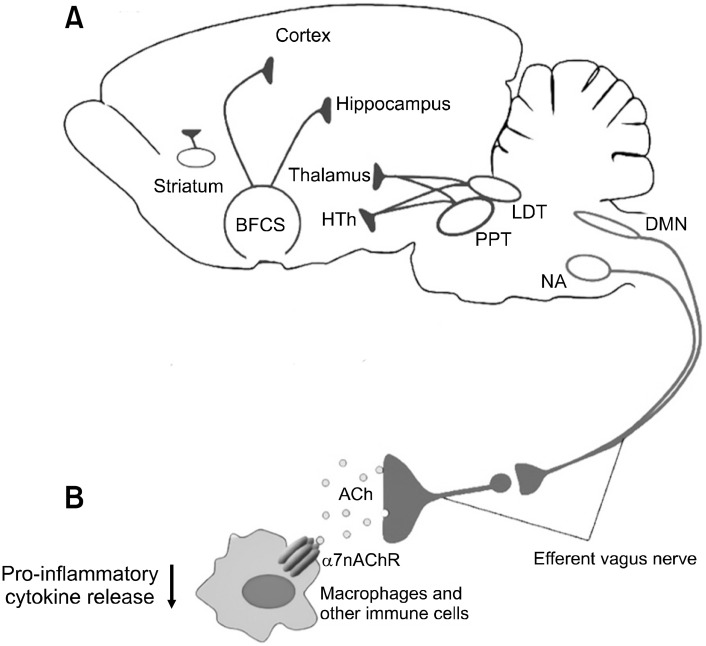
When looking at the mechanism of human inflam-mation, cytokines, which is a protein crucial for modulating the immune response, amplifies inflammation (i.e., pro-inflammatory cytokines) or inhibits inflammation (i.e., anti-inflammatory cytokines) depending on the type. Tumor-necrosis factor, one of pro-inflammatory cytokines, is released as a response to injurious stimuli. Tumor-necrosis factor activates and prolongs the inflammatory responses (e.g., heat, swelling, and pain) by stimulating cells to release other pro-inflammatory cytokines such as interleukin 1, leading to further inflammation [66]. Prior studies revealed that VNS may suppress proinflammatory cytokine synthesis by activating the cholinergic anti-inflammatory pathway [66-68], which is a long loop that runs from brain cholinergic system to the body organs (Fig. 3) [69]. Specifically, cholinergic neurons in the nucleus ambiguus and brainstem dorsal motor nucleus of the vagus project to the heart, liver, and other body organs within efferent vagus nerve fibers. And Acetylcholine released through efferent vagal ending binds to alpha-7 nicotinic acetylcholine receptors, suppressing pro-inflam-matory cytokines on immune cells [67,70]. Given this, VNS may play a putative role in controlling and preventing any development of inflammation related disorders such as rheumatoid arthritis (RA), irritable bowel syndrome, and fibromyalgia.
Fig. 3.
(A) Brain cholinergic system. Basal forebrain (BFCS), later-odorsal tegmental nuclei (LDT), mesopontine pedunculopontine (PPT), and striatum are important parts of brain cholinergic system. Cho-linergic neurons innervate to hypothalamus (HTh), thalamus, hippo-campus, and different cortex regions. (B) Cholinergic Anti-inflam-matory system. Cholinergic neurons in the nucleus ambiguus (NA) and brainstem dorsal motor nucleus of the vagus (DMN) within efferent vagus nerve fibers release acetylcholine (ACh). Ach released through efferent vagal ending binds to alpha 7 nicotinic acetylcholine receptors (α7nAChRs), suppressing pro-inflammatory cytokines on immune cells. Adapted from the article of Chang et al. (Front Neurosci 2019;13:263) [69].
For instance, a pilot study conducted a 4-day application of n-VNS for patients with low and high symptoms of RA, which is a chronic and inflammatory disease. The result showed that applying n-VNS reduced Disease Activity Score based on 28-joint count-C-reactive protein (i.e., 4.1 to 3.8), and pro-inflammatory cytokines interleukin-10 (i.e., 0.8 to 0.6 pg/ml) in high disease activity group [71]. In an animal experiment, arthritis rats under the condition of fifteen-day active VNS of the cholinergic anti-inflammatory pathway showed a significant reduction in joint swelling (i.e., 52% reduction in ankle diameter), and histological arthritis score (i.e., 46% reduction) compared to the sham group [72]. Irritable bowel syndrome is also one of inflammatory disorders that is characterized by chronic inflammation of gastrointestinal tract, dividing into Crohn’s disease and ulcerative colitis. Meregnani et al. [73] performed 5-day left vagus nerve stimulation with rats with colitis, and demonstrated a protective effect of VNS on inflammation induced weight loss. To address an issue of tolerability and effectiveness of invasive VNS for irritable bowel syndrome, a pilot study was conducted where 9 patients with irritable bowel syndrome (IBS) continuously underwent left VNS for 1 year. The results supported tolerability of VNS in treating irritable bowel syndrome as patients’ profile of pro-inflammatory cytokines (e.g., Interleukin 6, 12, and 23) transformed into more healthy standards in terms of gut mucosa metabolites [74]. Prior study also suggested that VNS may be useful in alleviating symptoms of fibromyalgia, which is often manifested as chronic pain, fatigue, and sleep problems. Lange et al. [75] performed an open-label longitudinal study with 14 patients with fibromyalgia. The results showed that 5 patients attained certain efficacy criteria set by the researcher, and two of the patients no longer fulfilled criteria for fibromyalgia [75]. Such results imply that VNS has potential for treating numerous inflammatory responses. However, most of the studies at present are pilot studies that need replication in a larger randomized controlled trial.
Treatment of VNS for Cognitive Disorders
After sensory information of vagal afferents are integrated in the NTS, it provides projection to the rest of the brain regions including the caudate nucleus, amygdala, thalamus, hippocampal formation, and brain cortex via LC and NTS. As some of these brain structures are involved in the memory process, it has been suggested that treatment of VNS may affect memory consolidation and performance [76,77]. One possible explanation of VNS enhancing memory may derive from its effect to facilitate long-term potentiation when stimulated with moderate intensity [78]. That is, the modulatory effect of VNS on synaptic plasticity in the hippocampus may underlie enhancement of retention performance observed after VNS treatment [78]. Studies conducted with a method of fMRI substantiate the effect of VNS on memory related brain regions. Specifically, studies using fMRI with implanted VNS manifested blood flow changes in brain structures engaged in cognitive processes [79,80].
A pilot study conducted with 10 patients with Alzheimer’s disease (AD) demonstrated the effect of VNS on cognition. Specifically, VNS may prevent progression of AD by increasing the level of noradrenaline [81] as AD patients suffer from noradrenaline loss due to atrophy within LC [82]. In addition, prior studies also investigated the effects of VNS on cognitive functions in patients with epilepsy. A study by Clark et al. [83] revealed that patients with epilepsy showed a 36% increase of words in recognition after VNS. Moreover, Martin et al. [84] examined the influence of VNS on decision-making process by conducting gambling test with 8 epileptic patients. Results confirmed that VNS improved decision making in gambling test. However, studies revealed that there was an inverted U- shaped correlation between cognitive performance and intensity of VNS [76,77,83] where VNS with too low (i.e., ~0.2 mA) or too high (i.e., 0.8 mA~) intensity was ineffective in enhancing cognitive functions of patients with epilepsy [83,85]. Given the general mechanism of VNS to facilitate memory consolidation by affecting LC, a study revealed a positive effect of VNS on enhancing episodic memory [86] and divergent thinking [87] in healthy individuals as well.
CONCLUSION
VNS has been utilized for the treatment of various neuropsychiatric conditions, employing both invasive and non-invasive approaches. The primary clinical application of VNS is invasive, involving the implantation of a pulse generator on the left upper chest or left axillary border to stimulate the mid-cervical left vagus nerve. Non-invasive methods of VNS, such as ta-VNS, are gaining popularity as they can selectively target the area of vagus nerve afferents without requiring surgery. The efficacy of VNS in treating drug-resistant epilepsy has been demonstrated, and studies conducted on children have shown similar antiseizure effect to those previously reported in adults. Non-invasive t-VNS has also been utilized as a safe and effective treatment for suppressing seizures, yielding comparable effects to traditional VNS. In the treatment of depression, VNS has been approved as an effective option for patients who do not respond to multiple medication trials. Studies using invasive VNS have confirmed the superior benefits of adjunctive VNS over TAU. Non-invasive t-VNS has also shown significant reduction in depressive symptoms, but further research is needed to determine optimal parameters and dosing principles. Invasive-VNS holds promise for treating inflammatory responses such as RA, IBS, and fibromyalgia. However, most studies on inflammatory diseases are currently in the pilot stages and require further validation through larger randomized controlled trials. Furthermore, VNS has demonstrated improvements in cognition for patients with AD, epilepsy, and even healthy individuals, despite the challenge of adjusting the intensity to maximize effective-ness. Overall, studies suggest that VNS has promising potential as an adjunctive therapy for various neuropsychiatric disorders in the future. Continuously verifying the effectiveness of VNS, alongside currently established pharmacological treatments through future research will broaden its utilization.
Funding Statement
Funding This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (1711138348, KD000169).
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Bori Jung, Seung-Hwan Lee. Original draft: Bori Jung. Critical revision: Chaeyeon Yang, Seung- Hwan Lee. Supervision: Seung-Hwan Lee.
References
- 1.Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4:1339–1368. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu ZJ, Weller RA, Sandidge K, Weller EB. Vagus nerve stimulation: Can it be used in adolescents or children with treatment-resistant depression? Curr Psychiatry Rep. 2008;10:116–122. doi: 10.1007/s11920-008-0021-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhu S, Zhang X, Zhou M, Kendrick KM, Zhao W. Therapeutic applications of transcutaneous auricular vagus nerve stimulation with potential for application in neurodevelopmental or other pediatric disorders. Front Endocrinol (Lausanne) 2022;13:1000758. doi: 10.3389/fendo.2022.1000758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley JO, DuBois FS. Quantitative studies of the vagus nerve in the cat: I. The ratio of sensory to motor fibers. J Nerv Ment Dis. 1937;86:587. doi: 10.1097/00005053-193711000-00019. [DOI] [Google Scholar]
- 5.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 6.Bonaz B, Picq C, Sinniger V, Mayol JF, Clarençon D. Vagus nerve stimulation: From epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil. 2013;25:208–221. doi: 10.1111/nmo.12076. [DOI] [PubMed] [Google Scholar]
- 7.Zagon A. Does the vagus nerve mediate the sixth sense? Trends Neurosci. 2001;24:671–673. doi: 10.1016/S0166-2236(00)01929-9. [DOI] [PubMed] [Google Scholar]
- 8.Henry TR. Therapeutic mechanisms of vagus nerve stimulation. Neurology. 2002;59(6 Suppl 4):S3–14. doi: 10.1212/WNL.59.6_suppl_4.S3. [DOI] [PubMed] [Google Scholar]
- 9.Terry RS, Tarver WB, Zabara J. The implantable neurocybernetic prosthesis system. Pacing Clin Electrophysiol. 1991;14:86–93. doi: 10.1111/j.1540-8159.1991.tb04052.x. [DOI] [PubMed] [Google Scholar]
- 10.Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia. 1998;39:677–686. doi: 10.1111/j.1528-1157.1998.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 11.Saper CB, Kibbe MR, Hurley KM, Spencer S, Holmes HR, Leahy KM, et al. Brain natriuretic peptide-like immunoreactive innervation of the cardiovascular and cerebrovascular systems in the rat. Circ Res. 1990;67:1345–1354. doi: 10.1161/01.RES.67.6.1345. [DOI] [PubMed] [Google Scholar]
- 12.De Ferrari GM, Crijns HJ, Borggrefe M, Milasinovic G, Smid J, Zabel M, et al. Chronic vagus nerve stimulation: A new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz PJ, De Ferrari GM. Sympathetic-parasympathetic interaction in health and disease: Abnormalities and relevance in heart failure. Heart Fail Rev. 2011;16:101–107. doi: 10.1007/s10741-010-9179-1. [DOI] [PubMed] [Google Scholar]
- 14.Busch V, Zeman F, Heckel A, Menne F, Ellrich J, Eichhammer P. The effect of transcutaneous vagus nerve stimulation on pain perception--an experimental study. Brain Stimul. 2013;6:202–209. doi: 10.1016/j.brs.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Dietrich S, Smith J, Scherzinger C, Hofmann-Preiß K, Freitag T, Eisenkolb A, et al. [A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI] BMT. 2008;53:104–111. doi: 10.1515/BMT.2008.022. German. [DOI] [PubMed] [Google Scholar]
- 16.Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm (Vienna) 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]
- 17.Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014;1:64–73. doi: 10.1007/s40473-014-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, et al. Vagus nerve stimulation: A new tool for brain research and therapy. Biol Psychiatry. 2000;47:287–295. doi: 10.1016/S0006-3223(99)00308-X. [DOI] [PubMed] [Google Scholar]
- 19.Dailey JW, Yan QS, Adams-Curtis LE, Ryu JR, Ko KH, Mishra PK, et al. Neurochemical correlates of antiepileptic drugs in the genetically epilepsy-prone rat (GEPR) Life Sci. 1996;58:259–266. doi: 10.1016/0024-3205(95)02286-4. [DOI] [PubMed] [Google Scholar]
- 20.Krahl SE, Senanayake SS, Handforth A. Seizure suppression by systemic epinephrine is mediated by the vagus nerve. Epilepsy Res. 2000;38:171–175. doi: 10.1016/S0920-1211(99)00089-3. [DOI] [PubMed] [Google Scholar]
- 21.Walker BR, Easton A, Gale K. Regulation of limbic motor seizures by GABA and glutamate transmission in nucleus tractus solitarius. Epilepsia. 1999;40:1051–1057. doi: 10.1111/j.1528-1157.1999.tb00818.x. [DOI] [PubMed] [Google Scholar]
- 22.Browning RA, Wang C, Faingold CL. Effect of norepinephrine depletion on audiogenic-like seizures elicited by microinfusion of an excitant amino acid into the inferior colliculus of normal rats. Exp Neurol. 1991;112:200–205. doi: 10.1016/0014-4886(91)90070-S. [DOI] [PubMed] [Google Scholar]
- 23.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of vagus nerve stimulation. Epilepsia. 1998;39:709–714. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia. 1994;35:616–626. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 25.Labar D. Vagus nerve stimulation for 1 year in 269 patients on unchanged antiepileptic drugs. Seizure. 2004;13:392–398. doi: 10.1016/j.seizure.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Morris GL, 3rd, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. Neurology. 1999;53:1731–1735. doi: 10.1212/WNL.53.8.1731. [DOI] [PubMed] [Google Scholar]
- 27.Amar AP, Apuzzo ML, Liu CY. Vagus nerve stimulation therapy after failed cranial surgery for intractable epilepsy: Results from the vagus nerve stimulation therapy patient outcome registry. Neurosurgery. 2004;55:1086–1093. doi: 10.1227/01.NEU.0000141073.08427.76. [DOI] [PubMed] [Google Scholar]
- 28.DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, et al. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41:1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 29.Kawai K, Tanaka T, Baba H, Bunker M, Ikeda A, Inoue Y, et al. Outcome of vagus nerve stimulation for drug-resistant epilepsy: The first three years of a prospective Japanese registry. Epileptic Disord. 2017;19:327–338. doi: 10.1684/epd.2017.0929. [DOI] [PubMed] [Google Scholar]
- 30.Orosz I, McCormick D, Zamponi N, Varadkar S, Feucht M, Parain D, et al. Vagus nerve stimulation for drug-resistant epilepsy: A European long-term study up to 24 months in 347 children. Epilepsia. 2014;55:1576–1584. doi: 10.1111/epi.12762. [DOI] [PubMed] [Google Scholar]
- 31.Patwardhan RV, Stong B, Bebin EM, Mathisen J, Grabb PA. Efficacy of vagal nerve stimulation in children with medically refractory epilepsy. Neurosurgery. 2000;47:1353–1357. discussion 1357–1358. doi: 10.1093/neurosurgery/47.6.1353. [DOI] [PubMed] [Google Scholar]
- 32.Elliott RE, Morsi A, Tanweer O, Grobelny B, Geller E, Carlson C, et al. Efficacy of vagus nerve stimulation over time: Review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS > 10 years. Epilepsy Behav. 2011;20:478–483. doi: 10.1016/j.yebeh.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson KJ, Koivikko MJ. Prevalence, classification, and severity of epilepsy and epileptic syndromes in children. Epilepsia. 1997;38:1275–1282. doi: 10.1111/j.1528-1157.1997.tb00064.x. [DOI] [PubMed] [Google Scholar]
- 34.Berg AT, Shinnar S, Levy SR, Testa FM, Smith-Rapaport S, Beckerman B. Early development of intractable epilepsy in children: A prospective study. Neurology. 2001;56:1445–1452. doi: 10.1212/WNL.56.11.1445. [DOI] [PubMed] [Google Scholar]
- 35.Helmers SL, Wheless JW, Frost M, Gates J, Levisohn P, Tardo C, et al. Vagus nerve stimulation therapy in pediatric patients with refractory epilepsy: Retrospective study. J Child Neurol. 2001;16:843–848. doi: 10.1177/08830738010160111101. [DOI] [PubMed] [Google Scholar]
- 36.Levy ML, Levy KM, Hoff D, Amar AP, Park MS, Conklin JM, et al. Vagus nerve stimulation therapy in patients with autism spectrum disorder and intractable epilepsy: Results from the vagus nerve stimulation therapy patient outcome registry. J Neurosurg Pediatr. 2010;5:595–602. doi: 10.3171/2010.3.PEDS09153. [DOI] [PubMed] [Google Scholar]
- 37.Barbella G, Cocco I, Freri E, Marotta G, Visani E, Franceschetti S, et al. Transcutaneous vagal nerve stimulatio (t-VNS): An adjunctive treatment option for refractory epilepsy. Seizure. 2018;60:115–119. doi: 10.1016/j.seizure.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Bauer S, Baier H, Baumgartner C, Bohlmann K, Fauser S, Graf W, et al. Transcutaneous vagus nerve stimulation (tVNS) for treatment of drug-resistant epilepsy: A randomized, double- blind clinical trial (cMPsE02) Brain Stimul. 2016;9:356–363. doi: 10.1016/j.brs.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Rong P, Liu A, Zhang J, Wang Y, Yang A, Li L, et al. An alternative therapy for drug-resistant epilepsy: Transcutaneous auricular vagus nerve stimulation. Chin Med J (Engl) 2014;127:300–304. doi: 10.3760/cma.j.issn.0366-6999.20131511. [DOI] [PubMed] [Google Scholar]
- 40.Rong P, Liu A, Zhang J, Wang Y, He W, Yang A, et al. Transcutaneous vagus nerve stimulation for refractory epilepsy: A randomized controlled trial. Clin Sci (Lond) 2014 Apr 1; doi: 10.1042/CS20130518. doi: 10.1042/CS20130518. [DOI] [PubMed] [Google Scholar]
- 41.He W, Jing X, Wang X, Rong P, Li L, Shi H, et al. Transcutaneous auricular vagus nerve stimulation as a complementary therapy for pediatric epilepsy: A pilot trial. Epilepsy Behav. 2013;28:343–346. doi: 10.1016/j.yebeh.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Lampros M, Vlachos N, Zigouris A, Voulgaris S, Alexiou GA. Transcutaneous vagus nerve stimulation (t-VNS) and epilepsy: A systematic review of the literature. Seizure. 2021;91:40–48. doi: 10.1016/j.seizure.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 43.He W, Wang XY, Zhou L, Li ZM, Jing XH, Lv ZL, et al. Transcutaneous auricular vagus nerve stimulation for pediatric epilepsy: Study protocol for a randomized controlled trial. Trials. 2015;16:371. doi: 10.1186/s13063-015-0906-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, et al. VNS therapy in treatment-resistant depression: Clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31:1345–1355. doi: 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 45.Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20:221–227. doi: 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- 46.Henry TR, Bakay RA, Votaw JR, Pennell PB, Epstein CM, Faber TL, et al. Brain blood flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy: I. Acute effects at high and low levels of stimulation. Epilepsia. 1998;39:983–990. doi: 10.1111/j.1528-1157.1998.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 47.Bohning DE, Lomarev MP, Denslow S, Nahas Z, Shastri A, George MS. Feasibility of vagus nerve stimulation-synchronized blood oxygenation level-dependent functional MRI. Invest Radiol. 2001;36:470–479. doi: 10.1097/00004424-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 48.Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: A randomized active-control trial. Neurology. 1998;51:48–55. doi: 10.1212/WNL.51.1.48. [DOI] [PubMed] [Google Scholar]
- 49.Harden CL, Pulver MC, Nikolov B, Halper JP, Labar DR. Effect of vagus nerve stimulation on mood in adult epilepsy patients. Neurology. 1999;52:A238. doi: 10.1006/ebeh.2000.0046. [DOI] [PubMed] [Google Scholar]
- 50.Goodwin FK, Jamison KR, Ghaemi SN. Manic-depressive illness: Bipolar disorders and recurrent depression. 2nd ed. Oxford University Press; 2007. [Google Scholar]
- 51.Post RM, Weiss SR, Chuang DM. Mechanisms of action of anticonvulsants in affective disorders: Comparisons with lithium. J Clin Psychopharmacol. 1992;12(1 Suppl):23S–35S. doi: 10.1097/00004714-199202001-00005. [DOI] [PubMed] [Google Scholar]
- 52.Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, et al. Vagus nerve stimulation (VNS) for treatment- resistant depressions: A multicenter study. Biol Psychiatry. 2000;47:276–286. doi: 10.1016/S0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 53.Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, et al. Vagus nerve stimulation for treatment-resistant depression: A randomized, controlled acute phase trial. Biol Psychiatry. 2005;58:347–354. doi: 10.1016/j.biopsych.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 54.Rush AJ, Sackeim HA, Marangell LB, George MS, Brannan SK, Davis SM, et al. Effects of 12 months of vagus nerve stimulation in treatment-resistant depression: A naturalistic study. Biol Psychiatry. 2005;58:355–363. doi: 10.1016/j.biopsych.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 55.Aaronson ST, Sears P, Ruvuna F, Bunker M, Conway CR, Dougherty DD, et al. A 5-year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: Comparison of response, remission, and suicidality. Am J Psychiatry. 2017;174:640–648. doi: 10.1176/appi.ajp.2017.16010034. [DOI] [PubMed] [Google Scholar]
- 56.Bottomley JM, LeReun C, Diamantopoulos A, Mitchell S, Gaynes BN. Vagus nerve stimulation (VNS) therapy in patients with treatment resistant depression: A systematic review and meta-analysis. Compr Psychiatry. 2019;98:152156. doi: 10.1016/j.comppsych.2019.152156. [DOI] [PubMed] [Google Scholar]
- 57.Kong J, Fang J, Park J, Li S, Rong P. Treating depression with transcutaneous auricular vagus nerve stimulation: State of the art and future perspectives. Front Psychiatry. 2018;9:20. doi: 10.3389/fpsyt.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rong P, Liu J, Wang L, Liu R, Fang J, Zhao J, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: A nonrandomized controlled pilot study. J Affect Disord. 2016;195:172–179. doi: 10.1016/j.jad.2016.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Austelle CW, O'Leary GH, Thompson S, Gruber E, Kahn A, Manett AJ, et al. A Comprehensive review of vagus nerve stimulation for depression. Neuromodulation. 2022;25:309–315. doi: 10.1111/ner.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oh J, Jeon S, Ha TH, Myung W, Lee SH, Ko YH, et al. Effect of home-based self-administered transcranial direct stimulation in patients with mild to moderate major depressive disorder: A single-arm, multicentral trial. Clin Psychopharmacol Neurosci. 2023;21:271–278. doi: 10.9758/cpn.2023.21.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh J, Jang KI, Jeon S, Chae JH. Effect of self-administered transcranial direct stimulation in patients with major depressive disorder: A randomized, single-blinded clinical trial. Clin Psychopharmacol Neurosci. 2022;20:87–96. doi: 10.9758/cpn.2022.20.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meron D, Hedger N, Garner M, Baldwin DS. Transcranial direct current stimulation (tDCS) in the treatment of depression: Systematic review and meta-analysis of efficacy and tolerability. Neurosci Biobehav Rev. 2015;57:46–62. doi: 10.1016/j.neubiorev.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 63.Razza LB, Palumbo P, Moffa AH, Carvalho AF, Solmi M, Loo CK, et al. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress Anxiety. 2020;37:594–608. doi: 10.1002/da.23004. [DOI] [PubMed] [Google Scholar]
- 64.Palm U, Hasan A, Strube W, Padberg F. tDCS for the treatment of depression: A comprehensive review. Eur Arch Psychiatry Clin Neurosci. 2016;266:681–694. doi: 10.1007/s00406-016-0674-9. [DOI] [PubMed] [Google Scholar]
- 65.Kamel LY, Xiong W, Gott BM, Kumar A, Conway CR. Vagus nerve stimulation: An update on a novel treatment for treatment-resistant depression. J Neurol Sci. 2022;434:120171. doi: 10.1016/j.jns.2022.120171. [DOI] [PubMed] [Google Scholar]
- 66.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 67.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol Med. 2003;9:125–134. doi: 10.1007/BF03402177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 69.Chang EH, Chavan SS, Pavlov VA. Cholinergic control of inflammation, metabolic dysfunction, and cognitive impairment in obesity-associated disorders: Mechanisms and novel therapeutic opportunities. Front Neurosci. 2019;13:263. doi: 10.3389/fnins.2019.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pavlov VA, Tracey KJ. Neural circuitry and immunity. Immunol Res. 2015;63:38–57. doi: 10.1007/s12026-015-8718-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drewes AM, Brock C, Rasmussen SE, Møller HJ, Brock B, Deleuran BW, et al. Short-term transcutaneous non-invasive vagus nerve stimulation may reduce disease activity and pro-inflammatory cytokines in rheumatoid arthritis: Results of a pilot study. Scand J Rheumatol. 2021;50:20–27. doi: 10.1080/03009742.2020.1764617. [DOI] [PubMed] [Google Scholar]
- 72.Levine YA, Koopman FA, Faltys M, Caravaca A, Bendele A, Zitnik R, et al. Neurostimulation of the cholinergic anti-inflammatory pathway ameliorates disease in rat collagen-induced arthritis. PLoS One. 2014;9:e104530. doi: 10.1371/journal.pone.0104530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meregnani J, Clarençon D, Vivier M, Peinnequin A, Mouret C, Sinniger V, et al. Anti-inflammatory effect of vagus nerve stimulation in a rat model of inflammatory bowel disease. Auton Neurosci. 2011;160:82–89. doi: 10.1016/j.autneu.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 74.Sinniger V, Pellissier S, Fauvelle F, Trocmé C, Hoffmann D, Vercueil L, et al. A 12-month pilot study outcomes of vagus nerve stimulation in Crohn's disease. Neurogastroenterol Motil. 2020;32:e13911. doi: 10.1111/nmo.13911. [DOI] [PubMed] [Google Scholar]
- 75.Lange G, Janal MN, Maniker A, Fitzgibbons J, Fobler M, Cook D, et al. Safety and efficacy of vagus nerve stimulation in fibromyalgia: A phase I/II proof of concept trial. Pain Med. 2011;12:1406–1413. doi: 10.1111/j.1526-4637.2011.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem. 1998;70:364–373. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- 77.Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem. 1995;63:213–216. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- 78.Zuo Y, Smith DC, Jensen RA. Vagus nerve stimulation potentiates hippocampal LTP in freely-moving rats. Physiol Behav. 2007;90:583–589. doi: 10.1016/j.physbeh.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sucholeiki R, Alsaadi TM, Morris GL, 3rd, Ulmer JL, Biswal B, Mueller WM. fMRI in patients implanted with a vagal nerve stimulator. Seizure. 2002;11:157–162. doi: 10.1053/seiz.2001.0601. [DOI] [PubMed] [Google Scholar]
- 80.Conway CR, Sheline YI, Chibnall JT, George MS, Fletcher JW, Mintun MA. Cerebral blood flow changes during vagus nerve stimulation for depression. Psychiatry Res. 2006;146:179–184. doi: 10.1016/j.pscychresns.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 81.Sjögren MJ, Hellström PT, Jonsson MA, Runnerstam M, Silander HC, Ben-Menachem E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer's disease: A pilot study. J Clin Psychiatry. 2002;63:972–980. doi: 10.4088/JCP.v63n1103. [DOI] [PubMed] [Google Scholar]
- 82.Bondareff W, Mountjoy CQ, Roth M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology. 1982;32:164–168. doi: 10.1212/WNL.32.2.164. [DOI] [PubMed] [Google Scholar]
- 83.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 84.Martin CO, Denburg NL, Tranel D, Granner MA, Bechara A. The effects of vagus nerve stimulation on decision-making. Cortex. 2004;40:605–612. doi: 10.1016/S0010-9452(08)70156-4. [DOI] [PubMed] [Google Scholar]
- 85.Helmstaedter C, Hoppe C, Elger CE. Memory alterations during acute high-intensity vagus nerve stimulation. Epilepsy Res. 2001;47:37–42. doi: 10.1016/S0920-1211(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 86.Jacobs HI, Riphagen JM, Razat CM, Wiese S, Sack AT. Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol Aging. 2015;36:1860–1867. doi: 10.1016/j.neurobiolaging.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 87.Colzato LS, Ritter SM, Steenbergen L. Transcutaneous vagus nerve stimulation (tVNS) enhances divergent thinking. Neuro-psychologia. 2018;111:72–76. doi: 10.1016/j.neuropsychologia.2018.01.003. [DOI] [PubMed] [Google Scholar]