Alopecia areata (AA) is a chronic, relapsing, immune-mediated disease affecting the hair follicles and resulting in non-scarring hair loss. Immune privilege mechanisms protect hair follicles from undesirable immune responses (1). Thus, the collapse of hair follicle immune privilege mediated by T cells in the anagen hair bulb is considered a key factor of AA pathogenesis. AA can lead to unpredictable hair loss, ranging from bald patches to complete hair loss, and imposes an immense psychosocial burden on patients (2). Some AA patients experience spontaneous hair regrowth, while others require treatment, including topical corticosteroid therapy, cryotherapy, phototherapy, intralesional corticosteroid therapy, and contact immunotherapy (1). Moreover, spontaneous remission is rarely observed in patients with alopecia totalis (AT, loss of entire scalp hair) or alopecia universalis (AU, loss of entire body hair). The overall rate of any regrowth and complete regrowth among patients with AA treated with immunotherapy was reportedly 65.5% and 32.3%, respectively (3). Therefore, there is currently an unmet need for treatments that can induce a durable response in moderate to severe AA.
The Janus kinases (JAKs), which are comprised of JAK1, JAK2, JAK3, and TYK2, are a family of intracellular tyrosine kinases (4). Because JAKs play a role in intracellular signalling of cytokines involved in AA immunopathogenesis, JAK inhibitors are garnering attention as a therapeutic target in moderate to severe AA (5). Baricitinib, a selective JAK 1/2 inhibitor, has been successfully used in phase II/III clinical trials to treat AA patients with a Severity of Alopecia Tool (SALT) score of 50 or higher and current AA lasting more than 6 months to less than 8 years (6). However, there are limited real-life data on JAK inhibitor use as a treatment for AA (7–10). Therefore, the current, multicentric, retrospective case series analysis of AA patients treated with baricitinib aimed to evaluate the effectiveness and safety of baricitinib as a treatment for moderate to severe AA.
MATERIALS AND METHODS
This study retrospectively reviewed medical record data, including age, sex, age at AA onset, duration of current episode of AA, AA subtype, prior treatments, nail involvement, trichoscopic findings (hair and scalp dermoscopy), and complications, in 95 Japanese patients with moderate to severe AA receiving baricitinib 4 mg between June 2022 and February 2023. Patients who had lost approximately 50% of their scalp hair for longer than 5 months were enrolled. Similarly, in a previous study hair loss was scored using SALT, with a SALT score of 20 or less (i.e., 80% or more scalp hair coverage) at weeks 12, 24, and 36 being considered a significant treatment outcome (11). The percentage of eyebrow and eyelash hair loss (patients with eyebrow and eyelash hair loss/all patients) at week 36 was then calculated (6). The χ2 test was used to determine statistical significance. The study was approved by the university’s ethics committee [approval code: T2023-0040].
RESULTS
The mean patient age was 38.7 years (range 18–65 years). The sex ratio was 1.64, with a predominance of female patients (36 males and 59 females). The mean age at AA onset was 19.3 years, and the mean duration of current episode of AA at baseline was 7.8 years. AA presented in several patterns, including patchy hair loss (patchy AA; n = 17), AT (n = 12), AU (n = 58) or a band-like distribution around the scalp (ophiasis pattern; n = 8). The median baseline SALT score was 89.2. Eyebrow and eyelash hair loss was observed in 71 and 59 patients, respectively. Previous treatments included topical corticosteroid (n = 2), intralesional corticosteroid (n = 4), oral corticosteroid (n = 3), dupilumab (n = 5), contact immunotherapy (n = 44), and phototherapy (n = 10). Twenty-seven, non-treated patients were enrolled > 6 months before the initiation of baricitinib therapy as well. Nail involvement, such as pitting and trachyonychia, was observed in 36 patients.
The percentage of patients in the entire cohort who achieved a SALT score of 20 or less at week 12, 24, and 36 was 6.4% (6/94), 35.4% (28/79), and 46.7% (21/45), respectively (Fig. 1a). The complete response rate (SALT 0) at week 24 and 36 was 1.3% (1/79) and 6.7% (3/45), respectively. Among these, the percentage of patients with patchy AA, AT/AU or the ophiasis subtype who achieved a SALT score of 20 or less at week 36 was 75.0%, 48.0%, and 75.0%, respectively (Fig. 1b–d). The percentage of patients with current AA of less than 4 years’ duration and a SALT score of 20 or lower at week 36 was greater than that of patients with AA of 4 years’ duration or longer (Fig. 1e). The percentage of patients with nail involvement who achieved a SALT score of 20 or less at week 36 was comparable to that of patients with no-nail involvement (Fig. 1f). The percentage of patients with black dot, broken hairs, and/or vellus hairs on trichoscopy who achieved a SALT score of 20 or less at week 24 differed significantly from that of patients with yellow dot and/or vacant follicular ostia on trichoscopy in AU or AT patients (Fig. 1g). The percentage of patients with eyebrow and eyelash hair loss at week 36 was 42.2% (19/45) and 37.8% (17/45), respectively. Infectious complications occurred in 6 patients during the initial 12 weeks. Herpes simplex and COVID-19 (SARS-CoV-2) occurred in 1 and 5 patients, respectively. No severe complications occurred during the entire 36-week course.
Fig. 1.
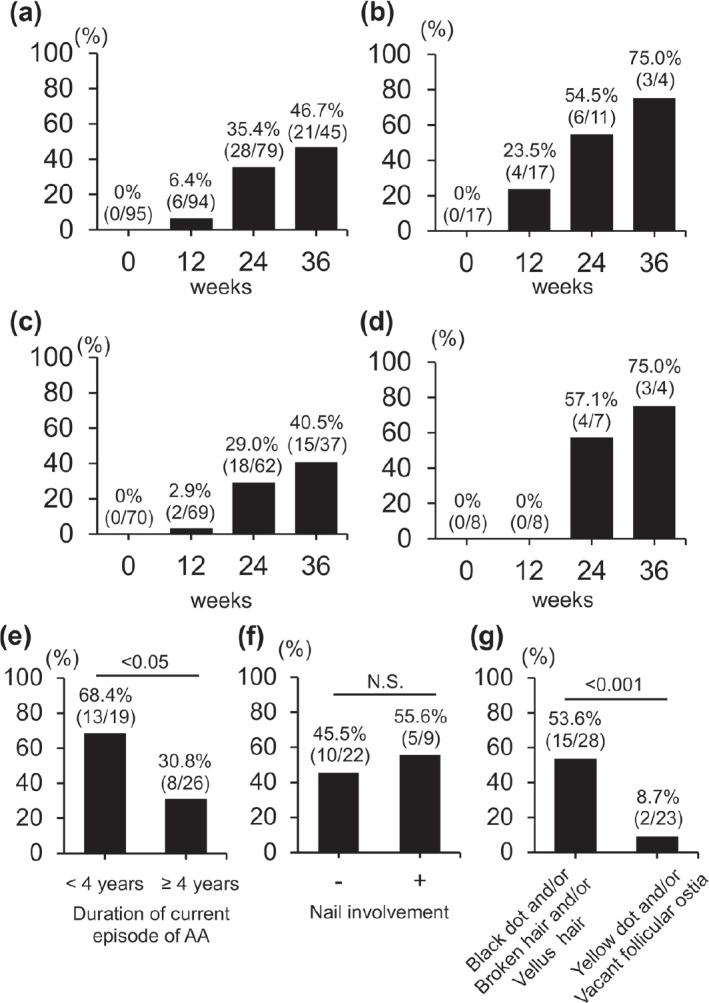
Percentage of patients with a Severity of Alopecia Tool (SALT) score of 20 or less. (a) SALT score of all the alopecia areata patients at weeks 0, 12, 24, and 36. (b) Scores of patchy alopecia areata patients. (c) Scores of alopecia totalis and alopecia universalis patients. (d) Score of ophiasis subtype alopecia areata patients. (e) Scores of patients with current AA < 4 years or ≥ 4 years at week 36. (f) Scores of patients with and without nail involvement at week 36. (g) Scores of alopecia totalis and alopecia universalis patients with trichoscopy findings at week 24.
DISCUSSION
Previous, real-life studies (7–10) and 1 clinical trial (6) revealed that approximately 40–50% of the AA patients achieved a SALT score of 20 or less at week 36, in line with the findings of the current study (Fig. 1a). Moreover, as previously described in another study (12), the current study found a shorter mean disease duration and current AA in the group with significant regrowth receiving baricitinib than in the group with no or minimal regrowth group (Fig. 1e). The percentage of nail involvement was higher among AU patients than among alopecia areata focalis or AT patients (13). Thus, baricitinib was thought to be unable to produce improvements in AA patients with nail involvement, but the percentage of patients in this group with a SALT score of 20 or less was comparable to that of the patients with no nail involvement (Fig. 1f). Black dot, broken hairs, and vellus hairs are highly specific to AA and correlate positively with disease activity. On the other hand, in the current study, the percentage of patients with these findings on trichoscopy before baricitinib treatment who achieved a SALT score of 20 or less was higher than the percentage of AU and AT patients with yellow dot and vacant follicular ostia (Fig. 1g). Based on these findings, confirmation of the presence of black dot, broken hairs or vellus hairs may predict a favourable treatment outcome. No severe adverse events were observed in the current study. The benefits and risks of baricitinib therapy were carefully explained to the patients before starting treatment prior to enrolment in this study. Thus, this process might have excluded elderly and/or high-risk patients and led to a greater proportion of favourable outcomes. In conclusion, trichoscopic findings, such as black dots, broken hairs, and vellus hairs, are usually regarded as indicators of AA activity. However, these, rather than yellow dots or empty follicles, are predictors of a favorable clinical outcome at the start of baricitinib treatment.
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Fukuyama M, Ito T, Ohyama M. Alopecia areata: current understanding of the pathophysiology and update on therapeutic approaches, featuring the Japanese Dermatological Association guidelines. J Dermatol 2022; 49: 19–36. [DOI] [PubMed] [Google Scholar]
- 2.Harries M, Macbeth AE, Holmes S, Chiu WS, Gallardo WR, Nijher M, et al. The epidemiology of alopecia areata: a population-based cohort study in UK primary care. Br J Dermatol 2022; 186: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, Kim BJ, Lee YB, Lee WS. Hair regrowth outcomes of contact immunotherapy for patients with alopecia areata: a systematic review and meta-analysis. JAMA Dermatol 2018; 154: 1145–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther 2021; 6: 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang EHC, Sallee BN, Tejeda CI, Christiano AM. JAK inhibitors for treatment of alopecia areata. J Invest Dermatol 2018; 138: 1911–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King B, Ohyama M, Kwon O, Zlotogorski A, Ko J, Mesinkovska NA, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med 2022; 386: 1687–1699. [DOI] [PubMed] [Google Scholar]
- 7.De Greef A, Thirion R, Ghislain PD, Baeck M. Real-life effectiveness and tolerance of baricitinib for the treatment of severe alopecia areata with 1-year follow-up data. Dermatol Ther (Heidelb) 2023; 13: 2869–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhan J, Cao J, Chen F, Jin Y, Huang C. Real-data on the use of baricitinib in adolescents with severe alopecia areata. J Eur Acad Dermatol Venereol 2023; 10.1111/jdv.19121. [DOI] [PubMed] [Google Scholar]
- 9.Moussa A, Eisman S, Sinclair RD, Bhoyrul B. Treatment of alopecia areata of the beard with baricitinib. J Am Acad Dermatol 2023; 88: 948–950. [DOI] [PubMed] [Google Scholar]
- 10.Gargiulo L, Ibba L, Vignoli CA, Ferrucci SM, Mercuri SR, Malagoli P, et al. Effectiveness and safety of baricitinib in patients with severe alopecia areata: a 36-week multicenter real-world experience. J Dermatolog Treat 2023; 34: 2268764. [DOI] [PubMed] [Google Scholar]
- 11.Olsen EA, Hordinsky MK, Price VH, Roberts JL, Shapiro J, Canfield D, et al. Alopecia areata investigational assessment guidelines – Part II. National Alopecia Areata Foundation. J Am Acad Dermatol 2004; 51: 440–447. [DOI] [PubMed] [Google Scholar]
- 12.Piraccini BM, Ohyama M, Craiglow B, Bewley A, Ding Y, Chen YF, et al. Scalp hair regrowth is associated with improvements in health-related quality of life and psychological symptoms in patients with severe alopecia areata: results from two randomized controlled trials. J Dermatolog Treat 2023; 34: 2227299. [DOI] [PubMed] [Google Scholar]
- 13.Roest YBM, van Middendorp HT, Evers AWM, van de Kerkhof PCM, Pasch MC. Nail involvement in alopecia areata: a questionnaire-based survey on clinical signs, impact on quality of life and review of the literature. Acta Derm Venereol 2018; 98: 212–217. [DOI] [PubMed] [Google Scholar]


