Abstract
Diabetes is a chronic disease potentially disabling. The purpose of the study was to investigate the effects of exercise in type II diabetes in obese subjects, both active and sedentary. The study demonstrates how exercise can be a useful approach to reducing blood glucose levels. In addition, we monitored changes in some hematochemical parameters at the end of the intervention. We recruited 90 people aged 35 to 40 years, both males and females. All were type II diabetics and were randomly randomized into group A (n=50, sedentary) and group B (n=40, active). Anthropometric parameters (BMI) and some hematochemical values (blood glucose, cholesterol, Hb1Ac) were assessed. At the end of the intervention, blood glucose values decreased from 160/150 (mg/dl) to 130 (mg/dl) in group A, and in group B from 140 (mg/dl) to 120/110 (mg/dl). BMI decreased by 60% in both groups, with greater significance in group B, of which 60% were obese. The training protocol used demonstrated that exercise combined with insulin therapy regulates blood glucose levels in obese and non-obese diabetics.
Key Words: diabetes type II, exercise, prevention
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Diabetes mellitus (DM) is a chronic disease that includes a group of conditions characterised by increased blood glucose concentration. Depending on the age of onset, it is classified into type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM).1 It is becoming an epidemic in some countries of the world, with the number of people affected expected to double in the next decade as the population ages. Is probably one of the oldest diseases known to man and it was first reported in Egyptian manuscript about 3000 years ago. T1DM is a chronic autoimmune disease characterised by a lack of or inadequate production and/or action of insulin, due to a dysfunction of the β cells of the endocrine pancreas and consequent hyperglycaemia.2 There are conflicting studies on the association between the onset of T1DM and a prolonged increase in body mass index (BMI) in the paediatric population, identified at the 85th percentile in growth curves. However, lifestyle modifications could delay the onset of the disease in these at-risk individuals.3 Although T1DM manifests itself as an acute event at a young age, the disease can reveal itself at any age and up to 50% of cases occur in adulthood.4 While, regarding T2DM was first described as a component of metabolic syndrome in 1988. It is estimated that by 2030, 552 million people will be affected by DM.5 In this metabolic alteration, age, gender, obesity, hypertension, genetics, diet, smoking, alcohol, sedentary lifestyle are risk factors. Several novel medications are in development, but the greatest need is for agents that enhance insulin sensitivity, halt the progressive pancreatic β-cell failure that is characteristic of T2DM and prevent or reverse the microvascular complications. Various lifestyle factors are known to be important to the development of the T2DM and of these obesity is neither an innocent bystander nor an occasional accomplice to diabetes, but it is a primary risk factor, in fact has been found to contribute to approximately 55% of cases of type 2 DM.2 Seventy percent of the western population is not active enough to maintain optimal health and weight. In the last twenty years obesity levels have tripled, (20% of men and 10-25% of women are obese). This tendency is caused by a decrease in physical activity (PA). In the last decade, the attention of the Italian diabetological community, has focused on the quality of life (QoL), PA and sports, thanks also to the promotional impulse given by the Italian Association of Diabetic Athletes. Various modalities of training can alter insulin action in a short period of time and an association of dietary programs, can improve the effects of the training to reverse the metabolism syndrome. In addition, numerous studies showed that the effects of obesity on glucose metabolism lead to impaired glucose tolerance, the development of insulin resistance and the consequent damage to the secretory function of β-cells.6-8 Keeping the weight fit is, therefore, a matter of intake and energy expenditure, because overweight and obesity develop when the energy intake is higher for a prolonged period. According to a World Health Organization (WHO) ranking, the prevalence of obesity in the United States increased from 30.5% to 41.9% during the period between from March 1999-2000 and during the same period between 2017-2020. The WHO defines a normal weight individual for an BMI 18.5 kg/m2 and < 25 kg/m2, underweight if < 18.5 kg/m2, overweight if 25 kg/m2 and obese if 30 kg/m2. The risk of the T2DM rises linearly with an increase in body mass index which, in concert, induces a constellation of metabolic abnormalities such as dyslipidaemia, cardiovascular morbidity and mortality. More than 95% of people with diabetes have T2DM, which is largely the result of excess body weight and physical inactivity. People with impaired glucose tolerance (IGT) or impaired fasting glycaemia (IFG) are at high risk of progressing to T2DM, although this is not inevitable9. The worldwide increase in the prevalence of obesity is likely responsible for the recent increase in the prevalence of type 2 diabetes. A correct diagnosis is the fundamental prerequisite to identify the persons to be addressed to the diagnostic and therapeutic paths of care (PDTA). In T2DM there is a relative insulin deficiency caused by pancreatic β-cell dysfunction and insulin resistance in target organs. This leads to a decrease in glucose transport into the liver, muscle cells, and fat cells. As a result of this dysfunction, glucagon and hepatic glucose levels that rise during fasting are not suppressed with a meal. Given inadequate levels of insulin and increased insulin resistance, hyperglycemia results. A diagnosis of diabetes is based on a fasting blood glucose concentration above 7·0 mmol/L (126 mg/dL), a random blood glucose concentrate on above 11·1 mmol/L (200 mg/dl) with symptoms, or an abnormal result from an oral glucose tolerance test.2 The incretins are important gut mediators of insulin release, and in the case of the glucagon-like peptide 1 (GLP-1), of glucagon suppression. GLP-1 insulinotropic effects are preserved, and thus GLP-1 represents a potentially beneficial therapeutic option.9 Both classes of agents have shown not only to have the ability to normalize fasting and post-prandial glucose levels, but also to improve the functioning and mass of β cells. Furthermore, based on the established health benefits of PA and a healthy diet in a recent review Proia et al, highlighted the role of these gut peptides in bone and glucose metabolism. Indeed, they are able to respond to food intake and trigger regulatory mechanisms in bone turnover by virtue of the expression of their receptors on immature human osteoblast cell lines.10 PA and exercise have beneficial effects on health such as improving osteo-articular function, metabolic control, and QoL of people. Autonomic nervous system (ANS) and heart rate (HR) parameters can be used to analyse subjects' health and are also used to investigate stress and exercise level. Specifically, HR spectral analysis clarified the nature of diabetic autonomic neuropathy and other neurological disorders that favor ANS.11 Walking is a convenient low-impact mode of PA and is reported to be the most performed activity for those with diabetes. 10,000 steps/day are effective at improving glucose tolerance and lowering blood pressure in overweight, inactive women at risk for T2DM.6 Epidemiological studies have suggested that environmental factors and lifestyle changes may be responsible for the increased incidence of Thyroid Cancer (TC). Potential modifiable TC risk factors include insulin resistance and hyperinsulinemia.12 PA has an important impact on cholesterol, blood sugar, BMI and hba1c in the diabetic. Prolonged exercise has therapeutic effects. That is why, we chose to apply in our study, motor protocols for a period of 6 months (Fig. 3, 4, 5, 6). The American College of Sports Medicine and the American Diabetes Association have recommended at least 150 min/wk of moderate (50%-70% of an individual’s maximum heart rate) to vigorous (> 70% of an individual’s maximum heart rate) PA for patients with T2DM. The intensity of PA is assessed using the MET (metabolic equivalent) parameter. 1 MET is equal to the basal resting metabolic rate and an oxygen uptake of 3.5 mL/kg per minute.13 Controlled and adapted exercise in diabetes is a therapy, which could prevent the occurrence of complications associated with disease progression.14 The aging process leads to a degeneration of muscles, ligaments, bones and joints, but in the diabetic, you amplify and add vascular complications that can aggravate the problem. The WHO states that health is a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity. PA and physical exercise play a fundamental role in promoting positive lifestyle behaviours, capable of promoting health and wellness. It reduces the risk of developing diabetes and prevents important complications that can be fatal for the patient's life or in any case strongly disabling. Regular and moderate exercise improves the function of the immune system, reduces environmental and psychological stress, decreases the demand and administration of immunosuppressants.15 Moreover, it plays a decisive role in improving glucometabolic compensation and, in general, the subject's perception of his or her state of health, leading to a significant reduction in healthcare expenditure. A study conducted on QoL in diabetic patients, who were administered the two tests Sickness Impact Profile (SIP) and Functional Living Index (FLI), an index of psychosocial, physical and motor function, showed that QoL is not only related to the severity of the disease, treatment and complications, but also to subcultural elements, representing in fact a surprising parameter.16 As for our study, we had previously conducted searches in the scientific literature, which were in line with our research objective. Although the results produced highlighted the benefits of sport or PA in diabetes, on the other hand, we did not find any studies that considered the interaction of other behaviours (active vs. sedentary). Therefore, we set out to investigate the effects of an aerobic exercise programme in reducing BMI and blood glucose levels in obese subjects with T2DM, whether active or sedentary.
Table 1.
Anthropometric characteristics of participants.
| Age (year) | BMI | Height | Gender | |
|---|---|---|---|---|
| Group A | 40 ± 4.6 | ±< 28.78 | 169.77 ± 18.10 cm | 20 f, 30 m |
| Group B | 35 ± 2.8 | ±< 23.95 | 172.88 ± 15.83 cm | 15 f, 25 m |
Materials and Methods
The study recruited 90 patients, 35 females and 55 males aged 45 years. All participants signed informed consent. The firm was carried out following the guidelines of Helsinki and with the con-sent of the ethical committee of the kore University of Enna (Prot. N. 12722). We have been recruiting diabetic patients for at least five years, excluding those who have serious complications from diabetes. Patients were randomly assigned to two groups: active or sedentar. Group A (sedentary) aged 40 ± 4.6, weight 70 ± 7.83, height: 149.77 ± 18.10 cm, and group B (active) aged 35 ± 2.8, weight 65 ± 2.3, height: 145.88 ± 15.83 cm. Participants were selected in relation to the inclusion criteria and thus type II diabetes being over the age of 35 years, being in a possible state of obesity and/or overweight, being available to undergo the study. The exclusion criteria did not consider individuals who reported ulcers, amputations in the limbs, age over 50, heart attack or stroke, neuropathies, vasculopathies, retinopathy, nephropathy, smokers (current or within one year). Anthropometric characteristics of participants are illustrated in Table 1.
The experimental group (n=90) performed the training by using adapted motor protocol, two times a week, at the same time of the day, between 10:30 and 12:45. The duration of the sessions was organized by consulting the Diabetes Associations.2 The protocol was designed by our multidisciplinary team, involving the sports medicine doctors, medical doctors, kinesiologists and posturologists. This choice made it possible to take charge of the 90 participants and follow them in full respect of the concept of prevention and health. The protocol involved active mobilization, muscle strengthening and stretching exercises. Regular exercise is a cornerstone of treatment for diabetes. Exercise training generally improves exercise function in T2D. In general, PA provides significant general health benefits and may improve disease outcomes.17 Specifically, the literature was consulted to define the protocol.18,19 Aerobic exercises of moderate and intense intensity were performed because it was noted that moderate to high volumes of aerobic activity are associated with significantly lower cardiovascular and overall mortality risks in both type 1 diabetes and T2D. In contrast, physical inactivity or sedentarism, is known to have deleterious health effects in people with diabetes. Prolonged sitting is typical among the habits of contemporary society; but the study conducted by Farì et al.20 points out that hours spent in a sitting position seem to be correlated with the incidence of articular and muscolar pain. From the scientific evidence, two different training sessions were planned for group A (inactive) and group B (active). Individuals with T2D had slower oxygen uptake kinetics with constant load exercise and had less ability to adapt to circumstances when oxygenation is required. This involved a high fatigue and effort for them, even when less physical exertion is required. The participants of "group B" did aerobic exercises of higher intensity, as they reacted well to the physical effort of medium intensity. So, they were offered aerobic exercises at 75% of the maximum heart rate, calculated according to the formula of Tanaka.21 The "group A" performed medium-intensity exercises in the first phase (first 2 months) and then at high intensity (<75%). Participants were asked to intensify glycemic self-monitoring before, during, and after exercise to avoid the risk of hypoglycaemia. The training sessions of both groups (duration 165 min) were articulated:
Fig 1.
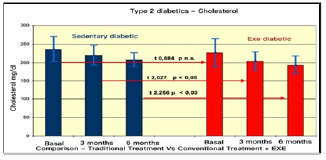
Comparison of Colesterol in type II diabetes Pre vs. Post treatment
Fig 2.
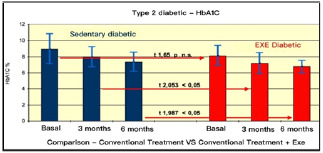
Comparison of HbA1C in type II Diabetes Pre vs. Post Treatments.
Fig 3.
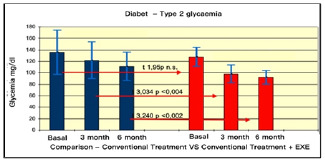
Comparison of glycemia in type II Diabetes Pre vs. Post Treatments.
Fig 4.
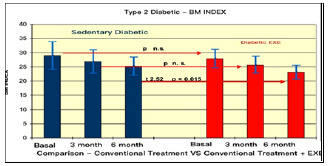
Comparison of glycemia in type II Diabetes Pre vs. Post Treatments.
• Warming-up (20 min) o Joint mobilizations (5 min)
o Walking (8 min)
o Total body strength (7 min)
Rest (3 min)
• Aerobic exercise (40 min)
o Total body strength (10 min)
o Core training (25 min)
o Lifting handheld weights (5 min)
3 series of 10 repetitions for each exercise with 3 minutes recovery between a series.
• Postural training (40 min) o Stretching (10 min)
o Balancing exercise (15 min)
o Core stability training (15 min)
• Propriocettive training (20 min)
Aerobic exercise of varying intensities is beneficial for improving dynamic postural control in T2DM people with/without neuropathy. People of group A were prescribed a low-calorie diet calculated on the ratio of basal metabolism to routine energy expenditure, (Harris and Benedect formula).22 So, in overweight people, caloric intake was reduced to obtain a negative balance of 500 kcal/d against energy expended. People of “group B” continued to follow the daily eating routine with-out any change in the conductors of the study. The checks were carried out at time T0, after three months (T1) and after six months (T2).
The biochemical parameters examined are Body Weight (BW), BMI, glycaemia, glycosylated haemoglobin (HbA1C), cholesterol. All dosages were made with the Clinical Chemestry System ilab 300 Plus- Instrumentation Laboratory. The HbA1c was measured with a high-performance liquid chromatography system using the Hi-AUTO A1C, TM HA 8121 system (DI, Daiichi, Kogaku, Japan).23 The glycaemia was dosed using the enzymatic method Test, GLUC (Glucose Oxidase). The cholesterol was measured using the enzymatic method IL Test CHOL - 00 18480200. The BMI was calculated according to the known formula: Weight (Kg) / height² (mt). In particular, the BMI value identifies: underweight (BMI <18.5 kg/m2), normal weight (BMI 18.5 to 24.9 kg/m2), overweight (BMI 25 to 29.9 kg/m2), and obese (BMI ≥30 kg/m2).24 The principal’s characteristics of diabetes are Glucose, BMI, HbA1C and cholesterol; for this reason, we analyzed this parameter both in sedentary and active participants. Obesity is a chronic disease associated to insulin resistance, diabetes mellitus and altered lipid profile.25 For this reason, we have evaluated in the study also obese men and women. To assess the reliability of the methodology, we performed a statistical evaluation of the comparisons of the methods and instrumental tests used.23 Correlations were measured using the Pearson’s product-moment correlation coefficient. When the data were "normally" distributed the comparison of the intragroup results was performed with parametric testing.26
Fig 5.
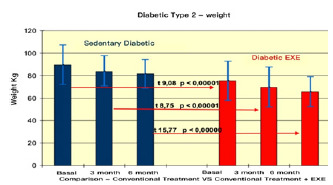
Comparison of weight in type II Diabetes Pre vs. Post Treatments.
Fig 6.
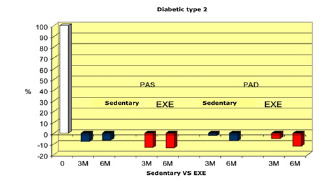
Sedentary vs active (EXE).
Differences between the assessments results before and after treatment were analyzed by t-test. Calculations and tables were all made in SPSS 23. Differences resulting in a p value < 0.05 were considered statistically significant.
Results
The results summarised in Table 2 indicate that, in group A (inactive) improvements were less noticeable than in group B (active). From the monitoring of the four parameters evaluated every 3 months, we noticed that cholesterol, blood sugar, BMI and Hb1Ac decreased. (as shown by the trend of the graphs Figures 1, 2, 3, 4, 5 and6) This is a very important fact because each of these elements is also influenced by other external factors and the change could thus be questioned. The graphs below show the pre/post of the examined parameters. Both sedentary and active subjects improved with evident reduction in blood sugar, BMI, HbAc1 and cholesterol. This study provides evidence of the positive effects of supplementation between exercise, diet and lifestyle changes on the parameters evaluated.
Comparing the results obtained before and after the treatments, we found that the glycemic values changed from 160/150 to 130 (mg/dl) in group A, and from 140 to 120/110(mg/dl) in group B. The BMI of group A changed from 28.78 to 27.33, while in group B, from ±< 23.95 to < 23.32.
Discussion
The results presented herein refer to patients completing the follow-up, since the baseline values carried forward analysis did not change the estimates. According to the training program, energy expenditure during the exercise sessions increased progressively throughout the study. Studies conducted by other authors,27,28 have shown the influence of exercise on blood sugar. In 2012 the Big Blue Test (BBT) confirmed that participation in varying types, intensities, and durations of exercise generally lowers blood glucose levels.29 Varying types, intensities, and durations of exercise generally lower blood glucose levels in most individuals, although exercise of longer duration is likely most effective, and elapsed time since eating should be considered.30 The results showed that PA deserves the same attention given to diet and insulin therapy; both in people with type I and type II diabetes. Exercise has significant health benefits. The 2020 WHO guidelines update previous WHO recommendations released in 2010, highlight the importance of regularly undertaking both aerobic and muscle strengthen-ing activities physical activity is better for optimal health outcomes and provide a new recommendation on reducing sedentary behaviours.31 Diet is also an important component that affects health and diabetes. Poor nutrition is one of the factors that leads to the development of diabetes. Attempts are being made to supplement diet and PA, along with health education, to reduce the negative effects of diabetes, but it is unclear how the combinations of these can have positive effects. The results show that activity and exercise must take place in conditions of good glucometabolic compensation and with the consequent insulin adjustment that it requires. The study highlights how exercise had a therapeutic effect (Figures 1,2,3,4,5,6). Active lifestyle has been shown to be able not only to significantly reduce the risk of developing diabetes, but also to prevent important complications that can be fatal for the patient’s life.32
The effects of exercise are beneficial for patients with T2D because exercise increases the concentration of GLUT-4 in the cell membrane and increases glucose uptake in skeletal muscles. Acute exercise increases glucose tolerance and insulin sensitivity and blood glucose reduction within 20–72 h.33 Studies have shown that acute moderate-intensity endurance training leads to a reduction in blood glucose levels, whereas light and short resistance and endurance training shows no effect. For this reason, it was decided to use a motor protocol of aerobic exercises of medium intensity. This study has several strengths and limitations. To our knowledge, this is the first parallel study that compared active and sedentary diabetics. Moreover, in the study participants did not follow only a sport or a specific type of exercise, but a motor and nutritional protocol of different intensity, which was adapted to the two groups, despite being the "same" exercises. Another strong point was the multi-discipline conduct of the study. The first limitation of the study was the choice of parameters to be evaluated, which were too numerous, and the number of participants involved. Initially they were at least double, but only 90 have completed the 6 months of research. The others were excluded because they were absent for more than 70% of the total hours planned, so their results were not reworked nor considered reliable. The second limitation was not having a QoL test. Subjects said that their daily lives improved with fewer limitations in daily activities, they are better off. PA has always been considered one of the cornerstones of the II types of diabetes therapy along with diet and drug therapy. Aerobic exercise reduces fat mass and weight, BMI, cholesterol and HbAC1 in 2TD people. Our findings suggested that the integration of exercise into the therapeutic protocols of diabetics, could be a promising option to improve glycaemic indices and still be recommended to be included in the routine of life. Active and healthier lifestyle is the best way to live healthy and improve your well-being. Both exercise and diet, in active and sedentary diabetics, is the right combination as a therapeutic treatment, to prevent diabetes from worsening the health of those suffering from it.
Table 2.
Pre vs. Post Treatment.
| Age (year) | BMI | HbAc1 | ||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Group A | 40 ± 4.6 | 40 ± 4.6 | ±< 28.78 | ±< 27.33 | ± 160/150 mg/dl | ± 130 mg/dl |
| Group B | 35 ± 2.8 | 35 ± 2.8 | ±< 23.95 | < 23.32 | ± 140 mg/dl | ± 120/110 mg/dl |
In conclusion we feel that an adequate motor protocol, adapted and variable dosage, can be associated with insulin therapy and should be prescribed as a drug for preventive and therapeutic purposes. Thus, we hope to have made an important contribution to the literature with the motor protocol proposed and tested.
Acknowledgments
The authors thank all persons who participated in the study and the entire multidisciplinary team consisting of the sports doctor, physicians, kinesiologist and posturologist who created the protocol.
List of acronyms
- ANS
Autonomic nervous system
- BBT
Big blue test
- BMI
Body mass index
- BW
Body weight
- DM
Diabetes mellitus
- FLI
Functional living index
- GLP-1
Glucagon-like peptide 1
- HbA1C
Glycosylated haemoglobin
- HR
Heart rate
- IFG
Impaired fasting glycaemia
- IGT
Impaired glucose tolerance
- MET
Metabolic equivalent
- PA
Physical activity
- PDTA
Path diagnostic therapeutic care
- QoL
Quality of life
- SIP
Sickness impact profile
- T1DM
Type 1 Diabetes mellitus
- T2DM
Type 2 Diabetes mellitus
- TC
Thyroid cancer
- WHO
World Health Organization
Funding Statement
Funding: This research received no external funding..
Contributor Information
Giuseppe Messina, Email: giuseppe.messina@uniroma5.it.
Anna Alioto, Email: annaalioto374@gmail.com.
Omar Mingrino, Email: omarmingrino@icloud.com.
Donatella Di Corrado, Email: donatella.dicorrado@unikore.it.
Caterina Crescimanno, Email: caterina.crescimanno@unikore.it.
Szimon Kuliś, Email: szymon.kulis@awf.edu.pl.
Fatma Nese Sahin, Email: nesesahin@ankara.edu.tr.
Elvira Padua, Email: elvira.padua@uniroma5.it.
Alberto Canzone, Email: alberto.canzone98@gmail.com.
Vincenzo C Francavilla, Email: vincenzo.francavilla@unikore.it.
References
- 1.Harreiter J, Roden M. Diabetes mellitus – Definition, Klassifikation, Diagnose, Screening und Prävention (Update 2019) [Diabetes mellitus-Definition, classification, diagnosis, screening and prevention (Update 2019)]. Wien Klin Wochenschr. 2019. May;131(Suppl 1):6-15. German. doi: 10.1007/s00508-019-1450-4. PMID: 30980151. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018. Jan;41(Suppl 1):S13-S27. doi: 10.2337/dc18-S002. PMID: 29222373. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara CT, Geyer SM, Liu YF, Evans-Molina C, Libman IM, Besser R, Becker DJ, Rodriguez H, Moran A, Gitelman SE, Redondo MJ; Type 1 Diabetes TrialNet Study Group. Excess BMI in Childhood: A Modifiable Risk Factor for Type 1 Diabetes Development? Diabetes Care. 2017. May;40(5):698-701. doi: 10.2337/dc16-2331. Epub 2017 Feb 15. PMID: 28202550; PMCID: PMC5399656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018. Feb;6(2):122-129. doi: 10.1016/S2213-8587(17)30362-5. Epub 2017 Nov 30. PMID: 29199115; PMCID: PMC5805861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamnan P, Simmons RK, Forouhi NG, Luben RN, Khaw KT, Wareham NJ, Griffin SJ. Incidence of type 2 diabetes using proposed HbA1c diagnostic criteria in the european prospective investigation of cancer-norfolk cohort: implications for preventive strategies. Diabetes Care. 2011. Apr;34(4):950-6. doi: 10.2337/dc09-2326. Epub 2010 Jul 9. PMID: 20622160; PMCID: PMC3064056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swartz AM, Strath SJ, Bassett DR, Moore JB, Redwine BA, Groër M, Thompson DL. Increasing daily walking improves glucose tolerance in overweight women. Prev Med. 2003. Oct;37(4):356-62. doi: 10.1016/s0091-7435(03)00144-0. PMID: 14507493. [DOI] [PubMed] [Google Scholar]
- 7.Bernardini AL, Vanelli M, Chiari G, Iovane B, Gelmetti C, Vitale R, Errico MK. Adherence to physical activity in young people with type 1 diabetes. Acta Biomed. 2004. Dec;75(3):153-7. PMID: 15796088. [PubMed] [Google Scholar]
- 8.Defrin R, Josefsberg Z, Karp M. [The effect of acute physical activity on blood glucose levels of children with insulin-dependent diabetes mellitus]. Harefuah. 2004. Dec;143(12):856-60, 912, 911. Hebrew. PMID: 15666702. [PubMed] [Google Scholar]
- 9.Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of Type 2 Diabetes Mellitus. Int J Mol Sci. 2020. Aug 30;21(17):6275. doi: 10.3390/ijms21176275. PMID: 32872570; PMCID: PMC7503727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proia P, Amato A, Drid P, Korovljev D, Vasto S, Baldassano S. The Impact of Diet and Physical Activity on Bone Health in Children and Adolescents. Front Endocrinol (Lausanne). 2021. Sep 13;12:704647. doi: 10.3389/fendo.2021.704647. PMID: 34589054; PMCID: PMC8473684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moscatelli F, Sessa F, Valenzano A, Polito R, Eronia S, Monda V, Scattarella F. The Influence of Physical Exercise, Stress and Body Composition on Autonomic Nervous System: A Narrative Review. Sport Mont. 2022;20(2), 131-134. DOI: 10.26773/smj.220620 [Google Scholar]
- 12.Malaguarnera R, Ledda C, Filippello A, Frasca F, Francavilla VC, Ramaci T, Parisi MC, Rapisarda V, Piro S. Thyroid Cancer and Circadian Clock Disruption. Cancers (Basel). 2020. Oct 24;12(11):3109. doi: 10.3390/cancers12113109. PMID: 33114365; PMCID: PMC7690860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamasaki H. Daily physical activity and type 2 diabetes: A review. World J Diabetes. 2016. Jun 25;7(12):243-51. doi:10.4239/wjd.v7.i12.243. PMID: 27350847; PMCID: PMC4914832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francavilla G, Francavilla C. Physical exercise is therapy. Med Sport. 2013;66, 625-628. [Google Scholar]
- 15.Francavilla VC, Polito R, Messina G, Parisi MC, Mingrino OGM, Campanozzi A, Daniele A, Messina A, Monda V, Valenzano A. Immune system and physical activity. Journal of human sport & exercise. 2020. doi:10.14198/ jhse.2020.15.Proc4.49 [Google Scholar]
- 16.Catalano D, Martines GF, Spadaro D, Di Corrado D, Crispi V, Di Nuovo S, Trovato GM. Qualità della vita nel paziente diabetico [Quality of life in diabetes]. Clin Ter. 2004. May;155(5):175-8. Italian. PMID: 15344564. [PubMed] [Google Scholar]
- 17.Patti A, Maggio MC, Corsello G, Messina G, Iovane A, Palma A. Evaluation of Fitness and the Balance Levels of Children with a Diagnosis of Juvenile Idiopathic Arthritis: A Pilot Study. Int J Environ Res Public Health. 2017. Jul 19;14(7):806. doi: 10.3390/ijerph14070806. PMID: 28753965; PMCID: PMC5551244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW, Hiatt WR, Regensteiner JG. Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care. 1999. Oct;22(10):1640-6. doi: 10.2337/diacare.22.10.1640. PMID: 10526728. [DOI] [PubMed] [Google Scholar]
- 19.Ring M, Eriksson MJ, Fritz T, Nyberg G, Östenson CG, Krook A, Zierath JR, Caidahl K. Influence of physical activity and gender on arterial function in type 2 diabetes, normal and impaired glucose tolerance. Diab Vasc Dis Res. 2015. Sep;12(5):315-24. doi: 10.1177/1479164115588548. Epub 2015 Jun 19. PMID: 26092821; PMCID: PMC4527971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farì G, Fischetti F, Zonno A, Marra F, Maglie A, Bianchi FP, Messina G, Ranieri M, Megna M. Musculoskeletal Pain in Gymnasts: A Retrospective Analysis on a Cohort of Professional Athletes. Int J Environ Res Public Health. 2021. May 20;18(10):5460. doi: 10.3390/ijerph18105460. PMID: 34065250; PMCID: PMC8160814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001. Jan;37(1):153-6. doi: 10.1016/s0735-1097(00)01054-8. PMID: 11153730. [DOI] [PubMed] [Google Scholar]
- 22 Luy SC, Dampil OA. Comparison of the Harris-Benedict Equation, Bioelectrical Impedance Analysis, and Indirect Calorimetry for Measurement of Basal Metabolic Rate among Adult Obese Filipino Patients with Prediabetes or Type 2 Diabetes Mellitus. J ASEAN Fed Endocr Soc. 2018;33(2):152-159. doi: 10.15605/jafes.033.02.07. Epub 2018 Sep 10. PMID: 33442121; PMCID: PMC7784146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung H-R, Sohn HE. Evaluation of VARIANTTM II Hemoglobin A1c Autoanalyzer. Korean J Clin Pathol. 2000; 20: 13-17. [Google Scholar]
- 24.Clinical Guidelines on the Identification Evaluation and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998. Sep;6 Suppl 2:51S-209S. Erratum in: Obes Res 1998. Nov;6(6): 464. PMID: 9813653. [PubMed] [Google Scholar]
- 25.Polito R, Monda V, Ametta A, Monda M, Messina A, Sessa F, Porro C, Pisanelli D, DiNunno N, DiMizio G, Asmundo A, Daniele A, Francavilla VC. Physical activity has numerous beneficial effects on metabolic and inflammatory processes. Journal of Human Sport and Exercise. 2000; 15(3proc), S815-S821. doi: 10.14198/jhse.2020.15. Proc3.32. [Google Scholar]
- 26.Krithikadatta J. Normal distribution. J Conserv Dent. 2014. Jan;17(1):96-7. doi: 10.4103/0972-0707.124171. PMID: 24554873; PMCID: PMC3915399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol (1985). 2005. Jul;99(1):338-43. doi: 10.1152/japplphysiol.00123.2005. PMID: 16036907. [DOI] [PubMed] [Google Scholar]
- 28.Röhling M, Herder C, Stemper T, Müssig K. Influence of Acute and Chronic Exercise on Glucose Uptake. J Diabetes Res. 2016;2016:2868652. doi: 10.1155/2016/2868652. Epub 2016 Mar 16. PMID: 27069930; PMCID: PMC4812462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colberg SR, Hernandez MJ. The big blue test: effects of 14 minutes of physical activity on blood glucose levels. Diabetes Care. 2013. Feb;36(2):e21. doi: 10.2337/dc12-1671. PMID: 23349154; PMCID: PMC3554301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colberg SR, Hernandez MJ, Shahzad F. Blood glucose responses to type, intensity, duration, and timing of exercise. Diabetes Care. 2013. Oct;36(10):e177. doi: 10.2337/dc13-0965. PMID: 24065851; PMCID: PMC3781559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput JP, Chastin S, Chou R, Dempsey PC, DiPietro L, Ekelund U, Firth J, Friedenreich CM, Garcia L, Gichu M, Jago R, Katzmarzyk PT, Lambert E, Leitzmann M, Milton K, Ortega FB, Ranasinghe C, Stamatakis E, Tiedemann A, Troiano RP, van der Ploeg HP, Wari V, Willumsen JF. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020. Dec;54(24):1451-1462. doi: 10.1136/bjsports-2020-102955. PMID: 33239350; PMCID: PMC7719906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francavilla VC, Mingrino O, Di Corrado D, Bellomo M, Lanza G, Crescimanno C, Orofino F, Parisi MC. Endothelial Reprogramming in Sports Traumatology: Role of the Widespread Neuro-Immuno-Endocrine-Endothelial System. Appl Sci. 2023; 13, 170. doi: 10.3390/app13010 170. [Google Scholar]
- 33.Yardley JE, Kenny GP, Perkins BA, Riddell MC, Malcolm JS. Greater fluctuations in blood glucose seen both during and after aerobic exercise as compared to resistance exercise or no exercise in type 1 diabetes: a study using continuous glucose monitoring. Appl Physiol Nutr Metab 2010; 35(Suppl.):S112. [Google Scholar]


