Abstract
Clinical isolates of Pseudomonas aeruginosa from blood adhered to and penetrated intestinal Caco-2 cell monolayers to a greater degree than did isolates from sputum, with a concomitant drastic decrease in transepithelial electrical resistance. PAO-PR1, an avirulent exotoxin A mutant of PAO1, did not cause a decrease in the resistance. The Caco-2 monolayer system may be useful for the evaluation of certain P. aeruginosa virulence factor activities.
Pseudomonas aeruginosa is an opportunistic pathogen; in immunocompromised hosts, it frequently causes severe septicemia, arising from their own endogenous intestinal flora (1, 5, 11, 13). We previously established an animal model of endogenous P. aeruginosa septicemia in neutropenic mice (11–13). This experimental model encompasses bacterial colonization, overgrowth, and invasion and consequently closely mimics the pathophysiology of septicemia in humans (11, 12). Clinical isolates of P. aeruginosa from blood are associated with a significantly higher rate of mortality of mice in this model than strains isolated from sputum (8). We also found that the blood isolates persisted in the intestines of mice (8). Moreover, we described that the rate of mortality of mice given P. aeruginosa exotoxin A mutant PAO-PR1 (2) was significantly lower than that of the parent strain, PAO1 (15), while the mice infected with elastase mutant PAO-E64 (18) died of septicemia, as did the mice given PAO1. The ability to adhere to and penetrate epithelial cell barriers is common to many pathogenic organisms (6) and may also contribute to the occurrence of septicemia in our animal model. Our findings suggest that pathogenic P. aeruginosa strains adhere to intestinal epithelial cells and invade intestinal barriers of mice to a greater extent than do nonpathogenic strains. However, the mechanism by which P. aeruginosa adheres to and penetrates the intestinal epithelial cells is unclear.
The human adenocarcinoma cell line Caco-2, although isolated from an adult human colon, expresses several markers characteristic of normal small intestinal villus cells, such as microvillar hydrolases (24). These cells are also thought to be highly analogous to enterocytes of the fetal colon (7). The transepithelial electrical resistance of Caco-2 cell monolayers on filters varies depending on their growth, indicative of the presence of tight junctions (7, 9, 16). Cells grown in such a manner also form regular microvilli and have a well-developed brush border (7). Using Caco-2 cells, bacterial adherence, invasion, and penetration and the role of virulence factors have been studied for several bacterial species, such as Salmonella spp. (7), Vibrio cholerae (20), Escherichia coli (17), and Klebsiella pneumoniae (3).
In the present study, we examined whether there are any differences in the abilities of P. aeruginosa isolates from blood and sputum to adhere to and penetrate Caco-2 cell monolayers in vitro. We also applied this system for evaluation of the role of P. aeruginosa exoenzymes in pathogenicity, using parent strain PAO1 (15) and its exotoxin A (2) and elastase (18) mutants, which were previously examined in our murine endogenous bacteremia model.
P. aeruginosa clinical isolates, including five blood isolates and five sputum isolates obtained at Nagasaki University Hospital, Nagasaki, Japan, were used. All of these isolates were serum resistant. As previously reported, the blood isolates caused >70% mortality of mice while the sputum isolates did not kill mice in the model of endogenous septicemia (8). P. aeruginosa PAO1 (15), its exotoxin A mutant, PAO-PR1 (2), and its elastase mutant, PAO-E64 (18), which were kindly provided by B. H. Iglewski, University of Rochester School of Medicine and Dentistry, Rochester, N.Y., were also used. P. aeruginosa PAO1 and PAO-E64 result in 70 to 100% mortality in the animal model, whereas PAO-PR-1 causes <10% mortality (11). Salmonella typhimurium SL1344 (14) and E. coli DH5α (7), used as positive and negative controls, respectively, for the Caco-2 cell monolayer penetration assay, were kindly provided by B. Brett Finlay, Biotechnology Laboratory, University of British Columbia, Vancouver, British Columbia, Canada. S. typhimurium SL1344 penetrates the Caco-2 cell monolayer and appears in the basolateral medium within 2 h, with a loss of transepithelial resistance by 3 to 4 h, whereas noninvasive E. coli DH5α does not normally penetrate the monolayer within 5 h unless the tight junctions are disrupted or the epithelial cells are physically damaged (7).
Monolayers of differentiated Caco-2 cells were prepared in Transwell filter units (no. 3415; Costar, Cambridge, Mass.) containing a 0.33-cm2 porous filter membrane with 3.0-μm pores in either 24-well tissue culture plates (7) or tissue culture chamber slides (Lab-Tek; Miles Laboratories, Inc., Naperville, Ill.) (13). The cells were seeded at a density of 105 per cm2 in Dulbecco modified Eagle medium (D-MEM; Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 20% fetal bovine serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml and incubated at 37°C in 5% CO2 for 14 days. Before addition of bacteria, the monolayers were rinsed with D-MEM without antibiotics.
A bacterial suspension of approximately 108 CFU/ml in antibiotic-free D-MEM was added to the tissue culture chamber slides. The chamber slides were incubated at 37°C for 60 min with gentle rocking and then washed five times with antibiotic-free D-MEM. The chambers were removed from the slides, stained with Giemsa stain, and examined microscopically under an oil immersion lens. The number of bacteria per Caco-2 cell was recorded by examining 100 cells, and the results were expressed as averages of data from at least three independent assays.
Bacteria were inoculated at 107 CFU/well onto the apical monolayer surface. The inoculum size and experimental conditions were those reported previously by Finlay and Falkow (7). Transmonolayer electrical resistance of Caco-2 cell monolayers grown in Transwell units was measured with a Millicell-ESR apparatus (Millipore Corp., Bedford, Mass.) at certain time intervals (7, 22). Area times resistance (Ωcm2) was calculated by multiplying the measured resistance by the area of the filter (7). The basolateral medium was removed at the time of assay and replaced with fresh 37°C antibiotic-free D-MEM. Bacteria in the basolateral medium were counted by plating appropriate dilutions on Trypticase soy agar (BBL Microbiology Systems, Cockeysville, Md.). The assay was performed in triplicate, and the results were expressed as an average. Analysis of variance was applied in multiple comparisons.
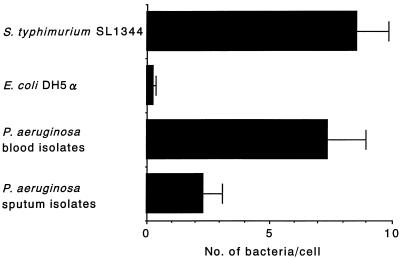
Figure 1 shows the results of studies of adherence of P. aeruginosa clinical isolates, S. typhimurium SL1344, and E. coli DH5α to Caco-2 cells. S. typhimurium SL1344 adhered to Caco-2 cells at an average ± standard deviation of 8.6 ± 1.3 bacteria/cell, while E. coli DH5α adhered at 0.3 ± 0.1 bacteria/cell. Adherence of P. aeruginosa clinical isolates from blood to Caco-2 cells was similar to that of S. typhimurium SL1344 (7.4 ± 1.6 bacteria/cell). In contrast, significantly fewer sputum isolates than blood isolates adhered to Caco-2 cells (2.3 ± 0.8 bacteria per cell; P < 0.02).
FIG. 1.
Adherence of P. aeruginosa to Caco-2 cell monolayers. P. aeruginosa clinical isolates, S. typhimurium SL1344, and E. coli DH5α were incubated with Caco-2 cell monolayers on tissue culture slides at 37°C for 60 min with gentle rocking. Each assay was performed at least in triplicate. The results for S. typhimurium SL1344 and E. coli DH5α are expressed as averages ± standard deviations of the data from replicate assays. The results for clinical isolates of P. aeruginosa are expressed as averages ± standard deviations of the values for five isolates. Clinical isolates of P. aeruginosa from blood adhered to Caco-2 cells to a greater extent than did sputum isolates (P < 0.02).
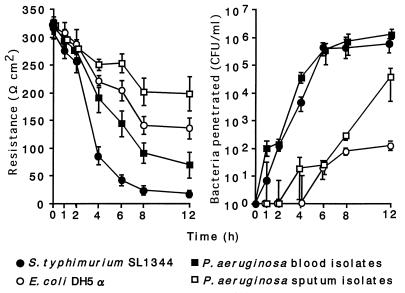
Figure 2 shows the changes in transepithelial resistance of the Caco-2 cell monolayer and the number of bacteria that have penetrated it over time. S. typhimurium SL1344 penetrated the Caco-2 cell monolayer and appeared in the basolateral medium 1 h after inoculation. Transepithelial resistance fell markedly between 2 and 4 h after bacterial inoculation and decreased to <50 Ωcm2, similar to resistance levels in unseeded filters. In contrast, E. coli DH5α could be detected in the basolateral medium after 6 h, and the transepithelial resistance decreased slowly over time. These findings are similar to those of Finlay and Falkow (7).
FIG. 2.
Penetration of P. aeruginosa through Caco-2 cell monolayers (right panel) and changes in transepithelial resistance (left panel). Bacteria were inoculated at 107 CFU/well to the apical surfaces of Caco-2 cell monolayers, and transepithelial electrical resistance of the monolayers and the numbers of bacteria in the basolateral medium were monitored over time. The results for S. typhimurium SL1344 and E. coli DH5α are expressed as averages ± standard errors of data from triplicate assays. The results for clinical isolates of P. aeruginosa are expressed as averages ± standard errors of the values for five isolates. Isolates of P. aeruginosa from blood penetrated the Caco-2 cell monolayers to a significantly greater extent than did sputum isolates (P < 0.05 for 2, 4, 6, and 12 h; P < 0.02 for 8 h after inoculation), with significant decreases in transepithelial resistance in Transwell units (P < 0.05 for 6 and 8 h; P < 0.02 for 12 h after inoculation).
P. aeruginosa isolates from blood penetrated monolayers similarly to S. typhimurium SL1344. Three of five isolates were detected in the basolateral medium 1 h after inoculation, and all isolates were present 2 h after inoculation. By contrast, it was 6 h after inoculation before all of the sputum isolates were detected in the basolateral medium. The numbers of bacteria that penetrated the Caco-2 cell monolayers were significantly higher for blood isolates than for sputum isolates (P < 0.05 for 2, 4, 6, 12 h after inoculation; P < 0.02 for 8 h after inoculation). The decrease in transepithelial resistance in Transwell units seen for blood isolates of P. aeruginosa was not as marked as for S. typhimurium SL1344 but significantly greater than that observed for sputum isolates (P < 0.05 for 6 and 8 h after inoculation; P < 0.02 for 12 h after inoculation).
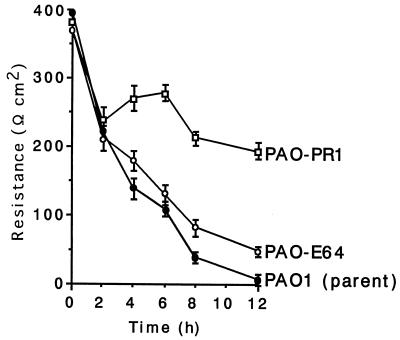
The transepithelial resistance of the Caco-2 cell monolayer infected with P. aeruginosa PAO1 decreased in a time-dependent manner, as shown in Fig. 3. When Caco-2 cells were infected with the elastase mutant PAO-E64, changes in transepithelial resistance did not differ from those observed with the parent strain. In contrast, the transepithelial resistance values for the Caco-2 cell monolayer infected with exotoxin A mutant PAO-PR1 were significantly higher than those determined after infection with the parent strain (P < 0.02 for 4 h, P < 0.002 for 6 h, P = 0.0002 for 8 h, and P < 0.0001 for 12 h after inoculation).
FIG. 3.
Changes in transepithelial resistance of Caco-2 cell monolayers infected with PAO1 and its exoenzyme mutants. Transepithelial resistances of Caco-2 cell monolayers infected with exotoxin A mutant PAO-PR1 were significantly higher than those of monolayers infected with the parent strain, PAO1 (P < 0.02 for 4 h, P < 0.002 for 6 h, P = 0.0002 for 8 h, and P < 0.0001 for 12 h after inoculation), while those of monolayers infected with elastase mutant PAO-E64 were equivalent to the values for the parent strain.
We previously showed that clinical isolates of P. aeruginosa from the blood of patients with bacteremia resulted in a higher rate of mortality for mice with endogenous bacteremia than did strains obtained from other infection sites, such as the respiratory tract (8). This implies that clinical isolates from blood carry potent virulence factors for septicemia. Our previous findings suggested that the ability of blood isolates to produce large amounts of exotoxin A and alkaline protease and to persist in the murine intestine contributes to the occurrence of septicemia in and death of mice (8). Although such host-parasite interactions are usually studied in animal models, animals are expensive and awkward to use for fine-structure studies (7).
In the present study, P. aeruginosa isolates from blood were found to be better able to adhere to Caco-2 cells than isolates from sputum. Although Caco-2 cells may be somewhat different from cells of the mouse intestine, our results suggest that blood isolates may be able to colonize the intestine, which is the first step in transintestinal endogenous bacteremia. Moreover, blood isolates penetrated the Caco-2 cell monolayer more readily and earlier than sputum isolates, with a decrease in transepithelial electrical resistance. Although it is still unclear whether P. aeruginosa invades and penetrates Caco-2 cells or passes through the junctions between cells, most organisms likely pass through broken tight junctions, as evidenced by a marked loss of transepithelial electrical resistance. A complete lipopolysaccharide (LPS) core, which is clearly needed for invasion of respiratory epithelial cells by P. aeruginosa (23, 25), is missing from most sputum isolates obtained from cystic fibrosis (CF) patients (10, 19, 21). It has recently been reported that the CF transmembrane conductance regulator (CFTR) is the cellular receptor used for invasion of epithelial cells by P. aeruginosa. Moreover, the CFTR is also the epithelial cell receptor of Salmonella typhi (26) and is expressed on the surface of Caco-2 cells (4) as well. The data from these reports suggest the possibility that P. aeruginosa strains also adhere to and penetrate the Caco-2 cell monolayer through the interaction between the complete LPS core and the CFTR and that the sputum isolates used in our study, like isolates from CF patients, also lack a complete LPS core. In the Caco-2 cell monolayer system, PAO-PR1, an exotoxin A mutant of strain PAO1 which does not kill mice in the endogenous septicemia model, did not elicit a decrease in the transepithelial electrical resistance and did not penetrate the monolayer within 4 h after inoculation like sputum isolates did, while the parent strain and elastase mutant strain, which are virulent in the animal model, caused a significant reduction in the transepithelial electrical resistance and penetrated the monolayer. Since adherence of strain PAO-PR1 to Caco-2 cells was similar to that of the parent strain (data not shown), the results suggest that exotoxin A may be involved in the penetration of bacteria. Moreover, the penetration of the monolayer by the parent strain and the concomitant decrease in its transepithelial electrical resistance were suppressed to the levels attained with strain PAO-PR1 in the presence of goat anti-exotoxin A antibody (List Biological Laboratories Inc., Campbell, Calif.) (data not shown).
Our study suggests that the in vitro Caco-2 cell monolayer system may provide insights into certain aspects of the virulence mechanisms used by P. aeruginosa. This system may also be useful for the evaluation of influences of various phenotypes of P. aeruginosa, such as the presence of flagella or pili, on its adherence to intestinal epithelial cells. Moreover, investigations performed in the presence of mucins may simulate the intestinal environment more closely. Clarification of the mechanism of colonization by P. aeruginosa of the intestinal epithelial cells by analysis of its adhesin and receptors in this in vitro model may contribute to the prevention of endogenous P. aeruginosa septicemia arising from the intestinal tract.
Acknowledgments
We thank B. H. Iglewski and B. B. Finlay for providing the bacterial strains. We are also grateful to N. Kajitani, J. Matsuda, and C. Mochida for their technical expertise.
REFERENCES
- 1.Bryan C S, Reynolds K L, Brenner E R. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effects of antimicrobial therapy. Rev Infect Dis. 1983;5:629–638. doi: 10.1093/clinids/5.4.629. [DOI] [PubMed] [Google Scholar]
- 2.Cryz S J, Jr, Friedman R L, Iglewski B H. Isolation and characterization of a Pseudomonas aeruginosa mutant producing nontoxic, immunologically crossreactive toxin A protein. Proc Natl Acad Sci USA. 1980;77:7199–7203. doi: 10.1073/pnas.77.12.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darfeuille-Michaud A, Jallat C, Aubel D, Sirot D, Rich C, Sirot J, Joly B. R-plasmid-encoded adhesive factor in Klebsiella pneumoniae strains responsible for human nosocomial infections. Infect Immun. 1992;60:44–55. doi: 10.1128/iai.60.1.44-55.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davenport S E, Mergey M, Cherqui G, Boucher R C, Gespach C, Gabriel S E. Deregulated expression and function of CFTR and Cl− secretion after activation of the Ras and Src/PyMT pathways in Caco-2 cells. Biochem Biophys Res Commun. 1996;229:663–672. doi: 10.1006/bbrc.1996.1861. [DOI] [PubMed] [Google Scholar]
- 5.Dick, J. D., V. Shull, J. E. Karp, and J. Valentine. 1988. Bacterial and host factors affecting Pseudomonas aeruginosa colonization versus bacteremia in granulocytopenic patients. Eur. J. Cancer Clin. Oncol. 25(Suppl. 1):S47–S54. [PubMed]
- 6.Finlay B B, Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989;53:210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finlay B B, Falkow S. Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis. 1990;162:1096–1106. doi: 10.1093/infdis/162.5.1096. [DOI] [PubMed] [Google Scholar]
- 8.Furuya N, Hirakata Y, Tomono K, Matsumoto T, Tateda K, Kaku M, Yamaguchi K. Comparison of mortality rates in mice with endogenous septicaemia due to Pseudomonas aeruginosa isolates from different clinical sources. J Med Microbiol. 1993;39:141–146. doi: 10.1099/00222615-39-2-141. [DOI] [PubMed] [Google Scholar]
- 9.Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux J F. Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol. 1984;247:260–267. doi: 10.1152/ajpcell.1984.247.3.C260. [DOI] [PubMed] [Google Scholar]
- 10.Hancock R E W, Mutharia L M, Chan L, Darveau R P, Speert D P, Pier G B. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable [sic] strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983;42:170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirakata Y, Furuya N, Tateda K, Kaku M, Yamaguchi K. In vivo production of exotoxin A and its role in endogenous Pseudomonas aeruginosa septicemia in mice. Infect Immun. 1993;61:2468–2473. doi: 10.1128/iai.61.6.2468-2473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirakata Y, Kaku M, Tomono K, Tateda K, Furuya N, Matsumoto T, Araki R, Yamaguchi K. Efficacy of erythromycin lactobionate for treating Pseudomonas aeruginosa bacteremia in mice. Antimicrob Agents Chemother. 1992;36:1198–1203. doi: 10.1128/aac.36.6.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirakata Y, Tomono K, Tateda K, Matsumoto T, Furuya N, Shimoguchi K, Kaku M, Yamaguchi K. Role of bacterial association with Kupffer cells in occurrence of endogenous systemic bacteremia. Infect Immun. 1991;59:289–294. doi: 10.1128/iai.59.1.289-294.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoiseth S K, Stocker B A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 15.Holloway B W, Krishnapillai V, Morgan A F. Chromosomal genetics of Pseudomonas. Microbiol Rev. 1979;43:73–102. doi: 10.1128/mr.43.1.73-102.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughson E J, Cutler D F, Hopkins C R. Basolateral secretion of kappa light chain in the polarized epithelial cell line, Caco-2. J Cell Sci. 1989;94:327–332. doi: 10.1242/jcs.94.2.327. [DOI] [PubMed] [Google Scholar]
- 17.Knutton S, Lloyd D R, McNeish A S. Adhesion of enteropathogenic Escherichia coli to human intestinal enterocytes and cultured human intestinal mucosa. Infect Immun. 1987;55:69–77. doi: 10.1128/iai.55.1.69-77.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohman D E, Cryz S J, Iglewski B H. Isolation and characterization of a Pseudomonas aeruginosa PAO mutant that produces altered elastase. J Bacteriol. 1980;142:836–842. doi: 10.1128/jb.142.3.836-842.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ojeniyi B, Baek L, Hoiby N. Polyagglutinability due to loss of O-antigenic determinants in Pseudomonas aeruginosa strains isolated from cystic fibrosis patients. Acta Pathol Microbiol Scand Sect B. 1985;93:7–13. doi: 10.1111/j.1699-0463.1985.tb02844.x. [DOI] [PubMed] [Google Scholar]
- 20.Panigrahi P, Tall B D, Russell R G, Detolla L J, Morris J G., Jr Development of an in vitro model for study of non-O1 Vibrio cholerae virulence using Caco-2 cells. Infect Immun. 1990;58:3415–3424. doi: 10.1128/iai.58.10.3415-3424.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penketh A, Pitt T, Roberts D, Hodson M E, Batten J C. The relationship of phenotype changes in Pseudomonas aeruginosa to the clinical condition of patients with cystic fibrosis. Am Rev Respir Dis. 1983;127:605–608. doi: 10.1164/arrd.1983.127.5.605. [DOI] [PubMed] [Google Scholar]
- 22.Perkins F M, Handler J S. Transport properties of total kidney epithelia in culture. Am J Physiol. 1981;241:154–159. doi: 10.1152/ajpcell.1981.241.3.C154. [DOI] [PubMed] [Google Scholar]
- 23.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaskas J R, Goldberg J B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinto M, Robine-Leon S, Appay M, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- 25.Zadai T S, Fleiszig S M, Preston M J, Goldberg J B, Pier G B. Lipopolysaccharide outer core is a ligand for corneal cell binding and ingestion of Pseudomonas aeruginosa. Invest Ophthalmol Vis Sci. 1996;37:976–986. [PubMed] [Google Scholar]
- 26.Zadai T S, Grout M, Lee C, Pier G B. Abstracts of the 97th General Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1997. Salmonella typhi, but not S. typhimurium, enters epithelial cells via the cystic fibrosis transmembrane conductance regulator (CFTR), abstr. D-98; p. 225. [Google Scholar]