Abstract
Objectives
Sex‐specific disparities in morbidity and mortality of COVID‐19 illness are not well understood. Neutralizing antibodies (Ab) may protect against severe COVID‐19 illness. We investigated the association of sex with disease progression and SARS‐CoV‐2 Ab response.
Methods
In this exploratory analysis of the phase 3, multicenter, randomized, placebo‐controlled Convalescent Plasma in Outpatients (C3PO) trial, we examined whether sex was associated with progression to severe illness, defined as a composite of all‐cause hospitalization, emergency/urgent care visit, or death within 15 days from study enrollment. Patients had a positive severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) test, symptom onset within 7 days, stable condition for emergency department discharge, and were either ≥50 years old or had at least one high‐risk feature for disease progression. Patients received blinded convalescent plasma or placebo in a 1:1 fashion and were evaluated on days 15 and 30 after infusion. Blood samples were collected on day 0 (pre‐/post‐infusion), 15, and 30 to measure Ab levels with the Broad Institute using the Plaque Reduction Neutralization Test assay.
Results
Of 511 patients enrolled (median age 54 [Iinterquartile range 41–62] years, 46% male, 66% white, 20% black, 3.5% Asian), disease progression occurred in 36.7% of males and 25.9% of females (unadjusted risk difference 10.8%, 95% confidence interval [CI], 2.8–18.8%). Sex‐disparities did not persist when adjusted for treatment group, age, viremic status, symptom onset, and tobacco use (adjusted risk difference 5.6%, 95% confidence interval [CI], −2.2% to 13.4%), but were present in the subgroup presenting 3 or more days after symptom onset (adjusted risk difference 12.6%, 95% CI, 3.4% to 21.9%). Mean baseline Ab levels (log scale) available for 367 patients were similar between sexes (difference 0.19 log units, 95% CI, −0.08 to 0.46). The log‐scale mean increase from baseline to day 15 after adjusting for treatment assignment and baseline levels was larger in males than females (3.26 vs. 2.67). A similar difference was noted when the groups were subdivided by outcome.
Conclusions
Progression of COVID‐19 was similar in males and females when adjusted for age, tobacco use, and viremia status in this study. However, in the cohort presenting 3 or more days after symptom onset, COVID‐19 outcomes were worse in males than females. Neutralizing Ab levels increased more in males but did not correlate with sex differences in outcomes.
Keywords: C3PO, Covid‐19, outcomes, sex disparity
1. INTRODUCTION
1.1. Background
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) emerged in December 2019 and quickly became a pandemic. 1 , 2 As of August 2022, there were over 700 million cases and nearly 7 million deaths worldwide. 3
Current epidemiologic studies show an increased risk of morbidity and mortality for males with COVID‐19, compared to females. 4 , 5 , 6 , 7 The Global Health 50/50 research initiative, which tracks COVID‐19 sex‐disaggregated data, observed that while males and females have similar rates of infection, males are 20% more likely to be hospitalized, 70% more likely to be admitted to the ICU, and 30% more likely to die compared to females. 8 Similarly, in a multi‐national meta‐analysis of over 3 million cases, males had twice as many ICU admissions and 39% more deaths compared to females. 9 These findings are consistent with prior coronavirus outbreaks in 2002 and in 2012, in which males suffered higher rates of death. 10
1.2. Importance
Characterizing sex‐dependent differences in clinical outcomes provides insights into COVID‐19 pathophysiology and may contribute to the development of more effective interventions. Sex differences in outcome after SARS‐CoV‐2 infection could be related to viral targeting or to different immune responses between males and females. 11 Sex is associated with differential responses to vaccines and infections. 12 Potential mechanisms include sex hormones: testosterone is described to suppress immune response, while estrogen has both immune stimulating and suppressing effects. 13 , 14 , 15 Sex chromosome related differences have also been proposed where females, owning two copies of chromosome X, may have an advantage over males for having the ability to express proteins from one or both genes as needed. This is especially important since angiotensin‐converting enzyme 2 (ACE2) and toll‐like receptor genes, purported to play critical roles in COVID‐19 pathogenesis, are on X chromosomes. 16 , 17 Further, differences in cell‐surface proteins may facilitate or impede viral entry or cytokine‐mediated response, which ultimately activates B and T cells and determines the titer of neutralizing antibodies (Ab). 18 Anti‐viral Abs are proposed to be fundamental to surviving the infection 19 , 20 , 21 and provide passive immunity to SARS‐CoV‐2‐naïve patients.
1.3. Goals of this investigation
To better understand sex‐dependent differences in outcomes for SARS‐CoV‐2 infection, we conducted a secondary analysis of the COVID‐19 in Convalescent Plasma in Outpatients (C3PO) trial. 22 We assessed for sex differences in COVID‐19 disease progression and Ab response. We hypothesized that females would have a higher titer of neutralizing Ab compared to males, and that this difference in Ab titer would account for sex difference in COVID‐19 outcomes.
2. METHODS
2.1. Design
The C3PO clinical trial was a phase 3, multicenter, randomized, placebo‐controlled investigation designed to determine whether an infusion of convalescent plasma, in patients at high risk for severe COVID‐19, would prevent progression to severe illness. The primary outcome of C3PO was progression to severe illness within 15 days of enrollment, which was a composite of all‐cause hospital admission, emergency or urgent care visit, or death. The trial showed no difference in disease progression in those treated with convalescent plasma versus saline. A central institutional review board approved the trial protocol for all participating sites, and an independent medical safety monitor reviewed and adjudicated all serious adverse events. The design and primary study results have been reported. 22
2.2. Setting and selection of subjects
Patients were enrolled at 48 hospitals in 21 states across the United States. Eligibility criteria included a nucleic acid assay test confirming the presence of SARS‐CoV‐2 infection, onset of symptoms within 7 days, and clinical stability for discharge from the emergency department without new supplemental oxygen. Enrolled patients were at least 50 years of age or had at least one Centers for Disease Control and Prevention(CDC)‐defined risk factor for disease progression. These included hypertension, diabetes, coronary artery disease, chronic lung disease, chronic kidney disease, immunosuppression, sickle cell disease, obesity (body mass index [BMI] > 30), or any condition determined to be “high risk” as per CDC guidance. We excluded patients if they were younger than 18 years, prisoners or wards of the state, unable to complete follow up assessments, had a history of adverse reactions from blood‐product transfusion or received any blood product within the past 120 days, could not receive up to 250 mL of intravenous infusion, or had received another investigational treatment for COVID‐19 (including vaccination).
2.3. Intervention
Patients were randomized in a 1:1 ratio to receive ABO‐compatible COVID‐19 convalescent plasma or placebo (normal saline mixed with multivitamin to resemble the color of convalescent plasma). Convalescent plasma was collected from donors at least 14 days after clinical recovery from COVID‐19 as per FDA guidance. Patients completed an interview in person or by telephone at 15 and 30 days post‐investigational drug administration to identify subsequent medical care and adverse events. Blood samples were collected on days 0 (before and 1 h after infusion), 15, and 30.
2.4. Measures/outcomes
This study is an exploratory analysis in which we examine the association between sex and disease progression, and sex differences in neutralizing Ab levels at baseline and at day 15. Ab levels were measured by the Broad Institute (Cambridge, MA) using their Plaque Reduction Neutralization Test assay. Viremia status was categorized as “reactive” or “non‐reactive” based on transcription mediated amplification assay. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology 23 reporting guideline.
2.5. Statistical analysis
Statistical analyses employed SAS software, version 9.4 or higher (SAS Institute, Cary, NC). A generalized linear model with an identity link was used to examine the relationship between sex and disease progression, adjusting for treatment group and potential prognostic variables of outcome identified by univariate analysis and manual backwards selection based on model fit. Variables examined included age, race, ethnicity, tobacco use, and comorbidities (eg, type 2 diabetes, hypertension, and coronary artery disease). We report unadjusted and adjusted risk differences, with 95% confidence intervals. Ab assay results were positively skewed and so were transformed to a natural log scale for analyses. The effect of sex on mean change in Ab assay from baseline to day 15 was evaluated using a generalized linear model with adjustment for the pre‐infusion Ab value and treatment arm.
3. RESULTS
Of 511 patients enrolled, median age was 54 (interquartile range[IQR]: 41–62) years, 46% were male, 66% white, 20% black, and 3.5% Asian. Table 1 summarizes clinical features for male and female patients. Males tended to be older, more likely to use tobacco, and more likely to have coronary artery disease (CAD). Males were also more likely to be viremic at presentation (unadjusted risk difference of 10.5%, 95% CI, 2% to 19%). The cohorts had similar median number of COVID‐19 symptoms and risk factors for progression to severe disease, but males presented 1 day later than females after symptom onset (median time to presentation was 4 vs. 3 days, respectively).
TABLE 1.
Demographics, baseline characteristics, and outcome.
| Male (n = 237) | Female (n = 274) | |
|---|---|---|
| Age in years—median (interquartile range) a | 56 (46, 62) | 51 (37, 60) |
| Race—no. (%) | ||
| Asian | 3 (1.3) | 15 (5.5) |
| Black | 43 (18.1) | 60 (21.9) |
| Other | 24 (10.1) | 29 (10.6) |
| White | 167 (70.5) | 170 (62) |
| Ethnicity—no. (%) | ||
| Hispanic or Latino | 69 (29.1) | 87 (31.8) |
| Not Hispanic or Latino | 167 (70.5) | 182 (66.4) |
| Unknown | 1 (0.4) | 5 (1.8) |
| Eligibility risk factors—no. (%) | ||
| Age ≥ 50 a | 162 (68.4) | 148 (54) |
| BMI greater than or equal to 30 | 131 (55.3) | 171 (62.4) |
| Hypertension | 102 (43) | 114 (41.6) |
| Tobacco use (current or former) a | 82 (34.6) | 70 (25.5) |
| Diabetes mellitus | 74 (31.2) | 68 (24.8) |
| COPD or asthma | 48 (20.3) | 76 (27.7) |
| Coronary artery disease a | 32 (13.5) | 19 (6.9) |
| Immunosuppression | 21 (8.9) | 29 (10.6) |
| Chronic lung disease | 14 (5.9) | 17 (6.2) |
| Chronic kidney disease | 16 (6.8) | 12 (4.4) |
| Congestive heart failure | 13 (5.5) | 7 (2.6) |
| Currently pregnant | 0 (0) | 6 (3.2) |
| Organ transplant | 3 (1.3) | 2 (0.7) |
| Active cancer | 2 (0.8) | 2 (0.7) |
| Sickle cell | 1 (0.4) | 0 (0) |
| Number of eligibility risk factors—no. (%) | ||
| 1 | 50 (21.1) | 67 (24.5) |
| 2 | 60 (25.3) | 70 (25.5) |
| 3 or more | 127 (53.6) | 137 (50) |
| Other comorbidities—no. (%) | ||
| Alcohol abuse (current or former) | 19 (8) | 17 (6.2) |
| Drug abuse (current or former) | 21 (8.9) | 14 (5.1) |
| Thromboembolic disorder | 10 (4.2) | 15 (5.5) |
| Liver disease | 10 (4.2) | 8 (2.9) |
| Other hematologic disorders | 4 (1.7) | 13 (4.7) |
| Number of symptoms at baseline—median (interquartile range) | 5 (4, 7) | 6 (4,8) |
| Post‐infection characteristics | ||
| Symptom duration prior to randomization (day)—median (interquartile range) | 4 (2, 6) | 3 (2, 5) |
| Time from randomization to infusion start (min)—median (interquartile range) | 80 (61, 109) | 81 (64, 111) |
| Viremia (denominator indicates those with viral data available) a | 162/233 (70) | 153/259 (59) |
| Outcome | ||
| Composite outcome a —Disease progression within 15 days | 87 (36.7) | 71 (25.9) |
| Outcome components | ||
| Seeking emergency or urgent care | 24 (10.1) | 26 (9.5) |
| Hospital admission for any reason | 63 (26.6) | 44 (16.1) |
| Death without hospitalization | 0 (0) | 1 (0.4) |
ap‐values < 0.05 (age: p = 0.002; age ≥ 50: p = 0.001; tobacco: p = 0.026; CAD: p = 0.01; viremia: p = 0.02; outcome: p = 0.001). Abbreviation: CAD, coronary artery disease.
Progression to severe disease occurred in 31% of patients, consisting of 36.7% of males (87 out of 237) and 25.9% of females (71 out of 274) with an unadjusted risk difference of 10.8% (95%CI, 2.8% to 18.8%). Figure 1 illustrates the risk of disease progression between males and females by subgroup. The risk difference between sexes was smaller when adjusted for baseline comorbidities (age, tobacco use; risk difference of 9.3%, 95% CI, 1.3% to 17.4%) and mitigated further when adjusted for baseline comorbidities and post‐infection mediators (treatment group, viremia status, symptom onset; risk difference 5.6%, 95% CI, −2.2% to 13.4%, adjusted relative risk 1.3, 95% CI, 0.9 to 1.7). The model was not affected by CAD status.
FIGURE 1.
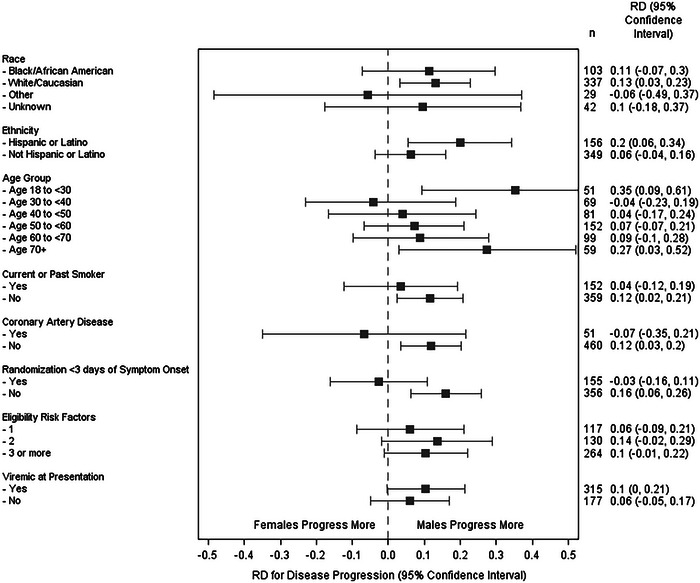
Subgroup analysis of disease progression between males and females. RD, risk difference.
A statistical interaction was identified between sex and symptom onset, regardless of symptom onset being treated as a continuous or categorical variable. The adjusted risk difference for those presenting 3 or more days after symptom onset was 12.6% (95% CI, 3.4% to 21.9%), while those presenting less than 3 days was −9.6% (95% CI, −26.5% to 7.3%). Males had a higher risk of disease progression across the oldest and youngest age groups (Figure 1).
Baseline and day 15 Ab levels were available for 367 of the randomized patients. Mean baseline levels were similar between males and females 4.24 (95% CI, 4.05 to 4.43) vs. 4.05 (95% CI, 3.86 to 4.24) (difference, 0.19, 95% CI, −0.08 to 0.46). However, at day 15, after adjusting for baseline levels and treatment assignment, the log‐scale mean increase from baseline was 3.26 (95% CI, 3.07 to 3.44) for males and 2.67 (95% CI, 2.49 to 2.85) for females. A similar difference was noted when groups were subdivided by outcomes (Figure 2A,B), with males having a larger increase in Ab levels than females, both among those who did and those who did not progress to the primary outcome at day 15. The differences between sex at baseline, and the change in Ab levels, were similar across age groups (Figure 3A,B).
FIGURE 2.
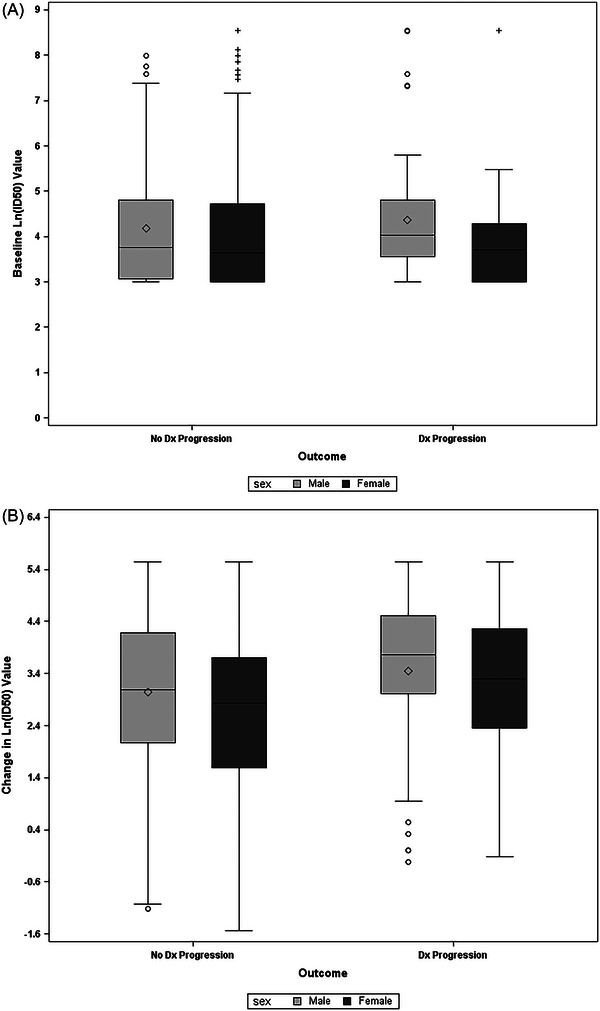
Antibody levels at (A) baseline and (B) change from baseline on day 15. Dx, disease; Ln, natural log.
FIGURE 3.
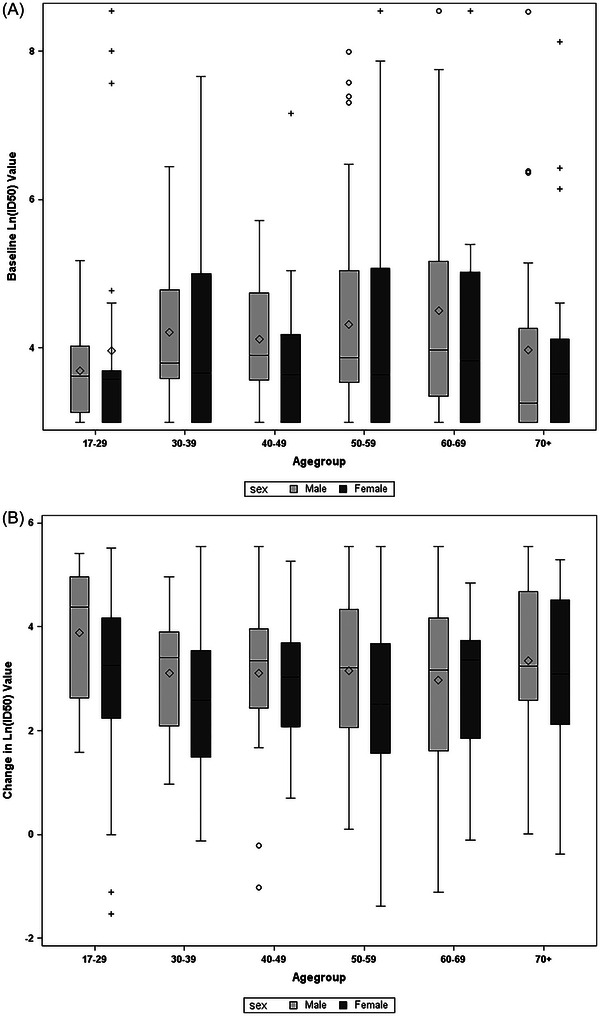
Antibody level stratified by age and sex: (A) baseline and (B) change from baseline on day 15.
Differences in the primary outcome between sexes in the subgroup that presented 3 or more days persisted when either the baseline Ab levels or change in Ab levels from baseline to day 15 were included in the model that also adjusted for baseline comorbidities (age and tobacco use) and post‐infection mediators (treatment group and viremia status).
4. LIMITATIONS
Our findings have limitations. First, the primary outcome of disease progression was a composite outcome of all‐cause emergency department or urgent care visits, hospitalization, and death, hence some of these events may have been due to baseline comorbid conditions and not COVID‐19 disease progression. Second, even though our comparative populations were relatively similar, undetected comorbid differences may have biased the results. Additionally, with numerically small numbers in each comorbid subset (e.g., there were only two males and two females, with active cancer), an underpowering of their representation may have resulted in an inability to detect a sex‐based outcome difference. Lastly, the difference in sex‐specific outcomes may be related to socioeconomic factors and these data were not captured in our clinical trial. For instance, being unhoused, having poor social support and transportation problems may lead to delayed presentation, and those could all influence poorer outcomes.
5. DISCUSSION
In this exploratory analysis of the C3PO study, a multicenter, randomized, placebo‐controlled trial to determine the effect of convalescent plasma on disease progression of COVID‐19, we found that males progressed to severe disease more frequently than females, with a 10.8% higher absolute risk of the primary outcome, even though the baseline Ab levels were similar. However, when adjusted for baseline comorbidities and post‐infection mediators, the difference only persisted in the subgroup that presented 3 or more days from symptom onset. Furthermore, we found that the rise in Ab level was higher in males, regardless of outcomes.
To our knowledge, this is the first study examining sex differences in Ab rise after SARS‐CoV‐2 infection and the association with disease progression. Although unadjusted studies have reported up to three times 24 , 25 mortality difference between males and females, 26 studies with adjusted risk analysis have seen a persistent but smaller difference. 27 , 28 In particular, a study by Vahidy et al., of 14,992 positive cases of COVID‐19 with 4785 hospitalizations, found an increased positivity rate (adjusted odds ration[aOR] 1.39) and a higher proportion of serious illness (aOR 1.31) in males after adjusting for demographics and comorbid conditions. In our study, we found that adjustment for baseline comorbidities and post‐infection mediators (i.e., viremia status) further mitigated the sex‐related differences in the composite outcome (emergency department visits, hospitalizations, and mortality). On average, males were 5 years older, with a 12% higher rate of tobacco use and 10.5% higher incidence of viremia and presented 1 day later than females. However, outcome differences persisted between males and females who presented 3 or more days after symptom onset.
Several mechanisms have been proposed to explain the disparity in sex‐specific outcomes. These include differences in baseline comorbidities, risky social behaviors (e.g., noncompliance with handwashing and wearing masks, smoking), and innate biological differences (e.g., protective effect of estrogen in females vs. high concentration of ACE2 receptors and thus high SARS‐CoV2 load in males). 29 , 30 , 31 Our cohort had similar baseline comorbidities except for age, tobacco use, and incidence of CAD (all higher in males). When adjusted for these differences, the outcomes were similar in males and females. Moreover, even though both males and females presented with a similar number of symptoms, the incidence of viremia was higher in males than females. When adjusted for viremia status, the difference in outcomes between males and females was further mitigated. Despite these considerations, we found that the association between sex and outcomes persisted among patients who presented 3 or more days after symptom onset signifying that there may be undetected mediators that require further investigation.
Sex hormones can play an important role in the immune response, and thus are hypothesized to contribute to the disparity in COVID‐19 outcomes. Although all humans produce estrogen, progesterone, and testosterone, the amount is variable based on biological sex, age, and comorbidities. 32 Estrogen can be immune stimulating or suppressing based on the circulating level, and it is proposed to play an important part in COVID‐19 outcome disparities. The release of inflammatory cytokines, and the subsequent cytokine storm that is well described in COVID‐19, is strongly associated with disease severity. 33 , 34 Estrogen has been considered as potential mediator due to its ability to modulate innate and adaptive immunity. 35 Importantly, estrogen levels can be variable based on age in females. Finally, in a large population‐based study, estrogen exposure (endogenous or in oral contraceptive form) was found to have a protective effect against Covid‐19 symptoms and hospitalizations. 36 However, in this study, the disease progression was uniform across age groups in females, even in their seventh decade of life when estrogen is known to be lower. Since our study did not aim to evaluate the effect of estrogen, we may not have collected all relevant parameters (e.g., menopausal state, exogenous estrogen use, and hormone replacement therapy) or powered it adequately. Nevertheless, it is worth noting that in this controlled trial the protective effect of estrogen in younger females was not appreciated and more studies are needed to understand the effect of estrogen.
Lastly, neutralizing Ab titer in response to an infection is the cornerstone of host defense. 37 The rapid rise in the SARS‐CoV‐2 infection rate, and its subsequent associated mortality, are thought to be the result of limited prior exposure to the virus. Thus, Ab levels pre‐ and post‐COVID‐19 infections are presumed to be important prognosticators of the disease progression. These theories are the basis for “immunity passports”, where a certificate showing positive detection of antibodies allows isolation or quarantine avoidance. The concept assumes that patients with high Ab levels are protected against re‐infection, and thus transmission as well as contracting severe disease. However, neutralizing Ab levels are dependent on several factors including sex, age, and severity of illness and maybe difficult to interpret. 38 , 39 We found a high level of neutralizing Ab in men, consistent with prior studies; however, we did not observe any protective effect. Our findings suggest that Ab levels do not explain the full effect of COVID‐19 disease severity, and that the effect of sex needs to be investigated further.
COVID‐19 affected males and females similarly after adjusting for baseline comorbidities, treatment with convalescent plasma, and viremia in this study. Males are affected more than females only if presenting 3 or more days after symptom onset. While males had higher titers of neutralizing antibodies at symptom onset, and a greater rise in Ab level as a response to infection, these were not associated with better outcomes. Ultimately, more studies are needed to fully understand the sex disparity in COVID‐19 outcomes.
ACKNOWLEDGMENTS
We acknowledge the SIREN‐C3PO Investigators for the hard work and dedication to this trial. The research was, in part, funded by the National Institutes of Health (NIH) Agreement 1OT2HL156812 through the National Heart, Lung, and Blood Institute (NHLBI) CONNECTS program under awards U24NS10065 and U24NS100655. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the official policies, either expressed or implied, of the NIH.
Biography
Zubaid Rafique, MD, is an Emergency Medicine physician at Henry J N Taub General Hospital and educator and researcher at Baylor College of Medicine in Houston, Texas.

Rafique Z, Durkalski‐Mauldin V, Peacock WF, Yadav K, Reynolds JC, Callaway CW. Sex‐specific disparities in COVID‐19 outcomes. JACEP Open. 2024;5:e13110. 10.1002/emp2.13110
REFERENCES
- 1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. doi: 10.1056/nejmoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. WHO Coronavirus (COVID‐19) Dashboard. WHO coronavirus (COVID‐19) dashboard with vaccination data . Accessed June 21, 2023. https://covid19.who.int/
- 4. Gebhard C, Regitz‐Zagrosek V, Neuhauser HK, Morgan R, Klein SL. Impact of sex and gender on COVID‐19 outcomes in Europe. Biol Sex Differ. 2020;11(1):1‐13. doi: 10.1186/S13293-020-00304-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gargaglioni LH, Marques DA. Let's talk about sex in the context of COVID‐19. J Appl Physiol. 2020;128(6):1533‐1538. doi: 10.1152/JAPPLPHYSIOL.00335.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meng Y, Wu P, Lu W, et al. Sex‐specific clinical characteristics and prognosis of coronavirus disease‐19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16(4):e1008520. doi: 10.1371/journal.ppat.1008520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leung GM, Hedley AJ, Ho LM, et al. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: An analysis of all 1755 patients. Ann Intern Med. 2004;141(9):662–673. https://annals.org [DOI] [PubMed] [Google Scholar]
- 8.Global Health 50/50. The sex, gender and COVID‐19 project | Accessed June 21, 2023. https://globalhealth5050.org/the‐sex‐gender‐and‐covid‐19‐project/
- 9. Peckham H, de Gruijter NM, Raine C, et al. Male sex identified by global COVID‐19 meta‐analysis as a risk factor for death and ITU admission. Nat Commun. 2020;11(1):6317. doi: 10.1038/s41467-020-19741-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu L, Zhong W, Bian Z, et al. A comparison of mortality‐related risk factors of COVID‐19, SARS, and MERS: a systematic review and meta‐analysis. J Infect. 2020;81(4):e18‐e25. doi: 10.1016/J.JINF.2020.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mauvais‐Jarvis F, Merz NB, Barnes PJ, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. 2020;396:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID‐19 outcomes. Nat Rev Immunol. 2020;20(7):442‐447. doi: 10.1038/s41577-020-0348-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rettew JA, Huet‐Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll‐like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod. 2008;78:432‐437. doi: 10.1095/biolreprod.107.063545 [DOI] [PubMed] [Google Scholar]
- 14. Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521‐574. doi: 10.1210/er.2007-0001 [DOI] [PubMed] [Google Scholar]
- 15. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313‐3318. doi: 10.1210/jc.2003-031069 [DOI] [PubMed] [Google Scholar]
- 16. Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V. COVID‐19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. might the double X‐chromosome in females be protective against SARS‐CoV‐2 compared to the single X‐chromosome in males? Int J Mol Sci. 2020;21:3474. doi: 10.3390/ijms21103474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Y, Jerkic M, Slutsky AS, Zhang H. Molecular mechanisms of sex bias differences in COVID‐19 mortality. Critical Care. 2020;24(1):405. doi: 10.1186/s13054-020-03118-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adli A, Rahimi M, Khodaie R, Hashemzaei N, Hosseini SM. Role of genetic variants and host polymorphisms on COVID‐19: from viral entrance mechanisms to immunological reactions. J Med Virol. 2022;94(5):1846‐1865. doi: 10.1002/JMV.27615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS‐CoV‐2 infection persist for months. Science (1979). 2020;370(6521):1227‐1230. doi: 10.1101/2020.06.28.20142190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Favresse J, Gillot C, Di Chiaro L, et al. Neutralizing antibodies in COVID‐19 patients and vaccine recipients after two doses of BNT162b2. Viruses. 2021;13(7). doi: 10.3390/V13071364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lucas C, Klein J, Sundaram ME, et al. Delayed production of neutralizing antibodies correlates with fatal COVID‐19. Nat Med. 2021;27(7):1178‐1186. doi: 10.1038/s41591-021-01355-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korley FK, Durkalski‐Mauldin V, Yeatts SD, et al. Early convalescent plasma for high‐risk outpatients with covid‐19. N Engl J Med. 2021;385(21):1951‐1960. doi: 10.1056/nejmoa2103784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495‐1499. doi: 10.1016/J.IJSU.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 24. Dio Stadio A, Ricci G, Greco A, Vincentiis M, Ralli M. Mortality rate and gender differences in COVID‐19 patients dying in Italy: a comparison with other countries. Eur Rev Med Pharmacol Sci. 2020;24(8):4066‐4067. doi: 10.1056/NEJMoa2004500 [DOI] [PubMed] [Google Scholar]
- 25. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846‐848. doi: 10.1007/s00134-020-05991-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tejpal A, Gianos E, Cerise J, et al. Sex‐based differences in COVID‐19 outcomes. J Womens Health (Larchmt). 2021;30(4):492‐501. doi: 10.1089/jwh.2020.8974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vahidy FS, Pan AP, Ahnstedt H, et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: cross‐sectional analysis from a diverse US metropolitan area. PLoS One. 2021;16(1 January):e0245556. doi: 10.1371/journal.pone.0245556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID‐19 disease outcomes: overview of the study design. Nature. 2020;588(7837):315‐320. doi: 10.1038/s41586-020-2700-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Walter LA, Mcgregor AJ. Sex‐and gender‐specific observations and implications for COVID‐19. West J Emerg Med. 2020;21(3):507‐509. doi: 10.5811/westjem.2020.4.47536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elgendy IY, Pepine CJ. Why are women better protected from COVID‐19: clues for men? Sex and COVID‐19. Int J Cardiol. 2020;315:105‐106. doi: 10.1016/j.ijcard.2020.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mauvais‐Jarvis F, Klein SL, Levin ER. Estradiol, progesterone, immunomodulation, and COVID‐19 outcomes. Endocrinology. 2020;161(9):1‐8. doi: 10.1210/endocr/bqaa127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu X, Han M, Li T, et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci U S A. 2020;117(20):10970‐10975. doi: 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63‐69. doi: 10.1016/j.cellimm.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Costeira R, Lee KA, Murray B, et al. Estrogen and COVID‐19 symptoms: associations in women from the COVID symptom study. PLoS One. 2021;16(9 September):e0257051. doi: 10.1371/journal.pone.0257051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yan LN, Liu PP, Li XG, et al. Neutralizing antibodies and cellular immune responses against SARS‐CoV‐2 sustained one and a half years after natural infection. Front Microbiol. 2022;12. doi: 10.3389/fmicb.2021.803031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Karuna S, Li SS, Grant S, et al. Neutralizing antibody responses over time in demographically and clinically diverse individuals recovered from SARS‐CoV‐2 infection in the United States and Peru: a cohort study. PLoS Med. 2021;18(12):e1003868. doi: 10.1371/JOURNAL.PMED.1003868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schlickeiser S, Schwarz T, Steiner S, et al. Disease severity, fever, age, and sex correlate with SARS‐CoV‐2 neutralizing antibody responses. Front Immunol. 2021;11. doi: 10.3389/fimmu.2020.628971 [DOI] [PMC free article] [PubMed] [Google Scholar]


