Abstract
Objective
This study investigated the association between non-suicidal self-injury (NSSI) and quantified electroencephalogram (QEEG) in patients with depression. We aimed to identify clinical features of NSSI and differences in QEEG findings.
Methods
This retrospective study used the medical records of 52 inpatients with major depressive episodes, aged from 15 to 30. The patients were categorized according to their history of NSSI. Their main diagnosis and sex were also considered. To evaluate clinical symptoms, self-reported scales were used. The absolute power and the Z-scores of various waves were included.
Results
NSSI was associated with suicidal ideations (p = 0.001) and trauma history (p = 0.014). In the binary logistic regression analysis, the Z-score of absolute alpha power was higher on the FP2 node (p = 0.029), lower on the F4 node (p = 0.029) in the NSSI group. The absolute high beta power in the NSSI group was higher on the FP2 and the F3 node, but lower on the F7 and F8 node. Patients with NSSI showed higher Z-score of the absolute delta power at the FP2 node (p = 0.044). The absolute gamma power was higher on the FP2 (p = 0.012) and the F3 node (0.043), lower on the FP1 (p = 0.019) and the F7 node (0.018) in the NSSI group. The absolute high gamma power at the FP2 (p = 0.017) and F8 nodes (p = 0.045) were higher in the NSSI group.
Conclusion
Patients with NSSI may have clinical features distinct from those of patients without NSSI. QEEG results have shown some differences, although it is less applicable due to some limitations.
Keywords: Non-suicidal self-injury, Quantitative electroencephalography, Depression
INTRODUCTION
Non-suicidal self-injury (NSSI) is the act of intentionally damaging and destroying parts of the body without the intention of committing suicide [1]. It includes the behaviors of cutting, skin scratching, self-biting, burning, and tying [2,3]. The prevalence of NSSI is rising rapidly worldwide, and it has become a critical issue in psychiatric research in recent decades. The incidence of NSSI varies between 7.5% and 46.5% in adolescents, approximately 38.9% in college students, and ranging from 4% to 23% in adults [4].
Even if there is no intention of suicide, NSSI not only leads to physical and mental sequelae [5] but also leads to suicidal ideations of increasingly destructive acts. In addition, self-injury is dangerous because it can lead to death [6]. Joiner [7] called this phenomenon “acquired capability for suicide”, insisting that NSSI is closely related to suicide and is as dangerous as suicide. Therefore, NSSI has its own clinical importance, supported by the fact that it was classified as “Conditions for Further Study” in the fifth edition of the diagnostic and statistical manual of mental disorders of the American Psychiatric Association, published in 2013 [8].
NSSI is a requirement for the diagnosis of borderline personality disorder, but it is also observed in many patients with mood disorders because it is related to emotional dysregulation [9,10]. Several studies have been conducted to determine the risk factors for NSSI in patients with mood disorders. Some investigators have attempted to establish a link between childhood trauma, serum cortisol levels, and NSSI in patients with major depressive disorder (MDD) [11]. Previous research has reported a noteworthy link between NSSI and levels of tumor necrosis factor [12]. Moreover, studies utilizing quan-tified electroencephalogram (QEEG) have revealed a positive correlation between suicide attempts and elevated high gamma power on the frontal and temporal lobes [13]. Several studies have indicated that there is a correlation between the intensity of depression and the amplitude of alpha power [14-17]. Additional research has shown that the amplitude and asymmetry of alpha power are connected to NSSI. However, very few studies have focused on the QEEG patterns of patients with NSSI or suicidality, and the results of these studies have been incon-sistent. Therefore, this study investigated the clinical characteristics of patients with depression who experienced NSSI. In this study, we aimed to identify the QEEG and clinical features of patients with NSSI by comparatively analyzing patients with and without NSSI. To our knowledge, this is the first study to investigate the relationship between NSSI and QEEG among individuals who have been diagnosed with mood disorders.
METHODS
Subjects and Study Design
We conducted a retrospective study, utilizing the medical records of 52 patients with mood disorders (major depressive disorder, bipolar I disorder, and bipolar II disorder) who experienced major depressive episodes. We collected data on suicidal ideation, suicide attempts, and NSSI reported within 6 months of hospitalization. Patients diagnosed with neurological disorders such as epilepsy or a history of head trauma, brain surgery, cerebrovascular accidents, or hemorrhage were excluded. A total of 52 patients were categorized into two groups based on their history of NSSI: those who had not committed NSSI (non-NSSI group, n = 29) and those who had committed NSSI (NSSI group, n = 23). As QEEG results could be influenced by aging and since the prevalence of NSSI is especially higher in adolescents and young adults, we limited the age of the patients from 15 to 30. Main diagnosis of the patients were considered, where we divided patients into two groups: MDD (n = 31) and bipolar disorder (BD, n = 21). We compared the QEEG and scores on scales such as the Beck Depression Inventory (BDI), Suicidal Ideation Questionnaire (SIQ), and Korean-Childhood Trauma Que-stionnaire (K-CTQ) between the two groups. The BDI is a self-report questionnaire that comprises 21 items, each rated on a three-point scale, and is used to assess the presence and severity of depression [18]. If a person scores 20 or above on the BDI, out of a total score of 63, it suggests that they have moderate depression, whereas a score of 29 or above implies severe depression. The SIQ is a self- report assessment tool that measures the presence and intensity of suicidal ideation. It consists of 30 items, each worth 3 points, for a total of 90 points. A total score of 41 or higher indicates the presence of suicidal ideation. The K-CTQ is a self-reported questionnaire about adverse childhood experiences before the age of 18 years, consisting of 28 questions with a total score of 140 points. It is divided into the following five areas: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. Out of a total score of 140, a total score of 51 or more indicates moderate abuse, 68 or more indicates severe abuse, and 125 or more indicates extreme abuse. The Inje University Ilsan Paik Hospital Ethics Com-mittee granted approval for the study protocol (IRB 2022- 02-038).
QEEG Procedure
We used E-prime software (Psychology Software Tools) to synchronize the start of the QEEG recordings and stimulation presentation. We collected the QEEG data using a Neuroscan SynAmps amplifier, with a total of 64 Ag-AgCl electrodes attached to a Quik-Cap, following the extended 10−20 system. The protocols have been described previously [19,20]. The Z-scores of the absolute power of delta power (1.0−4.0 Hz), theta power (4.0−8.0 Hz), alpha power (8.0−12.0 Hz), beta power (12.0−25.0 Hz), gamma power (25.0−40.0 Hz), and high gamma power (> 40 Hz) were calculated as 95% confidence intervals. The nodes in the frontal lobe included FP1, FP2, F3, F4, F7, and F8.
Statistical Analyses
Based on their experience with current NSSI, a total of 52 patients were classified into two groups: non-NSSI group, n = 29; NSSI group, n = 23. The ttest and Mann–Whitney Utest were conducted. We performed a chi- square test to determine whether a history of suicide attempts was related to a history of NSSI. Binary logistic regression analysis was performed with age and sex as covariates, and the two groups were considered fixed factors. Binary logistic regression was conducted on the clusters of each QEEG wave. The amplitudes and Z-scores of the absolute power of all wave types were included. A significance level of p < 0.05 was chosen for all tests, and two-tailed tests were employed. The SPSS software package was utilized to carry out statistical analyses (version 25; IBM Co.).
RESULTS
Demographic Characteristics
Of the 52 patients, 15 were male and 37 were female. The non-NSSI group had a sex ratio of 12:17 (male:female), and the NSSI group of 3:20 (p = 0.025) (Table 1). The mean age of the NSSI group was 21.00 years old, while the mean age of the non-NSSI group was 22.79 years old (p = 0.048) (Table 1). A ratio of MDD:BD was 14:9 in the NSSI group, while that in the non-NSSI group was 17:12 (p = 0.871) (Table 1). Compared to the non-NSSI group, the NSSI group exhibited notably higher SIQ scores (p = 0.001) (Table 1). The scores on the K-CTQ were also found to be higher in the NSSI group compared to the non-NSSI group, with statistical significance (p = 0.014) (Table 1). Further, 7 out of 29 in the non-NSSI group and 11 out of 23 in the NSSI group had previously attempted suicide. This difference was not statistically significant (p = 0.075) (Table 1). The average absolute powers of each wave in the QEEG were compared between the groups using the Mann–Whitney Utest, and none showed a significant difference.
Table 1.
Demographic characteristics and rating scale scores of groups without and with NSSI
| Variable | Group without NSSI (n = 29) | Group with NSSI (n = 23) | t, z | p value |
|---|---|---|---|---|
| Sex (M/F)‡ | 12/17 | 3/20 | −2.218 | 0.025* |
| Age† | 22.79 ± 4.53 | 21.00 ± 3.41 | 1.575 | 0.048* |
| Diagnosis (MDD/BD)‡ | 17/12 | 14/9 | −0.163 | 0.871 |
| BDI† | 28.90 ± 11.23 | 36.22 ± 10.75 | −2.379 | 0.749 |
| SIQ | 79.14 ± 49.46 | 122.87 ± 35.62 | −3.225 | 0.001* |
| K-CTQ | 47.03 ± 15.91 | 57.87 ± 17.91 | −2.453 | 0.014* |
| History of suicidal attempt (N/Y)‡ | 22/7 | 12/11 | −1.766 | 0.075 |
| Aavr. | 24.30 ± 21.07 | 20.74 ± 20.91 | −0.930 | 0.352 |
| Bavr. | 10.7 ± 10.89 | 11.20 ± 6.50 | −0.967 | 0.333 |
| HBavr. | 1.63 ± 1.51 | 1.38 ± 0.79 | −0.636 | 0.525 |
| Tavr. | 17.46 ± 15.68 | 20.35 ± 26.29 | −0.028 | 0.978 |
| Davr.† | 16.24 ± 7.01 | 17.28 ± 8.71 | −0.341 | 0.594 |
| Gavr. | 1.431 ± 1.08 | 1.41 ± 0.68 | −0.193 | 0.847 |
| HGavr. | 0.79 ± 0.65 | 0.77 ± 0.50 | −0.728 | 0.467 |
Values are presented as number only or mean ± standard deviation.
MDD, major depressive disorder; BD, bipolar disorder; NSSI, non-suicidal self-injury; BDI, Beck Depression Inventory; SIQ, Suicidal Ideation Questionnaire; K-CTQ, Korean-Childhood Trauma Questionnaire; Aavr, the average absolute power of alpha wave; Bavr, the average absolute power of beta wave; HBavr, the average absolute power of high beta wave; Tavr, the average absolute power of theta wave; Davr, the average absolute power of delta wave; Gavr, the average absolute power of gamma wave; HGavr, the average absolute power of high gamma wave.
*p < 0.05; †ttest; ‡c2-test. For the other variables, mean and standard deviation are based on ttest results, and z and p values are based on Mann–Whitney test.
Comparison of QEEG Data between the NSSI and Non-NSSI Groups
Binary logistic regression analyses, controlled for sex; age; diagnosis; and the BDI, SIQ, and K-CTQ scores as covariates, were conducted for comparisons between the two groups. Binary logistic regression of the presence of NSSI with absolute alpha power showed that sex was associated with a history of NSSI (p = 0.018) (Table 2). In addition, SIQ (p = 0.009) and K-CTQ (p = 0.042) scores were positively correlated with NSSI history (Table 2). The amplitude of the absolute alpha power showed no significant relationship with a history of NSSI. However, the Z-scores of the absolute alpha powers at certain locations were associated with a history of NSSI. The mean Z-score of absolute alpha power at the FP2 node (located in the right prefrontal cortex) was higher in the NSSI group (p = 0.029) (Table 2), while that at the F4 node (located in the right dorsolateral prefrontal cortex) was lower in the NSSI group (p = 0.029) (Table 2).
Table 2.
Binary logistic regression of absence or presence of NSSI groups with alpha power
| History of NSSI | Coefficient | Standard error | p value | Exp. coefficient | 95% lower CI Exp. coefficient | 95% upper CI Exp. coefficient |
|---|---|---|---|---|---|---|
| Sex | 2.848 | 1.203 | 0.018* | 17.262 | 1.633 | 182.417 |
| SIQ | 0.032 | 0.012 | 0.009** | 1.032 | 1.008 | 1.057 |
| K-CTQ | 0.065 | 0.032 | 0.042* | 1.067 | 1.002 | 1.136 |
| AF4 | 0.192 | 0.107 | 0.073 | 1.212 | 0.982 | 1.495 |
| Aavr. | −0.221 | 0.125 | 0.076 | 0.802 | 0.627 | 1.025 |
| ZAFP2 | 3.167 | 1.453 | 0.029* | 23.732 | 1.375 | 409.502 |
| ZAF4 | −4.166 | 1.906 | 0.029* | 0.016 | 0.000 | 0.650 |
Exp. coefficient, exponentiated coefficient; CI, confidence interval; NSSI, non-suicidal self-injury; SIQ, Suicidal Ideation Questionnaire; K-CTQ, Korean-Childhood Trauma Questionnaire; AF4, the amplitude of the absolute alpha power at the F4 node; Aavr, the average absolute power of alpha wave; ZAFP2, the mean Z-score of absolute alpha power at the FP2 node; ZAF4, the mean Z-score of absolute alpha power at the FP4 node.
*p < 0.05; **p < 0.01.
Binary logistic regression of the presence of NSSI with absolute high beta power showed that history of suicidal attempt was associated with a history of NSSI (p = 0.013) (Table 3). Also, BDI was highly related to the history of NSSI (p = 0.009) (Table 3). The amplitude of the absolute high beta power on the FP2 (p = 0.043) and the F3 node (p = 0.004) was higher in the NSSI group, but the absolute high beta power on the F7 (p = 0.018) and F8 node (p = 0.019) was lower in the NSSI group (Table 3). The mean Z-score of absolute high beta power at the FP1 node was lower in the NSSI group (p = 0.002) (Table 3).
Table 3.
Binary logistic regression of absence or presence of NSSI groups with high beta power
| History of NSSI | Coefficient | Standard error | p value | Exp. coefficient | 95% lower CI Exp. coefficient | 95% upper CI Exp. coefficient |
|---|---|---|---|---|---|---|
| History of suicidal attempt (N/Y) | 2.690 | 1.085 | 0.013* | 14.731 | 1.757 | 123.497 |
| BDI | 0.146 | 0.056 | 0.009** | 1.157 | 1.037 | 1.290 |
| HBFP2 | 3.215 | 1.587 | 0.043* | 24.894 | 1.110 | 558.228 |
| HBF3 | 6.770 | 2.338 | 0.004** | 871.665 | 8.913 | 85,244.562 |
| HBF7 | −3.099 | 1.304 | 0.018* | 0.045 | 0.003 | 0.581 |
| HBF8 | −3.353 | 1.431 | 0.019* | 0.035 | 0.002 | 0.578 |
| ZHBFP1 | −9.050 | 2.874 | 0.002** | 0.000 | 0.000 | 0.033 |
Exp. coefficient, exponentiated coefficient; CI, confidence interval; NSSI, non-suicidal self-injury; BDI, Beck Depression Inventory; HBFP2, the amplitude of absolute high beta power at the FP2 node; HBF3, the amplitude of absolute high beta power at the F3 node; HBF7, the amplitude of absolute high beta power at the F7 node; HBF8, the amplitude of absolute high beta power at the F8 node; ZHBFP1, the mean Z-score of absolute high beta power at the FP1 node.
*p < 0.05; **p < 0.01.
Binary logistic regression of the presence of NSSI with absolute delta power showed that sex was associated with a history of NSSI (p = 0.018) (Table 4). In addition, SIQ (p = 0.019) and K-CTQ (p = 0.015) scores were signifi-cantly positively correlated with a history of NSSI (Table 4). The amplitude of the absolute delta power showed no significant relationship with a history of NSSI. However, the mean Z-score of the absolute delta power at the FP2 node (located in the right prefrontal cortex) was higher in the NSSI group (p = 0.044).
Table 4.
Binary logistic regression of absence or presence of NSSI groups with delta power
| History of NSSI | Coefficient | Standard error | p value | Exp. coefficient | 95% lower CI Exp. coefficient | 95% upper CI Exp. coefficient |
|---|---|---|---|---|---|---|
| Sex | 2.691 | 1.136 | 0.018* | 14.752 | 1.591 | 136.793 |
| SIQ | 0.027 | 0.012 | 0.019* | 1.027 | 1.004 | 1.051 |
| K-CTQ | 0.078 | 0.032 | 0.015* | 1.081 | 1.015 | 1.151 |
| ZDFP2 | 3.162 | 1.566 | 0.044* | 23.616 | 1.096 | 508.708 |
| ZDF8 | −1.210 | 0.718 | 0.092 | 0.298 | 0.073 | 1.217 |
Exp. coefficient, exponentiated coefficient; CI, confidence interval; NSSI, non-suicidal self-injury; SIQ, Suicidal Ideation Questionnaire; K-CTQ, Korean-Childhood Trauma Questionnaire; ZDFP2, the mean Z-score of absolute delta power at the FP2 node; ZDF8, the mean Z-score of absolute delta power at the F8 node.
*p < 0.05.
Binary logistic regression of the presence of NSSI with absolute gamma power showed that age was associated with a history of NSSI (p = 0.030) (Table 5). In addition, SIQ (p = 0.011) and K-CTQ (p = 0.027) scores were significantly positively correlated with a history of NSSI (Table 5). The amplitude of absolute gamma power on the FP2 node (p = 0.012) and the F3 node (p = 0.043) was higher in the NSSI group (Table 5). The NSSI group had a significantly lower amplitude of absolute gamma power on the FP1 (p = 0.019) and the F7 node (p = 0.018) (Table 5).
Table 5.
Binary logistic regression of absence or presence of NSSI groups with gamma power
| History of NSSI | Coefficient | Standard error | p value | Exp. coefficient | 95% lower CI Exp. coefficient | 95% upper CI Exp. coefficient |
|---|---|---|---|---|---|---|
| Age | −0.355 | 1.640 | 0.030* | 0.701 | 0.509 | 0.966 |
| SIQ | 0.042 | 0.017 | 0.011* | 1.043 | 1.010 | 1.077 |
| K-CTQ | 0.069 | 0.031 | 0.027* | 1.072 | 1.008 | 1.140 |
| GFP1 | −6.039 | 2.580 | 0.019* | 0.002 | 0.000 | 0.375 |
| GFP2 | 5.165 | 2.065 | 0.012* | 174.967 | 3.059 | 10,006.284 |
| GF3 | 4.624 | 2.283 | 0.043* | 101.904 | 1.162 | 8,938.143 |
| GF7 | −3.594 | 1.519 | 0.018* | 0.027 | 0.001 | 0.540 |
Exp. coefficient, exponentiated coefficient; CI, confidence interval; NSSI, non-suicidal self-injury; SIQ, Suicidal Ideation Questionnaire; K-CTQ, Korean-Childhood Trauma Questionnaire; GFP1, the amplitude of absolute gamma power at the FP1 node; GFP2, the amplitude of absolute gamma power at the FP2 node; GF3, the amplitude of absolute gamma power at the F3 node; GF7, the amplitude of absolute gamma power at the F7 node.
*p < 0.05.
Binary logistic regression of the presence of NSSI with absolute high gamma power; sex; age; diagnosis and BDI, SIQ, and K-CTQ scores revealed that neither sex nor age differed significantly between the two groups (Table 6). However, SIQ (p = 0.03) and K-CTQ (p = 0.017) scores showed significant positive relationships with a history of NSSI (Table 6). The amplitudes of absolute high gamma power on the FP2 node (located on the right prefrontal cortex) (p = 0.017) and F8 node (located on the right ventrolateral prefrontal cortex) (p = 0.045) were higher in the NSSI group (Table 6).
Table 6.
Binary logistic regression of absence or presence of NSSI groups with high gamma power
| History of NSSI | Coefficient | Standard error | p value | Exp. coefficient | 95% lower CI Exp. coefficient | 95% upper CI Exp. coefficient |
|---|---|---|---|---|---|---|
| Sex | 2.032 | 1.261 | 0.107 | 7.627 | 0.644 | 90.335 |
| Age | −0.227 | 0.136 | 0.095 | 0.797 | 0.610 | 1.040 |
| SIQ | 0.025 | 0.011 | 0.030* | 1.025 | 1.002 | 1.048 |
| K-CTQ | 0.085 | 0.036 | 0.017* | 1.089 | 1.015 | 1.168 |
| HGFP1 | −3.788 | 2.116 | 0.073 | 0.023 | 0 | 1.432 |
| HGFP2 | 4.555 | 1.910 | 0.017* | 95.062 | 2.252 | 4,012.260 |
| HGF7 | −3.887 | 1.990 | 0.051 | 0.020 | 0 | 1.014 |
| HGF8 | 5.216 | 2.604 | 0.045* | 184.111 | 1.118 | 30,329.620 |
Exp. coefficient, exponentiated coefficient; CI, confidence interval; NSSI, non-suicidal self-injury; SIQ, Suicidal Ideation Questionnaire; K-CTQ, Korean-Childhood Trauma Questionnaire; HGFP1, the amplitude of absolute high gamma power at the FP1 node; HGFP2, the amplitude of absolute high gamma power at the FP2 node; HGF7, the amplitude of absolute high gamma power at the F7 node; HGF8, the amplitude of absolute high gamma power at the F8 node.
*p < 0.05.
DISCUSSION
This study attempted to determine the relationship between NSSI and QEEG. When the QEEG results of the NSSI and non-NSSI groups were subjected to the Student’s ttest, no statistically significant results were found. How-ever, after controlling for sex, age, diagnosis and BDI, SIQ, and K-CTQ scores in the binary logistic regression analyses, an association between the presence of NSSI and QEEG outcomes in terms of the alpha, high beta, delta, gamma, and high gamma waves was revealed (Fig. 1).
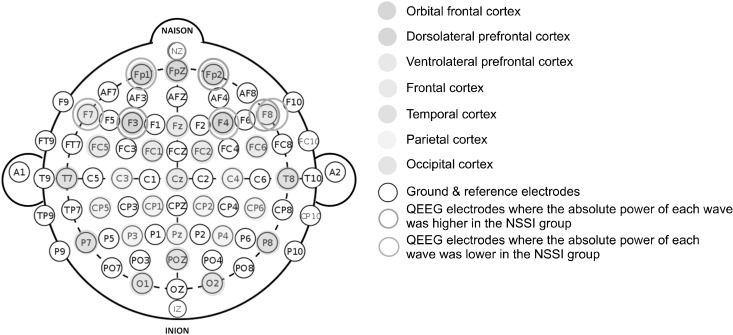
Fig. 1.
Quantified electroencephalo-gram (QEEG) electrode placement and corresponding brain cortical areas. Electrodes showing signifi-cant differences between the two groups are marked separately.
NSSI, non-suicidal self-injury.
The current study revealed differences in the Z-scores of absolute alpha power between the NSSI and non-NSSI groups. There have been limited studies investigating the connection between frontal alpha waves and NSSI so far. However, there have been studies on alpha power in relation to depression severity and suicidal ideation. Under normal conditions, higher levels of alpha power in the frontal lobe suggest a person is in a state of relaxation. Conversely, when there are abnormal conditions, such as attention deficits, hyperactivity disorder, and depressive states, higher levels of alpha power in the frontal lobe may indicate a different meaning [21]. There is extensive research supporting the idea that patients with depression exhibit a general increase in alpha power in their frontal lobe [22,23]. A different research study discovered that individuals with MDD, especially males, displayed higher levels of alpha power in the frontal and parietal regions, as well as lower levels of activity in the left midfrontal region [24]. Iznak’s study, conducted in 2021, showed greater left hemisphere activation in patients with suicide attempts, whereas the right hemisphere was relatively more active in the NSSI-only group [25]. In our study, the Z- scores of absolute alpha power in the NSSI group were higher on the FP2 node, while those on the F4 node were lower. The FP2 node was located on the orbitofrontal cortex, whereas the F4 node was located on the dorsolateral prefrontal cortex. The expression and control of social and emotional processes, as well as instinctive reactions, are mediated by the orbital frontal cortex. On the other hand, the dorsolateral prefrontal cortex is crucial for executive processes, such as working memory and the temporal organization of goal-directed behaviors for behavior, cognition, and language [26]. Considering these neuroanatomical features, the QEEG outcomes in the NSSI group in this study could be related to impaired cognition (low alpha power in the F4 node) and increased instinct-ual and impulsive behaviors (high alpha power in the FP2 node).
In terms of high beta waves, some studies have shown that high beta activity increased in the frontal region of MDD patients [27]. It has been explained in relation with psychomotor agitation and rumination [28]. In consistent with those studies, the current study shows increase of high beta activity in FP2 node and F3 node in the NSSI group. However, high beta activity in F7 and F8 node of the NSSI group were lower than non-NSSI group. It might be related to hyperthymic temperament, referring to the study which showed anger is related to the increased high beta activity in F7, F8 node and some nodes in the occipital region [29].
There are a few studies on delta power related to depression and NSSI; however, in one study, strong delta waves were observed in the bilateral prefrontal lobes of victims of domestic violence, indicative of emotional conflict, anger, and disrupted communication between the partners or between the perpetrator and victim [30]; these results may be related to the higher Z-scores of delta power on the FP2 node of patients and the higher K-CTQ in the NSSI group. In turn, we can speculate that the traumatic experiences of patients with NSSI are associated with increased delta power.
Gamma power increases when a person is highly alert and conscious while thinking and concentrating simul-taneously. Low levels of gamma power in the brain can induce memory and learning problems, as well as mental performance, attention, and concentration problems [31]. Recent research has suggested that decreased gamma power amplitude may contribute to depression [32]. In the current study, there was inconsistency within the result. We observed a decreased amplitude of gamma waves on the FP1 node and F3 node in the left prefrontal lobe in the NSSI group, correlated with depression severity, but on the F7 node, we observed increased amplitude.
To our knowledge, there is no study on the relationship between high gamma power and NSSI, but some studies have focused on the association between the former and suicidal ideation. In one study, high gamma power in the prefrontal, frontal and temporal regions was considerably higher in patients with suicidal ideation than in other patients [13]. This is consistent with the increase in absolute high gamma power at the FP2 and F8 nodes in the NSSI group in our study. The right frontal lobe exhibited higher absolute high gamma power in the NSSI group, along with higher SIQ scores. In this study, higher depression severity and trauma scores were observed in the NSSI group. The results of this study are similar to previous research that has examined the relationship between depression, trauma, and NSSI in patients. Both PTSD and BPD were significantly associated with hospital admissions for self-harm, according to censored regression analysis [33].
This study has some limitations. This study aimed to identify the clinical features of NSSI in patients with depression. In addition, our analyses were not controlled for other confounding factors, such as education and medications. Comorbid psychiatric disorders like personality disorders were not taken into consideration. The goal of this study was to investigate the characteristics of NSSI in individuals with depression. We found some differences in the QEEG outcomes between patient groups with and without NSSI, while controlling for other variants that could influence the QEEG outcomes, such as sex, age, diagnosis, severity of depressive symptoms, history of childhood trauma, and suicide attempts. However, differences of epidemiologic characteristics between two groups was evident, so it remains as a limitation of this study even after the adjustment. Prospective studies with larger sample sizes and the ability to control for other confounding variables, such as education and medications, are required to further investigate the clinical features of NSSI in patients with depression.
ACKNOWLEDGEMENTS
The authors express their gratitude to Kim B.E. for her support in collecting the data.
Funding Statement
Funding None.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Eun-Jin Park, Young-Min Park. For-mal analysis: Soh Yeon Hong. Methodology: Eun-Jin Park, Young-Min Park. Software: Soh Yeon Hong. Writing—original draft: Soh Yeon Hong. Writing—review & editing: Young-Min Park.
References
- 1.Klonsky ED, Muehlenkamp JJ. Self-injury: A research review for the practitioner. J Clin Psychol. 2007;63:1045–1056. doi: 10.1002/jclp.20412. [DOI] [PubMed] [Google Scholar]
- 2.Klonsky ED, Glenn CR. Assessing the functions of non-suicidal self-injury: Psychometric properties of the inventory of statements about self-injury (ISAS) J Psychopathol Behav Assess. 2009;31:215–219. doi: 10.1007/s10862-008-9107-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andover MS, Primack JM, Gibb BE, Pepper CM. An examination of non-suicidal self-injury in men: Do men differ from women in basic NSSI characteristics? Arch Suicide Res. 2010;14:79–88. doi: 10.1080/13811110903479086. [DOI] [PubMed] [Google Scholar]
- 4.Cipriano A, Cella S, Cotrufo P. Nonsuicidal self-injury: A systematic review. Front Psychol. 2017;8:1946. doi: 10.3389/fpsyg.2017.01946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muehlenkamp JJ, Gutierrez PM. An investigation of differences between self-injurious behavior and suicide attempts in a sample of adolescents. Suicide Life Threat Behav. 2004;34:12–23. doi: 10.1521/suli.34.1.12.27769. [DOI] [PubMed] [Google Scholar]
- 6.Oh YS, Jang YJ. A qualitative study of counselors' experiences working with non-suicidal self-injury adolescents. The Korea Journal of Youth Counseling. 2021;29:175–204. [Google Scholar]
- 7.Joiner TE. Why people die by suicide. Harvard University Press; 2009. [Google Scholar]
- 8.American Psychiatric Association, author. Diagnostic and statistical manual of mental disorders, 5th edition (DSM-5) American Psychiatric Association; 2013. pp. 803–806. [DOI] [Google Scholar]
- 9.Kleindienst N, Bohus M, Ludäscher P, Limberger MF, Kuenkele K, Ebner-Priemer UW, et al. Motives for nonsuicidal self-injury among women with borderline personality disorder. J Nerv Ment Dis. 2008;196:230–236. doi: 10.1097/NMD.0b013e3181663026. [DOI] [PubMed] [Google Scholar]
- 10.Brown MZ, Comtois KA, Linehan MM. Reasons for suicide attempts and nonsuicidal self-injury in women with borderline personality disorder. J Abnorm Psychol. 2002;111:198–202. doi: 10.1037/0021-843X.111.1.198. [DOI] [PubMed] [Google Scholar]
- 11.Peng B, Li J, Liu H, Fang H, Zhao W, Chen G, et al. Childhood maltreatment, low serum cortisol levels, and non-suicidal self- injury in young adults with major depressive disorders. Front Pediatr. 2022;10:822046. doi: 10.3389/fped.2022.822046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JS, Kang ES, Bahk YC, Jang S, Hong KS, Baek JH. Exploratory analysis of behavioral impulsivity, pro-inflammatory cytokines, and resting-state frontal EEG activity associated with non-suicidal self-injury in patients with mood disorder. Front Psychiatry. 2020;11:124. doi: 10.3389/fpsyt.2020.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arikan MK, Gunver MG, Tarhan N, Metin B. High-gamma: A biological marker for suicide attempt in patients with depres-sion. J Affect Disord. 2019;254:1–6. doi: 10.1016/j.jad.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20:125–151. doi: 10.1016/0278-2626(92)90065-T. [DOI] [PubMed] [Google Scholar]
- 15.Davidson RJ, Schaffer CE, Saron C. Effects of lateralized presentations of faces on self-reports of emotion and EEG asymmetry in depressed and non-depressed subjects. Psychophy-siology. 1985;22:353–364. doi: 10.1111/j.1469-8986.1985.tb01615.x. [DOI] [PubMed] [Google Scholar]
- 16.Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. J Abnorm Psychol. 1991;100:535–545. doi: 10.1037/0021-843X.100.4.535. [DOI] [PubMed] [Google Scholar]
- 17.Tucker DM, Stenslie CE, Roth RS, Shearer SL. Right frontal lobe activation and right hemisphere performance: Decrement during a depressed mood. Arch Gen Psychiatry. 1981;38:169–174. doi: 10.1001/archpsyc.1981.01780270055007. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 19.Jin MJ, Jung W, Hyun MH, Lee SH. Effect of behavioral inhibition system and childhood emotional neglect on serotonergic activity, negative affect, and rejection sensitivity in non- clinical adults. PLoS One. 2018;13:e0207746. doi: 10.1371/journal.pone.0207746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YR, Park YM. Mismatch negativity and loudness dependence of auditory evoked potentials among patients with major depressive disorder, bipolar II disorder, and bipolar I disorder. Brain Sci. 2020;10:789. doi: 10.3390/brainsci10110789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demos JN. Getting started with neurofeedback. WW Norton & Company; 2005. p. 115. [Google Scholar]
- 22.Bauer LO, Hesselbrock VM. Lateral asymmetries in the frontal brain: Effects of depression and a family history of alcoholism in female adolescents. Alcohol Clin Exp Res. 2002;26:1662–1668. doi: 10.1111/j.1530-0277.2002.tb02468.x. [DOI] [PubMed] [Google Scholar]
- 23.Ricardo-Garcell J, González-Olvera JJ, Miranda E, Harmony T, Reyes E, Almeida L, et al. EEG sources in a group of patients with major depressive disorders. Int J Psychophysiol. 2009;71:70–74. doi: 10.1016/j.ijpsycho.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Jaworska N, Blier P, Fusee W, Knott V. α Power, α asymmetry and anterior cingulate cortex activity in depressed males and females. J Psychiatr Res. 2012;46:1483–1491. doi: 10.1016/j.jpsychires.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iznak AF, Iznak EV, Damyanovich EV, Oleichik IV. Differen-ces of EEG frequency and spatial parameters in depressive female adolescents with suicidal attempts and non-suicidal self-injuries. Clin EEG Neurosci. 2021;52:406–413. doi: 10.1177/1550059421991685. [DOI] [PubMed] [Google Scholar]
- 26.Tung S, Gupta R, Vitagliano H, Meyer FL. Neuropsychiatric complications of cancer and its treatments. In: Silbersweig D, Safar LT, Daffner KR, editors. Neuropsychiatry and behavioral neurology: Principles and practice. McGraw Hill Professional; 2020. pp. 692–693. [Google Scholar]
- 27.Pizzagalli DA, Nitschke JB, Oakes TR, Hendrick AM, Horras KA, Larson CL, et al. Brain electrical tomography in depression: The importance of symptom severity, anxiety, and melancholic features. Biol Psychiatry. 2002;52:73–85. doi: 10.1016/S0006-3223(02)01313-6. [DOI] [PubMed] [Google Scholar]
- 28.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 29.Kesebir S, Yosmaoglu A, Tarhan N. A dimensional approach to affective disorder: The relations between Scl-90 subdimen-sions and QEEG parameters. Front Psychiatry. 2022;13:651008. doi: 10.3389/fpsyt.2022.651008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weon HW, Byun YE, Lim HJ. Quantitative EEG (QEEG) analysis of emotional interaction between abusers and victims in intimate partner violence: A pilot study. Brain Sci. 2021;11:570. doi: 10.3390/brainsci11050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roh SC, Park EJ, Shim M, Lee SH. EEG beta and low gamma power correlates with inattention in patients with major depressive disorder. J Affect Disord. 2016;204:124–130. doi: 10.1016/j.jad.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald PJ, Watson BO. Gamma oscillations as a biomarker for major depression: An emerging topic. Transl Psychiatry. 2018;8:177. doi: 10.1038/s41398-018-0239-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellesdal L, Gjestad R, Johnsen E, Jørgensen HA, Oedegaard KJ, Kroken RA, et al. Borderline personality disorder and posttraumatic stress disorder at psychiatric discharge predict general hospital admission for self-harm. J Trauma Stress. 2015;28:556–562. doi: 10.1002/jts.22053. [DOI] [PubMed] [Google Scholar]