Abstract
Enteropathogenic Escherichia coli (EPEC) induces formation of actin pedestals in infected host cells. Agents that inhibit the activity of Rho, Rac, and Cdc42, including Clostridium difficile toxin B (ToxB), compactin, and dominant negative Rho, Rac, and Cdc42, did not inhibit formation of actin pedestals. In contrast, treatment of HeLa cells with ToxB inhibited EPEC invasion. Thus, Rho, Rac, and Cdc42 are not required for assembly of actin pedestals; however, they may be involved in EPEC uptake by HeLa cells.
We investigated the roles of Rho, Rac, and Cdc42 in enteropathogenic Escherichia coli (EPEC)-induced pedestal formation and in EPEC invasion. To do so, we applied three different treatments that inhibit the activity of these small GTP-binding proteins. The first method was to utilize compactin; compactin competitively inhibits hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase, which catalyzes the reduction of HMG-CoA to mevalonic acid. The latter serves as a precursor for the biosynthesis of isoprenyl. Since small GTP-binding proteins require isoprenylation of CAAX sequences at their C termini, compactin treatment causes inactivation of these of GTP-binding proteins (reference 1 and references therein). The second method was to utilize Clostridium difficile toxin B (ToxB). ToxB catalyzes the glucosylation of Rho, Rac, and Cdc42 at specific sites, causing their inactivation (5). The third method was to use HeLa cells transiently expressing dominant negative mutants of Rho, Rac, and Cdc42 (reference 10 and references therein).
HeLa cells were incubated with Dulbecco minimal essential medium supplemented with either compactin (50 μM for 18 h) (Sigma) or ToxB (10 ng/ml for 3 h) and infected with wild-type EPEC E2348/69 (2) or the EPEC JPN15 strain (4). The effects of ToxB and compactin on several parameters were examined; these included (i) the viability of HeLa cells; (ii) the efficiency of attachment of wild-type EPEC and JPN15 to HeLa cells; (iii) the formation of EPEC-induced actin pedestals, and (iv) the morphology of the actin pedestals. The JPN15 strain was used because, in contrast to wild-type EPEC, it is incapable of aggregating and forming microcolonies on the surfaces of host cells. However, like wild-type EPEC, it is still capable of inducing the formation of actin pedestals (8). The lack of aggregated microcolonies on the surfaces of JPN15-infected cells enabled the quantification of actin pedestals per infected HeLa cell and allowed examination of the morphology of individual pedestals.
Compactin, under the conditions used in our experiments, caused only a small decrease in the viability of HeLa cells and in the attachment of wild-type EPEC and JPN15 to HeLa cells (data not shown). ToxB treatment did not reduce HeLa cell viability or bacterial attachment. In fact, we observed consistently a slight increase in the viability of HeLa cells and in the attachment levels of JPN15 in ToxB-treated cells (data not shown). ToxB and compactin were not toxic to EPEC, as determined by comparisons of growth rates of treated and untreated wild-type and JPN15 strains (data not shown).
HeLa cells treated with compactin or ToxB rounded up, and actin structures were disrupted (Fig. 1 and 2; also data not shown). In contrast, compactin and ToxB did not block the formation of JPN15-induced actin pedestals (Fig. 1 and 2). The average numbers of actin pedestals per infected HeLa cell were similar in cells treated with compactin or ToxB or in untreated cells (Fig. 3A). Relatively high standard deviations were obtained in these experiments (Fig. 3A). This is because of uneven distributions of pedestals among HeLa cells due to the tendency of EPEC to form microcolonies on the surfaces of infected cells. Similar results were obtained with wild-type EPEC (data not shown). Pedestal formation was not observed when an EPEC sepA mutant deficient in signaling for pedestal formation (7) was used instead of the wild-type strain (data not shown). This confirms that pedestal formation in ToxB- or compactin-treated HeLa cells requires EPEC-mediated signaling.
FIG. 1.
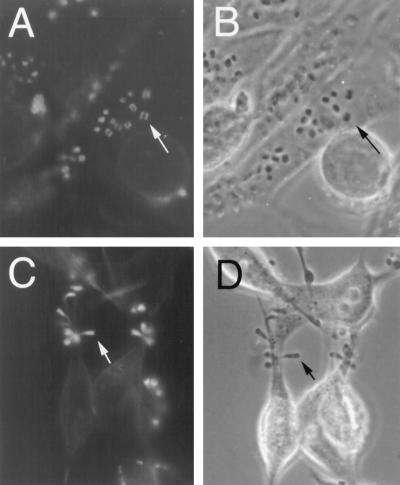
Formation of actin pedestals in HeLa cells treated with compactin. Untreated HeLa cells (A and B) or cells treated for 18 h with 50 μM compactin (C and D) were infected with JPN15 for 2 h. The infected cells were fixed and stained with phalloidin-rhodamine, and the fluorescent images (A and C), as well as the corresponding phase-contrast images (B and D), were photographed. Arrows indicate the EPEC-induced actin structures (A and C) and the corresponding bacteria above this structure (B) or at the tip of the actin pedestal (D). All images represent only one optical plane focusing on some of the EPEC-induced actin pedestals. The actin stress fibers in panel A are layered in a different optical plane and thus cannot be seen.
FIG. 2.
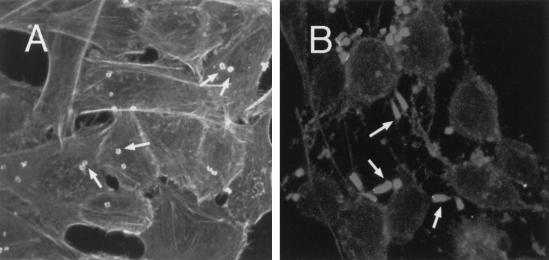
Formation of actin pedestals in HeLa cells treated with ToxB. Untreated HeLa cells (A) or cells treated with ToxB (10 ng/ml) for 3 h (B) were infected with JPN15 for 2 h. Then, cells were fixed and stained with phalloidin-rhodamine and analyzed by confocal microscopy. The cells were scanned at 0.5-μm intervals, and all optical sections were projected to form one image. The flat EPEC-induced actin structures in panel A and the elongated actin pedestals in panel B are indicated by arrows. The untreated cells are rich in stress fibers (A) that disappeared after treatment with ToxB (B).
FIG. 3.
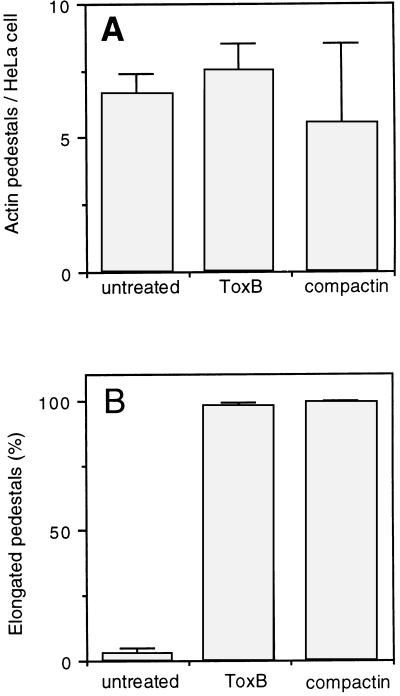
Formation of actin pedestals in HeLa cells treated with compactin or ToxB. The microscopic preparations described in the legends to Fig. 1 and 2 were used to quantify the formation of actin pedestals in infected HeLa cells (A), including untreated HeLa cells (n = 320), cells treated with compactin (n = 60), and cells treated with ToxB (n = 112). In addition, we determined the percentages of elongated pedestals (B) in untreated HeLa cells (n = 309), cells treated with compactin (n = 332), and cells treated with ToxB (n = 133). Standard deviations are indicated by error bars.
The typical morphology of EPEC-induced actin structures evolves within a few hours from flat, cup-like structures into short actin pedestals and then into elongated pedestals (8, 9). After 2 h of JPN15 infection of untreated HeLa cells, most of the actin pedestals were still flat and less than 2% of them were elongated (Fig. 1A, 2A, and 3B). In contrast, in infected HeLa cells that had been pretreated with ToxB or compactin, more than 95% of the cell-associated JPN15 bacteria induced actin structures which appeared as elongated pedestals (Fig. 1C, 2B, and 3B). Similar results were obtained when wild-type EPEC was used instead of JPN15 (data not shown). These results indicate that compactin and ToxB treatments accelerate the kinetics of actin pedestal elongation, possibly because these treatments cause the formation of high concentrations of actin monomers and actin-associated proteins that serve as building blocks for the assembly of the actin pedestals.
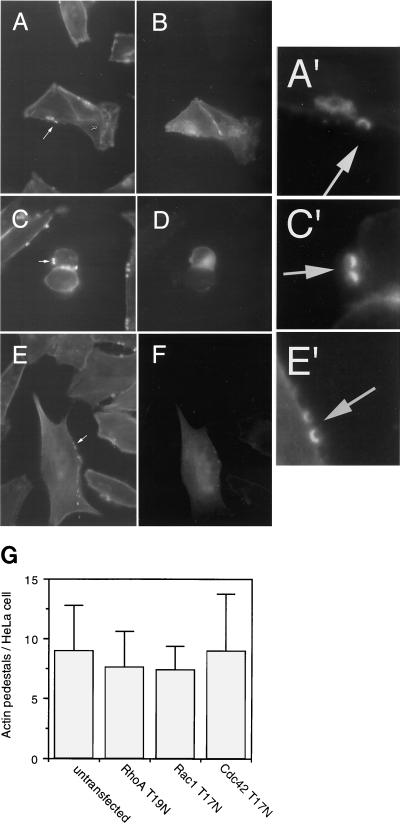
HeLa cells transiently expressing dominant negative forms of either Rho, Rac, or Cdc42, including RhoA-T19N, Rac1-T17N, and Cdc42-T17N, were infected with JPN15, fixed, stained with phalloidin-rhodamine, and analyzed by fluorescent microscopy. To enable distinguishing of the transiently transfected cells from the untransfected cells, the mutated small GTP-binding proteins were expressed (via a cytomegalovirus promoter in the pcDNA3 vector) as fusions with green fluorescence protein (GFP) (S65T) (3). JPN15 induced the formation of actin pedestals in HeLa cells expressing dominant negative Rho, Rac, or Cdc42 (Fig. 4). The frequencies of formation of actin pedestals in the transfected cells were similar to those of the untransfected cells (Fig. 4G). Similar results were observed when wild-type EPEC was used instead of JPN15 (data not shown).
FIG. 4.
Formation of actin pedestals in HeLa cells expressing dominant negative Rho, Rac, and Cdc42. HeLa cells were transiently transfected with plasmids encoding dominant negative small GTP-binding proteins fused to GFP, including Rac1-T17N-GFP (A, A′, and B), Cdc42-T17N-GFP (C, C′, and D), and RhoA-T19N-GFP (E, E′, and F). The transfected cultures were infected with JPN15 and fixed, and the actin filaments were stained with phalloidin-rhodamine. Phalloidin stained all of the HeLa cells in the culture (A, C, and E). Among these, the transfected cells were identified as green fluorescing cells (B, D, and F). The arrows in panels A, C, and E indicate the localization of typical actin pedestals. These pedestals can be better seen in close-ups of the arrow-indicated regions (A′, C′, and E′). Only some of the actin pedestals associated with each cell could be visualized in a given optical plane, including those shown in panels A, C, and E. Additional actin pedestals were detected in other optical planes (not shown). (G) The average numbers of actin pedestals per HeLa cell were determined for untransfected cells (n = 128) and cells expressing either RhoA-T19N-GFP (n = 80), Rac1-T17N-GFP (n = 83), or Cdc42-T17N-GFP (n = 100). Standard deviations are indicated by error bars.
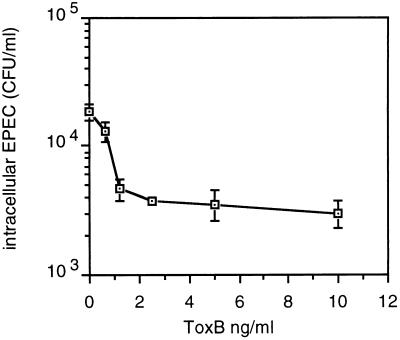
ToxB inhibited EPEC invasion in a dose-dependent manner (Fig. 5). Inhibition of invasion was evident also when compactin was used instead of ToxB (data not shown). Cells transiently transfected with dominant negative mutants of Rho, Rac, or Cdc42 could not be used in invasion assays because the transfected culture consisted of a mixture of transfected and untransfected cells and the frequency of transfection did not exceed 15%.
FIG. 5.
Inhibition of EPEC invasion by ToxB treatment of HeLa cells. HeLa cells, in a 24-well plate, were treated for 2 h with 500 μl of Dulbecco minimal essential medium containing different concentrations of ToxB and 10% fetal calf serum. Then, 5 μl of a fresh overnight bacterial culture was added, followed by incubation (37°C, 5% CO2) for an additional 3 h. The cells were washed twice and incubated for an additional 90 min in a medium containing 100 μg of gentamicin per ml; the monolayers were washed and then lysed with 1% Triton X-100, and appropriate dilutions were plated to determine the numbers of intracellular bacteria. The assays were carried out in duplicate.
Several EPEC mutants are incapable of both invasion and pedestal formation (7). This apparent genetic linkage suggests that invasion and formation of actin pedestals may be different manifestations of the same biochemical process. In contrast to this hypothesis, we demonstrated that ToxB inhibits EPEC invasion but not pedestal formation. These results might indicate that actin polymerization and invasion are different, unlinked processes and that activity of Rho, Rac, or Cdc42 is involved in EPEC uptake but not in formation of EPEC-induced actin pedestals. In agreement with this notion, Rabinowitz et al. (6) demonstrated that an EPEC strain carrying a mutation in the sepZ gene, whose product, SepZ, is required for protein secretion via the type III secretion system, is noninvasive but still induces assembly of actin pedestals. An alternative interpretation is that invasion occurs accidentally as a result of some rare event occurring during EPEC-induced actin rearrangement. For example, association of an individual bacterium with several pedestals, resulting in bacterial phagocytosis, might be such an event. ToxB, by accelerating pedestal elongation, may further reduce the frequency of this putative event. At this point, further work is required to determine whether or not the processes of invasion and formation of actin pedestals are biochemically linked.
Acknowledgments
E.H. was supported by grants from the Israeli Academy of Science, G.M.B. by NIH grants GM44428 and GM39434, K.M.H. by NIH grants AI34929 and AI34643, and I.R. by the Israeli Academy of Science, the Israel-United States Binational Foundation, and the Israeli Ministry of Health.
REFERENCES
- 1.Chong L D, Kaplan T A, Bokoch G M, Schwartz M A. The small GTP-binding protein rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- 2.Donnenberg M S, Calderwood S B, Donohue-Rolfe A, Keusch G T, Kaper J B. Construction and analysis of TnphoA mutants of enteropathogenic Escherichia coli unable to invade HEp-2 cells. Infect Immun. 1990;58:1565–1571. doi: 10.1128/iai.58.6.1565-1571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heim R, Cubitt A B, Tsien R Y. Improved green fluorescence protein. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- 4.Jerse A E, Yu J, Tall B D, Kaper J B. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci USA. 1990;87:7839–7843. doi: 10.1073/pnas.87.20.7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Just I, Selzer J, Wilm M, Von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 6.Rabinowitz R P, Lai L C, Jarvis K, McDaniel T K, Kaper J B, Stone K D, Donnenberg M S. Attaching and effacing of host cells by enteropathogenic Escherichia coli in the absence of detectable tyrosine kinase mediated signal transduction. Microb Pathog. 1996;21:157–171. doi: 10.1006/mpat.1996.0051. [DOI] [PubMed] [Google Scholar]
- 7.Rosenshine I, Donnenberg M S, Kaper J B, Finlay B B. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 1992;11:3551–3560. doi: 10.1002/j.1460-2075.1992.tb05438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenshine I, Ruschkowski S, Stein M, Reinsceid D, Mills D S, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 9.Sanger J M, Chang R, Ashton F, Kaper J B, Sanger J W. Novel form of actin-based motility transports bacteria on the surfaces of infected cells. Cell Motil Cytoskelet. 1996;34:279–287. doi: 10.1002/(SICI)1097-0169(1996)34:4<279::AID-CM3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]