Abstract
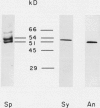
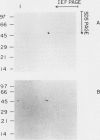
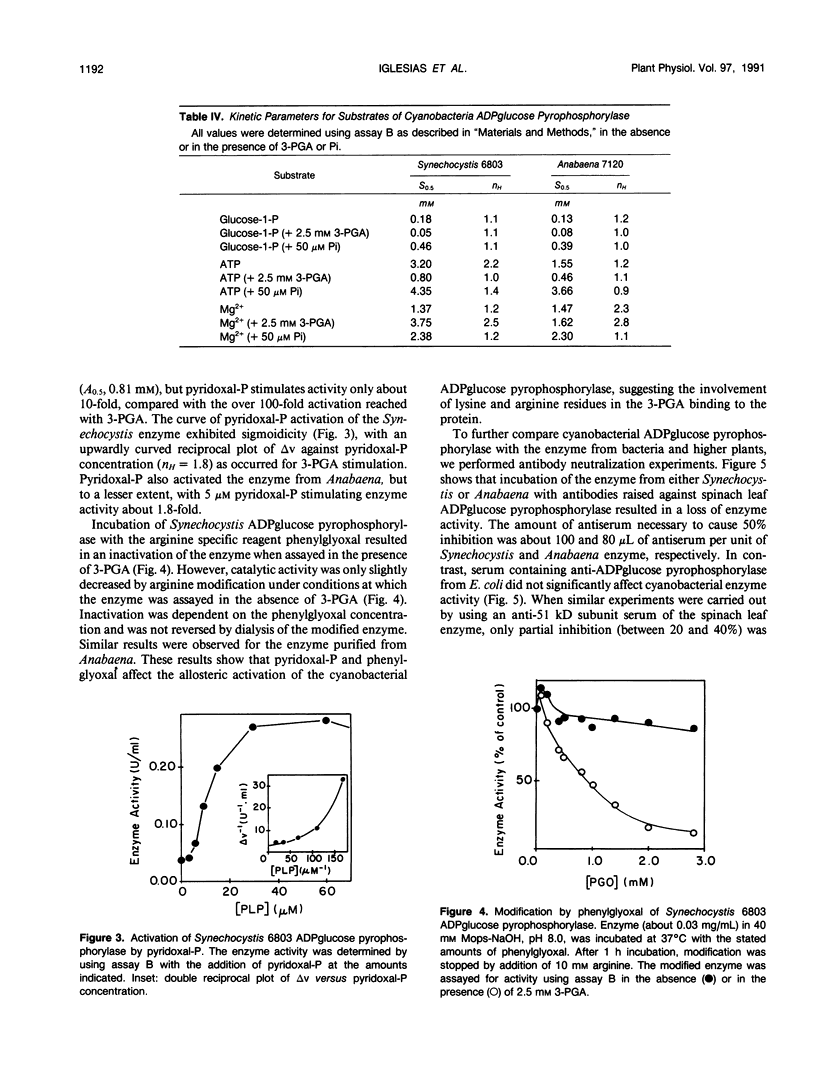
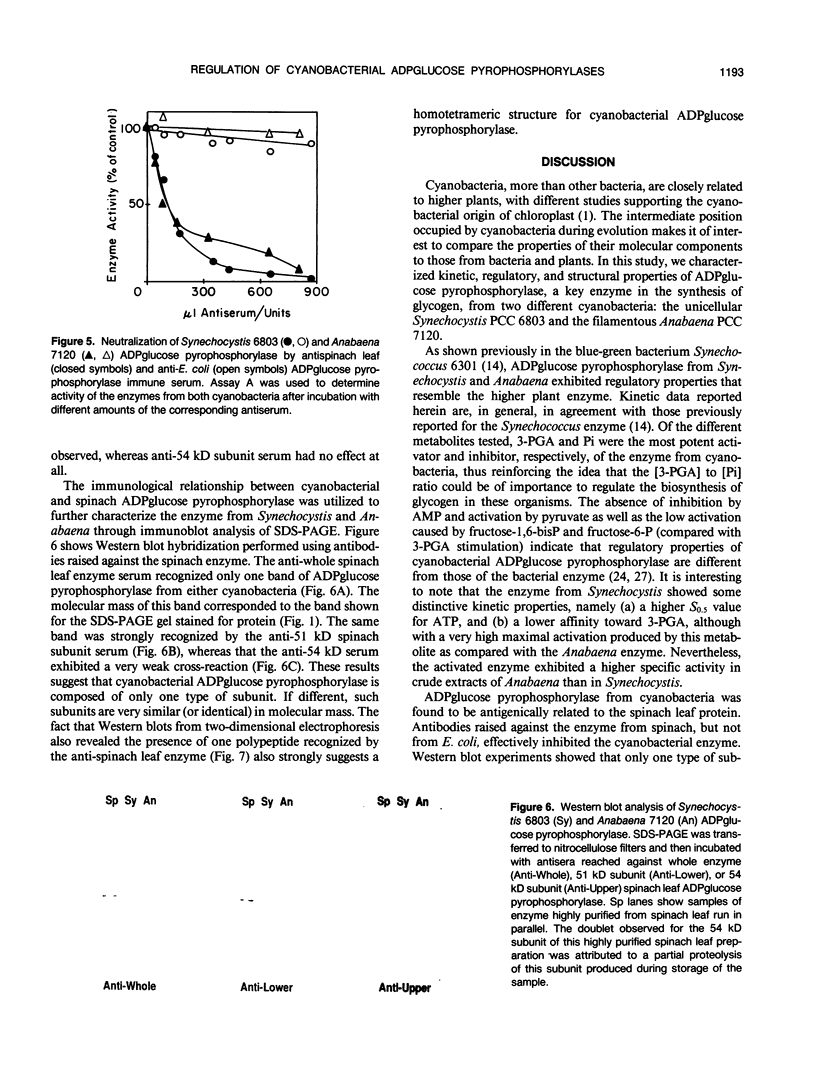
ADPglucose pyrophosphorylase (EC 2.7.7.27) has been purified from two cyanobacteria: the filamentous, heterocystic, Anabaena PCC 7120 and the unicellular Synechocystis PCC 6803. The purification procedure gave highly purified enzymes from both cynobacteria with specific activities of 134 (Synechocystis) and 111 (Anabaena) units per milligram protein. The purified enzymes migrated as a single protein band in sodium dodecyl sulfate-polyacrylamide gel electrophoresis with molecular mass corresponding to 53 (Synechocystis) and 50 (Anabaena) kilodaltons. Tetrameric structures were determined for the native enzymes by analysis of gel filtrations. Kinetic and regulatory properties were characterized for the cyanobacterial ADPglucose pyrophosphorylases. Inorganic phosphate and 3-phosphoglycerate were the most potent inhibitor and activator, respectively. The Synechocystis enzyme was activated 126-fold by 3-phosphoglycerate, with saturation curves exhibiting sigmoidicity (A0.5 = 0.81 millimolar; nH = 2.0). Activation by 3-phosphoglycerate of the enzyme from Anabaena demonstrated hyperbolic kinetics (A0.5 = 0.12 millimolar; nH = 1.0), having a maximal stimulation of 17-fold. I0.5 values of 95 and 44 micromolar were calculated for the inhibition by inorganic phosphate of the Synechocystis and Anabaena enzyme, respectively. Pyridoxal-phosphate behaved as an activator of the cyanobacterial enzyme. It activated the enzyme from Synechocystis nearly 10-fold with high apparent affinity (A0.5 = 10 micromolar; nH = 1.8). Phenylglyoxal modified the cyanobacterial enzyme by inactivating the activity in the presence of 3-phosphoglycerate. Antibody neutralization experiments showed that anti-spinach leaf (but not anti-Escherichia coli) ADPglucose pyrophosphorylase serum inactivated the enzyme from cyanobacteria. When the cyanobacterial enzymes were resolved on sodium dodecyl sulfate- and two-dimensional polyacrylamide gel electrophoresis and probed with Western blots, only one protein band was recognized by the anti-spinach leaf serum. The same polypeptide strongly reacted with antiserum prepared against the smaller spinach leaf 51 kilodalton subunit, whereas the anti-54 kilodalton antibody raised against the spinach subunit reacted weakly to the cyanobacterial subunit. Regulatory and immunological properties of the cyanobacterial enzyme are more related to the higher plant than the bacterial enzyme. Despite this, results suggest that the ADPglucose pyrophosphorylase from cyanobacteria is homotetrameric in structure, in contrast to the reported heterotetrameric structures of the higher plant ADPglucose pyrophosphorylase.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A. Protein sequences as taxonomic probes of cyanobacteria. Methods Enzymol. 1988;167:145–154. doi: 10.1016/0076-6879(88)67016-9. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carlson C. A., Preiss J. Involvement of arginine residues in the allosteric activation of Escherichia coli ADP-glucose synthetase. Biochemistry. 1982 Apr 13;21(8):1929–1934. doi: 10.1021/bi00537a036. [DOI] [PubMed] [Google Scholar]
- Copeland L., Preiss J. Purification of Spinach Leaf ADPglucose Pyrophosphorylase. Plant Physiol. 1981 Nov;68(5):996–1001. doi: 10.1104/pp.68.5.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Haugen T. H., Ishaque A., Preiss J. Biosynthesis of bacterial glycogen. Characterization of the subunit structure of Escherichia coli B glucose-1-phosphate adenylyltransferase (EC 2.7.7.27). J Biol Chem. 1976 Dec 25;251(24):7880–7885. [PubMed] [Google Scholar]
- Haugen T., Ishaque A., Preiss J. ADPGlucose pyrophosphorylase: evidence for a lysine residue at the activator site of the Escherichia coli B enzyme. Biochem Biophys Res Commun. 1976 Mar 22;69(2):346–353. doi: 10.1016/0006-291x(76)90528-3. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Bassham J. A. Light-Dark Regulation of Starch Metabolism in Chloroplasts: II. Effect of Chloroplastic Metabolite Levels on the Formation of ADP-Glucose by Chloroplast Extracts. Plant Physiol. 1979 Jan;63(1):109–113. doi: 10.1104/pp.63.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H. B., Reeves C. D., Okita T. W. ADPglucose Pyrophosphorylase Is Encoded by Different mRNA Transcripts in Leaf and Endosperm of Cereals. Plant Physiol. 1986 Jun;81(2):642–645. doi: 10.1104/pp.81.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi C., Preiss J. Regulatory Properties of the ADP-Glucose Pyrophosphorylase of the Blue-Green Bacterium Synechococcus 6301. Plant Physiol. 1976 Dec;58(6):753–756. doi: 10.1104/pp.58.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C. R., Preiss J. A Starch Deficient Mutant of Arabidopsis thaliana with Low ADPglucose Pyrophosphorylase Activity Lacks One of the Two Subunits of the Enzyme. Plant Physiol. 1988 Dec;88(4):1175–1181. doi: 10.1104/pp.88.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell M. K., Bloom M., Knowles V., Preiss J. Subunit Structure of Spinach Leaf ADPglucose Pyrophosphorylase. Plant Physiol. 1987 Sep;85(1):182–187. doi: 10.1104/pp.85.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell M., Bloom M., Preiss J. Affinity labeling of the allosteric activator site(s) of spinach leaf ADP-glucose pyrophosphorylase. J Biol Chem. 1988 Jan 15;263(2):633–637. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Okita T. W., Nakata P. A., Anderson J. M., Sowokinos J., Morell M., Preiss J. The Subunit Structure of Potato Tuber ADPglucose Pyrophosphorylase. Plant Physiol. 1990 Jun;93(2):785–790. doi: 10.1104/pp.93.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C., Preiss J. Purification and Properties of Nonproteolytic Degraded ADPglucose Pyrophosphorylase from Maize Endosperm. Plant Physiol. 1987 Jan;83(1):105–112. doi: 10.1104/pp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- Preiss J., Danner S., Summers P. S., Morell M., Barton C. R., Yang L., Nieder M. Molecular Characterization of the Brittle-2 Gene Effect on Maize Endosperm ADPglucose Pyrophosphorylase Subunits. Plant Physiol. 1990 Apr;92(4):881–885. doi: 10.1104/pp.92.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Romeo T. Physiology, biochemistry and genetics of bacterial glycogen synthesis. Adv Microb Physiol. 1989;30:183–238. doi: 10.1016/s0065-2911(08)60113-7. [DOI] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]