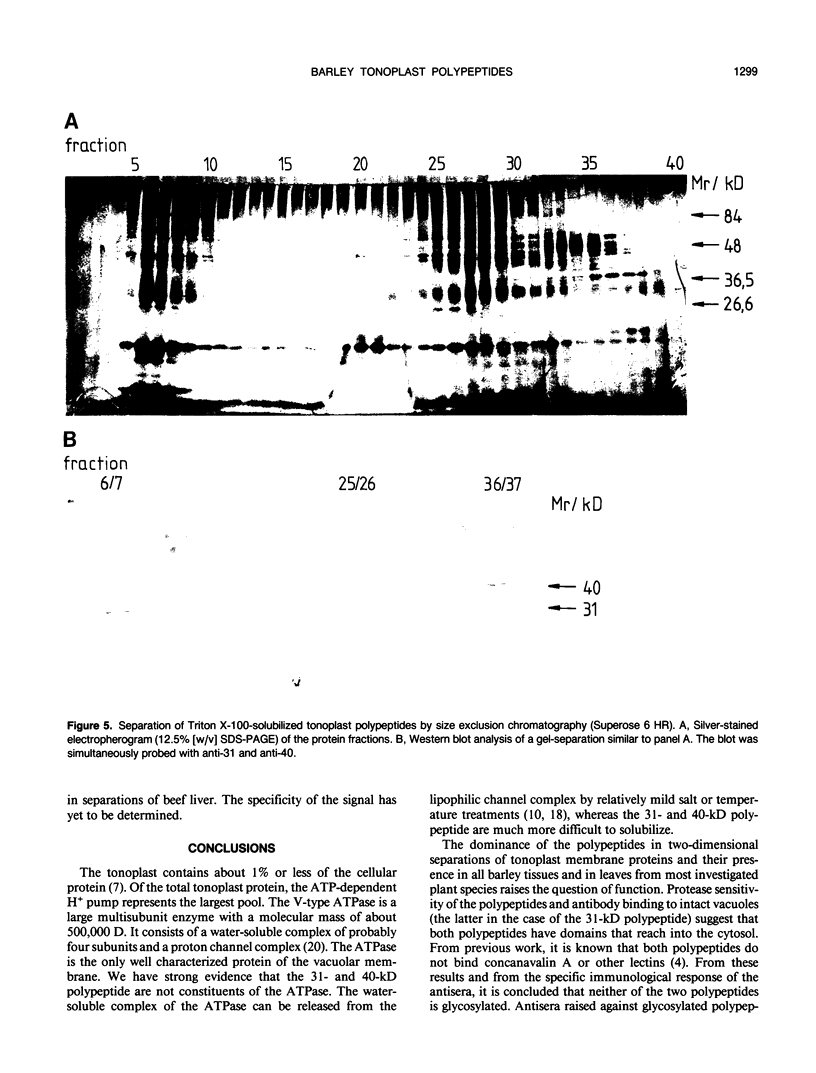
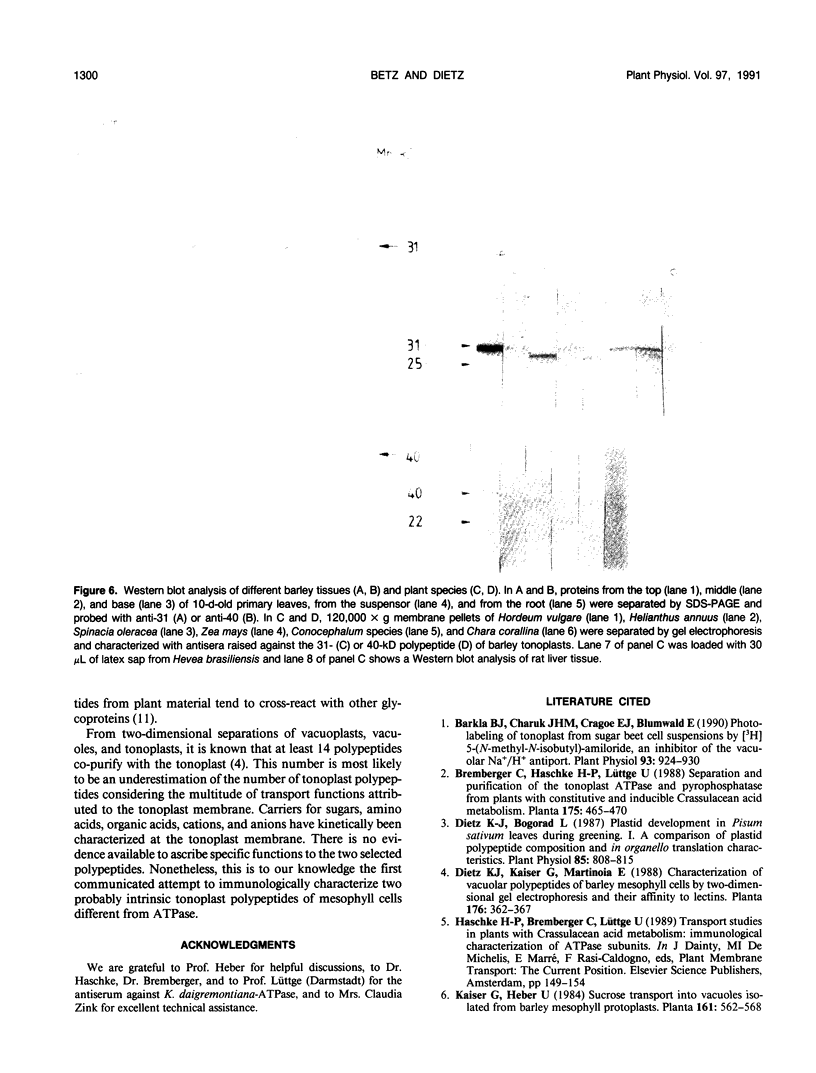
Abstract
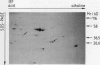
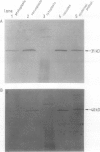
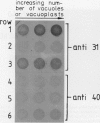
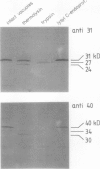
At least 14 distinct polypeptides reside in the tonoplast of barley (Hordeum vulgare) mesophyll vacuoles. Two of the polypeptides were isolated from two-dimensional separations of vacuoplast membrane proteins and used for immunization. With the antisera, the localization on the membrane and the distribution of the polypeptides in the plant kingdom and in various tissues of barley plants was studied. The polypeptides have an apparent molecular mass of 31 and 40 kilodaltons. After freeze-thaw cycles or washing of the membranes with 4.5 millimolar NaCl, the polypeptides were still sedimented with the membranes, suggesting an intrinsic localization. The antiserum against the 31-kilodalton polypeptide bound to the outer surface of isolated intact vacuoles. In chromatographic separations of Triton X-100-solubilized membrane fractions, the residual activities of various acid hydrolases eluted distinct from the 31- and 40-kilodalton polypeptides. Both polypeptides tend to form larger aggregates, however smaller than the tonoplast ATPase. Cross-reactive polypeptides were present in higher and lower plants (the green alga Chara corallina and the liverwort Conocephalum) and in liver tissue from rat and beef, but were not detected in other animal tissues tested so far. The results indicate a wide distribution of these tonoplast polypeptides in vacuole-containing organisms.
Full text
PDF
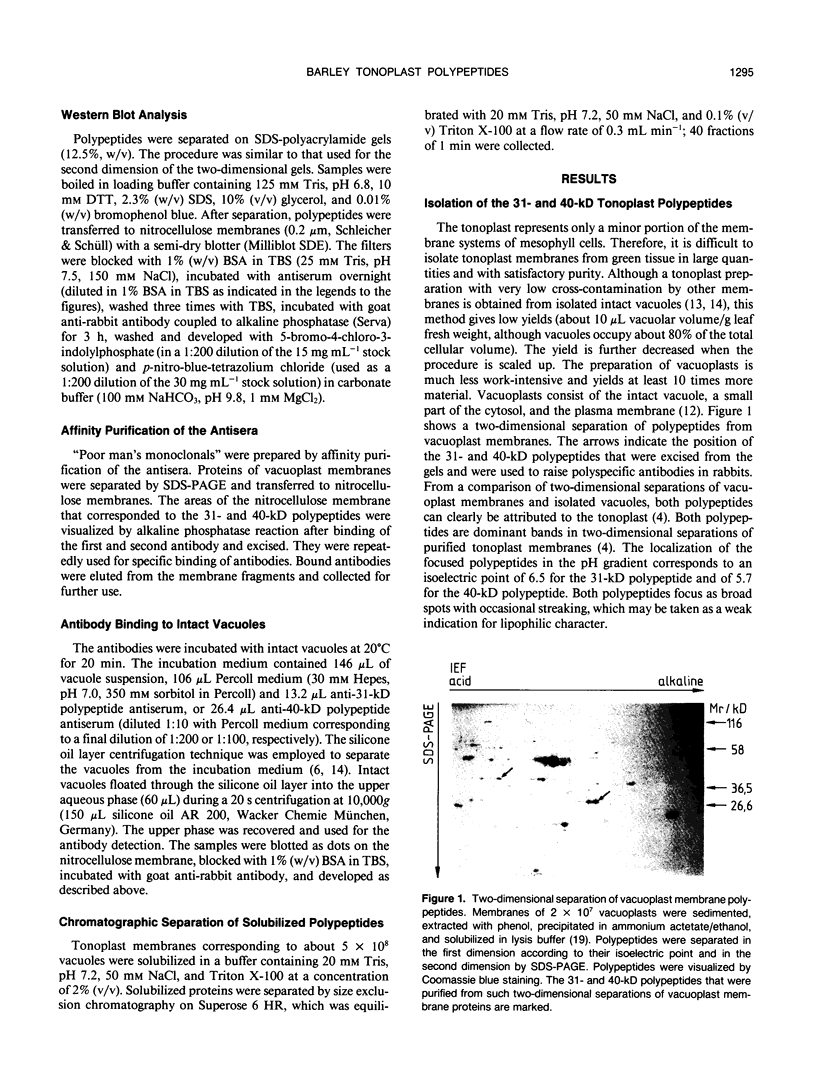

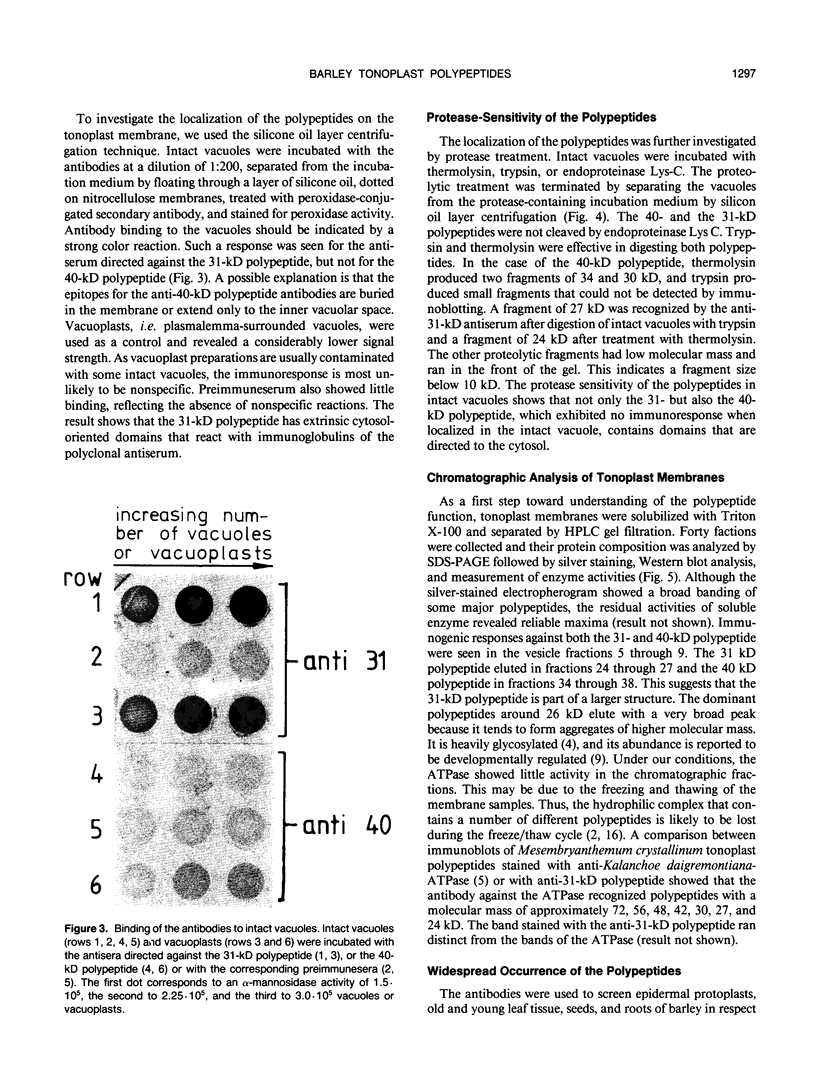
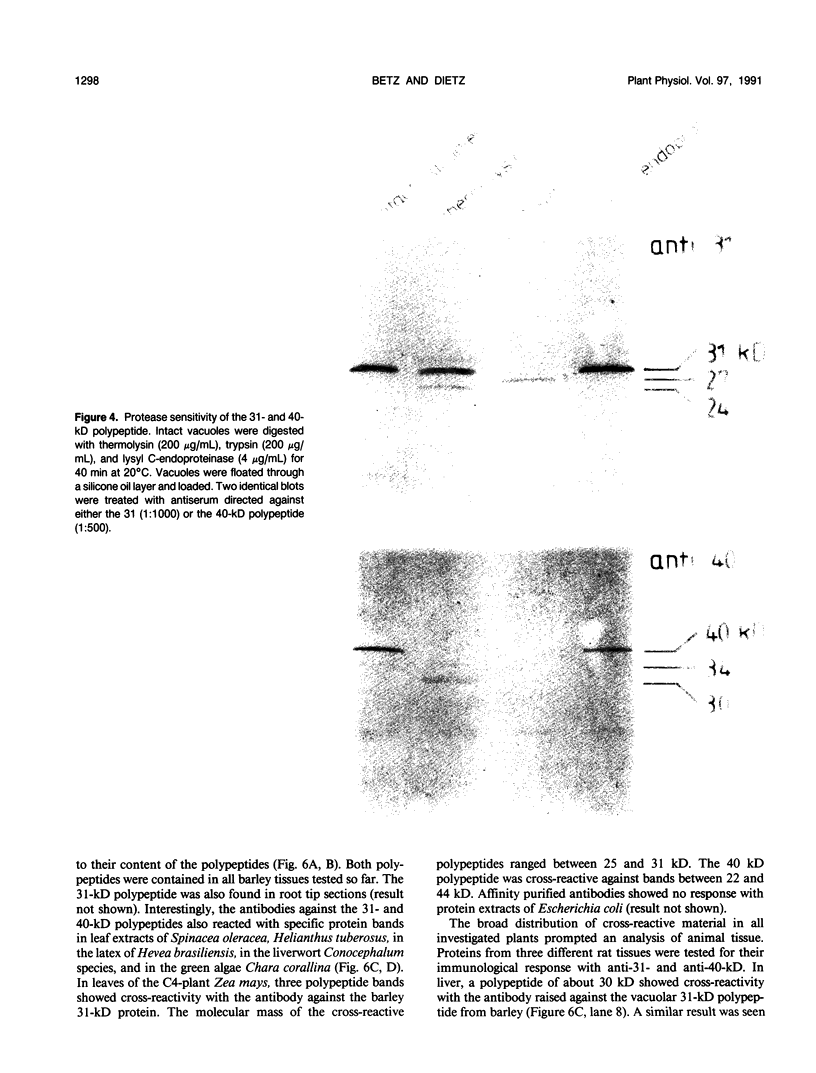

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkla B. J., Charuk J. H., Cragoe E. J., Blumwald E. Photolabeling of tonoplast from sugar beet cell suspensions by [h]5-(N-methyl-N-isobutyl)-amiloride, an inhibitor of the vacuolar na/h antiport. Plant Physiol. 1990 Jul;93(3):924–930. doi: 10.1104/pp.93.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz K. J., Bogorad L. Plastid Development in Pisum sativum Leaves during Greening : I. A Comparison of Plastid Polypeptide Composition and in Organello Translation Characteristics. Plant Physiol. 1987 Nov;85(3):808–815. doi: 10.1104/pp.85.3.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon W. H., Black C. C. Electrophoretic analysis of protoplast, vacuole, and tonoplast vesicle proteins in crassulacean Acid metabolism plants. Plant Physiol. 1986 Dec;82(4):916–924. doi: 10.1104/pp.82.4.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S. P., Randall S. K., Sze H. Peripheral and integral subunits of the tonoplast H+-ATPase from oat roots. J Biol Chem. 1988 Nov 15;263(32):16731–16737. [PubMed] [Google Scholar]
- Laurière M., Laurière C., Chrispeels M. J., Johnson K. D., Sturm A. Characterization of a xylose-specific antiserum that reacts with the complex asparagine-linked glycans of extracellular and vacuolar glycoproteins. Plant Physiol. 1989 Jul;90(3):1182–1188. doi: 10.1104/pp.90.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura-Endo C., Maeshima M., Yoshida S. Subunit composition of vacuolar membrane H(+)-ATPase from mung bean. Eur J Biochem. 1990 Feb 14;187(3):745–751. doi: 10.1111/j.1432-1033.1990.tb15362.x. [DOI] [PubMed] [Google Scholar]
- Moriyama Y., Nelson N. Cold inactivation of vacuolar proton-ATPases. J Biol Chem. 1989 Feb 25;264(6):3577–3582. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]