Abstract
Objectives:
Dysphagia is prevalent in older adults and impacts health and quality of life. However, relationships between dysphagia and social determinants of health (SDOH) are uncertain. Our objective was to evaluate relationships between dysphagia and SDOH in older adults ≥65 years.
Methods:
Cross-sectional analyses were performed in community-dwelling Medicare beneficiaries included in the National Health & Aging Trends Study (NHATS). The primary exposure was self-reported difficulty chewing/swallowing in the prior month. Dependent measures were a variety of SDOH outcomes (e.g., food insecurity (FI), transportation access, being homebound, meal assistance). Associations between dysphagia and demographics were evaluated with weighted Chi-Square tests. Potential risk factors for dysphagia were assessed and purposeful selection was used in an unweighted logistic regression model to determine a final set of risk factors. Weighted logistic regression models were estimated to determine associations between dysphagia and SDOH outcomes. Control variables included demographic/health characteristics and dysphagia risk factors.
Results:
Of 4,041 participants, 428 (10.6%) self-reported dysphagia in the prior month. Risk factors for dysphagia included a number of health conditions (e.g., dementia), fall/balance concern/event, sleep problems, pain, unintended weight loss, and body mass index. In the adjusted model, dysphagia was associated with significantly increased odds for FI (odds ratio [OR] =1.48, 95% confidence interval [CI] =1.06 to 2.07, p=0.023) and being homebound (OR=1.32, 95% CI=1.13 to 1.55, p=<0.001).
Discussion:
Older adults with dysphagia had increased odds of FI and being homebound. These associations have implications for health-promoting interventions at the individual and policy levels in older adults.
Keywords: swallowing impairment, social determinants of health, National Health & Aging Trends Study (NHATS)
Swallowing is the sensorimotor process of moving boluses from the mouth to the stomach. Dysphagia (i.e., difficulty swallowing) prevalence rates increase with age (Cohen at al., 2021; Holland et al., 2011; Yang et al., 2013) and difficulty swallowing occurs in up to one-third of older adults living in the community (Yang et al., 2013). Older adults with dysphagia are at increased risk for health complications including malnutrition, dehydration, aspiration and non-aspiration pneumonia, hospital readmission and institutionalization which, collectively, lead to frailty, morbidity and mortality (Baijens et al. 2016). Individuals with swallowing difficulties also experience reduced quality of life and loss of independence and negative impacts on psychological well-being including embarrassment, anxiety, depression, social isolation, and decreased pleasure from eating (Ekberg et al., 2002; Farri et al., 2007; Han et al, 2011).
Social determinants of health (SDOH), which encompass the social, economic, and environmental conditions in which people live and age, are nonmedical factors that influence health outcomes and can significantly impact health disparities. The lack of economic security, safe and affordable housing, reliable transportation, access to healthcare services, and consistent access to sufficient food for an active and healthy life (i.e., food insecurity [FI]), can be particularly impactful on the health and quality of life of older Americans. For example, older adults with low income are at increased risk for earlier mortality (Cherry et al., 2016; Tucker-Seeley et al., 2009), frailty (Lee et al., 2018; Woo et al., 2005), disability (Lynch et al., 1997), being homebound (Ornstein et al., 2015; Ornstein et al., 2020), and FI (Goldberg & Mawn, 2015; Tucher et al., 2021). Given the impact of SDOH on health outcomes, intervening on these factors, in addition to applying traditional healthcare solutions, may more effectively address overall wellness and health disparities (Braveman et al., 2011). In order to promote healthy aging and meet the unique needs of older Americans, research that promotes an understanding of the relationships between social factors and health outcomes is vital to developing innovative intervention models. While dysphagia is common in older adults and impacts health and quality of life, little is known about the relationship between SDOH and dysphagia among all populations, especially in older adults at particular risk for swallowing difficulty (Leiman et al., 2022). Therefore, in a nationally-representative sample of US Medicare beneficiaries ≥65 years, we assessed associations between self-reported dysphagia and a number of SDOH outcomes including food insecurity (FI), transportation access, being homebound, use of meal assistance, neighborhood physical disorder, immediate built environment, community social cohesion, and technology access.
Methods
SAMPLE
Data are derived from 2019 Round 9 data of the National Health & Aging Trends Study (NHATS). NHATS is a cohort study of US Medicare beneficiaries ages 65 years and older. Patients were originally enrolled in NHATS in 2011 during Round 1 or in 2015 during Round 5 of the survey study. We included community-dwelling participants with non-missing responses to the question about difficulty chewing/swallowing in the month prior to the interview. We accounted for the NHATS sampling design weights (DeMatteis et al., 2020a) and multiply imputed income values (DeMatteis et al., 2020b).
MEASURES
The primary exposure was self-reported difficulty chewing or swallowing when eating in the month prior to the interview. Dependent measures were SDOH outcomes including FI, transportation access, being homebound, use of meal assistance, neighborhood physical disorder, immediate built environment, community social cohesion, and technology access based on established protocols within NHATS.
Food insecurity was derived from participants’ responses to the following five items: going without groceries, going without hot meals, going without eating due to lack of ability or social support in the prior month, skipping meals due to financial limitations in the prior month, and the number of days in the last month meals were skipped. A positive response to any of these items led to a classification of being food insecure (Tucher et al., 2021).
Transportation access was derived from participants’ responses to four items about driving, transportation being provided by family and/or friends, use of public transportation systems, or use of another mode of transportation such as a van, shuttle, or taxi. Responses were scored dichotomously (yes/no) and transportation access was classified as present with a positive response to any of these items (Keeney & Jette, 2019).
Being homebound was determined based on participants’ responses to three items: the frequency that they left the home, difficulty leaving the home, and whether help was needed to leave the home (Orstein et al., 2015). Participants were defined as homebound if they never or rarely left the home, semi-homebound if they left the home but required assistance or reported difficulty doing so, or not homebound, otherwise.
Meal assistance use was derived from participants’ responses about receiving Meals on Wheels services and responses were scored dichotomously.
Neighborhood physical disorder was derived from direct observation by the interviewer of physical disorder in the vicinity of the home including litter, broken glass, or trash on the sidewalks and streets, graffiti on buildings and walls, and vacant or deserted houses or storefronts, where each component was measured with a four-point scale (0=none, 1=a little, 2=some, and 3=a lot) (Latham & Clarke, 2018). We imputed each missing component score with the mean of the non-missing values, then dichotomized overall neighborhood physical disorder as present if any of the component scores was greater than or equal to 0.5.
Immediate built environment was assessed from direct observation by the interviewer of uneven walking surfaces or broken steps in the area leading to the home/building and responses were scored dichotomously (Clarke, 2014).
Community social cohesion was derived from participants’ responses to three questions about their residential community and how well people in the community know each other, are willing to help each other, and can be trusted. Using a three-point scale (1=agree a lot, 2=agree a little, 3=do not agree), the mean score across these three items was used to represent community social cohesion. Each missing component score was imputed with the mean of the non-missing values. If the average of the three component scores was greater than or equal to the sample 90th percentile, community social cohesion was low (set to 0); otherwise, community social cohesion was high (set to 1) (Latham & Clarke, 2018). Technology access was derived from participants’ responses to three items about cell phone, computer, and tablet access (1=yes, 2=no). Responses were scored dichotomously and negative responses to all three items led to lack of technology access classification.
STATISTICAL ANALYSIS
Subject demographics were summarized as unweighted frequencies and percentages by whether or not the subject reported experiencing dysphagia in the preceding month. Associations between demographics and self-reported dysphagia were evaluated with weighted Chi-Square tests.
As the first step in a two-stage analysis, potential risk factors for dysphagia (Yang et al., 2013; Madhavan et al., 2016) were posited, including demographic (age, sex, race/ethnicity, marital status, education, income, health insurance coverage, metropolitan status) and health characteristics (comorbid diseases, falls, fall concern, hip/other fracture, use of medical device, sleep problems, pain, overall health, frailty, Short Physical Performance Battery [SPPB], body mass index [BMI], weight loss). To determine a final set of risk factors for dysphagia, purposeful selection was used in an unweighted logistic regression model25. For each potential variable, simple logistic regression was performed and variables with a significant Wald test at α=0.25 were selected as candidates for multiple logistic regression. A multiple regression model was fit by iteratively removing non-significant covariates at α=0.1 level which were also not confounders. Confounders were defined as variables whose removal causes any remaining coefficient to change by at least 15%. To the resulting model, each variable not originally selected for consideration was separately added and evaluated for inclusion at the α=0.1 significance level. Finally, the multivariable model with any additionally added covariates was fit and reduced using the same backward selection method previously described. Since income was multiply imputed with five iterations, purposeful selection was conducted for each of the imputations. The final model retained only those variables included in all five models as risk factors. As a sensitivity analysis, stepwise selection with inclusion significance level α=0.3 and retaining level α=0.35 was also used to determine a set of risk factors, and the resulting models were compared using area under the curve.
In the second stage of the analysis, weighted unadjusted and adjusted logistic regression models were estimated to determine associations between self-reported dysphagia and SDOH outcomes. Control variables included demographic and health characteristics (age, sex, race/ethnicity, marital status, education, income, insurance, metropolitan status, number of comorbid diseases, overall health, frailty) and risk factors for dysphagia identified with purposeful selection.
Results
In this sample of 4,041 community-dwelling participants, self-reported dysphagia was present in 428 respondents (10.6%). Descriptive summary statistics with and without dysphagia are summarized in Table 1. A greater proportion of participants reported dysphagia than not among Hispanics (14.4% vs 6.5%), as well as those who were separated/divorced/widowed/never married (55.2% vs 43.7%), had less than high school education (26.3% vs 13.0%), had total income less than $27,600 (32.9% vs 20.9%), received Medicaid (24.3% vs 10.8%) and Tricare (7.1% vs 6.4%), did not have long-term care insurance (76.1% vs 70.1%), were rural residents (24.6% vs 17.5%), and received payment from the Department of Veterans Affairs (VA) in the prior month (10.1% vs 6.6%).
Table 1.
Descriptive summary statistics
| Dysphagia | ||||
|---|---|---|---|---|
| Characteristic | Total (N=4041) | Yes (N=428) | No (N=3613) | P-value |
| Age | 0.084 | |||
| 65 to 69 | 43 (2.3%) | 4 (2.4%) | 39 (2.3%) | |
| 70 to 74 | 898 (40.0%) | 76 (36.1%) | 822 (40.4%) | |
| 75 to 79 | 1117 (27.0%) | 99 (23.6%) | 1018 (27.4%) | |
| 80 to 84 | 916 (16.9%) | 100 (19.4%) | 816 (16.6%) | |
| 85 to 89 | 645 (8.9%) | 80 (10.9%) | 565 (8.7%) | |
| 90+ | 422 (4.9%) | 69 (7.6%) | 353 (4.6%) | |
| Sex | 0.243 | |||
| Male | 1710 (45.0%) | 189 (48.7%) | 1521 (44.6%) | |
| Female | 2331 (55.0%) | 239 (51.3%) | 2092 (55.4%) | |
| Race/Ethnicity | <.001 | |||
| White Non-Hispanic | 2794 (78.1%) | 277 (72.6%) | 2517 (78.7%) | |
| Black Non-Hispanic | 845 (8.0%) | 87 (6.9%) | 758 (8.1%) | |
| Other Non-Hispanic | 104 (4.0%) | 6 (2.7%) | 98 (4.2%) | |
| Hispanic | 239 (7.3%) | 49 (14.4%) | 190 (6.5%) | |
| More Than One and DKRF primary, DKRF | 59 (2.6%) | 9 (3.3%) | 50 (2.5%) | |
| Marital Status | <.001 | |||
| Separated/Divorced/Widowed/Never Married | 2123 (44.9%) | 256 (55.2%) | 1867 (43.7%) | |
| Married or Living with Partner | 1918 (55.1%) | 172 (44.8%) | 1746 (56.3%) | |
| Education | <.001 | |||
| None-12 grade | 770 (14.4%) | 121 (26.3%) | 649 (13.0%) | |
| HS/GED/Vocational Certificate/Some College | 1888 (46.7%) | 186 (43.2%) | 1702 (47.1%) | |
| Associate, Bachelor, Master Degree or Higher | 1327 (36.7%) | 112 (27.1%) | 1215 (37.8%) | |
| Total Income* | <.001 | |||
| < $27,600 | 1021 (22.1%) | 144 (32.9%) | 877 (20.9%) | |
| $27,600-$41,999 | 530 (13.1%) | 53 (13.9%) | 477 (13.1%) | |
| $42,000-$63,999 | 453 (12.3%) | 46 (12.3%) | 407 (12.3%) | |
| $64,000-$107,999 | 513 (16.5%) | 40 (11.1%) | 473 (17.1%) | |
| >= $108,000 | 338 (10.5%) | 20 (4.9%) | 318 (11.1%) | |
| Medicare Part D | 0.536 | |||
| No | 1022 (27.6%) | 105 (24.7%) | 917 (27.9%) | |
| Yes | 2808 (67.9%) | 300 (70.5%) | 2508 (67.6%) | |
| Medicare Gap/Supplemental | 0.423 | |||
| No | 1270 (29.6%) | 137 (30.5%) | 1133 (29.5%) | |
| Yes | 2621 (67.5%) | 269 (65.3%) | 2352 (67.7%) | |
| Medicaid | <.001 | |||
| No | 3342 (86.2%) | 316 (73.3%) | 3026 (87.7%) | |
| Yes | 618 (12.2%) | 98 (24.3%) | 520 (10.8%) | |
| Tricare | <.001 | |||
| No | 3732 (92.6%) | 388 (89.8%) | 3344 (92.9%) | |
| Yes | 266 (6.5%) | 26 (7.1%) | 240 (6.4%) | |
| Long-Term Care Insurance | 0.050 | |||
| No | 2775 (70.7%) | 310 (76.1%) | 2465 (70.1%) | |
| Yes | 1042 (24.5%) | 86 (19.0%) | 956 (25.1%) | |
| Urban Resident | 0.028 | |||
| No | 792 (18.2%) | 99 (24.6%) | 693 (17.5%) | |
| Yes | 3249 (81.8%) | 329 (75.4%) | 2920 (82.5%) | |
| Veteran Affairs Payment Last Month | 0.027 | |||
| No | 3735 (92.4%) | 384 (89.2%) | 3351 (92.7%) | |
| Yes | 274 (6.9%) | 39 (10.1%) | 235 (6.6%) | |
Non-imputed values only, DKRF=Don’t know or refused to answer, column percentage totals may not total 100% due to missing values.
The final set of predictors and related confounders identified as risk factors for dysphagia are presented in Table 2 with parameter estimates, 95% confidence intervals. Additionally, risk factors for dysphagia were identified with stepwise selection as a sensitivity analysis. The resulting models were compared using AUC, yielding mean (SD) values of 0.741 (0.0006) for purposeful selection and 0.743 (0.0005) for stepwise selection. Due to the comparable predictive performance and inclusion of more potential co-variates, the risk factors identified by purposeful selection were used to yield a more conservative estimate of the relationship between dysphagia and SDOH in the remainder of the analysis.
Table 2.
Risk factors for dysphagia identified with purposeful selection
| Effect | Level | OR (95% CI) | T | P-value |
|---|---|---|---|---|
| Age | 65 to 74 | 1.26 (0.98,1.62) | 1.78 | 0.076 |
| 75 to 79 | 0.91 (0.67,1.22) | -0.63 | 0.529 | |
| 80 to 84 | 1.02 (0.74,1.40) | 0.11 | 0.912 | |
| 85 to 89 | 0.98 (0.74,1.30) | -0.14 | 0.885 | |
| ≥ 90 | Reference | |||
| Sex | Female | 0.79 (0.65,0.95) | -2.45 | 0.014 |
| Race | Black Non-Hispanic | 0.86 (0.57,1.30) | -0.71 | 0.480 |
| Hispanic | 1.56 (1.14,2.14) | 2.77 | 0.006 | |
| Other Non-Hispanic, More than one, DKRF | 0.57 (0.29,1.12) | -1.63 | 0.103 | |
| White Non-Hispanic | Reference | |||
| Highest Education Level | Associate, Bachelor, Master Degree or Higher | 0.92 (0.70,1.21) | -0.59 | 0.558 |
| HS/GED/Vocational Certificate/Some College | 0.88 (0.68,1.14) | -0.95 | 0.340 | |
| Less than HS | Reference | |||
| Total Income | < $27,600 | 1.10 (0.81,1.51) | 0.63 | 0.531 |
| $27,600-$41,999 | 1.06 (0.72,1.57) | 0.31 | 0.760 | |
| $42,000-$63,999 | 1.35 (0.94,1.95) | 1.63 | 0.107 | |
| $64,000-$107,999 | 1.00 (0.67,1.49) | -0.00 | 0.996 | |
| ≥ $108,000 | Reference | |||
| Medicaid | 1.20 (0.96,1.49) | 1.61 | 0.108 | |
| Long-Term Care Insurance | 0.93 (0.77,1.14) | -0.69 | 0.489 | |
| Urban resident | 0.85 (0.67,1.07) | -1.37 | 0.172 | |
| Heart Attack (new) | 1.44 (1.07,1.93) | 2.44 | 0.015 | |
| Heart Disease | 0.91 (0.80,1.03) | -1.52 | 0.129 | |
| High Blood Pressure | 0.86 (0.72,1.03) | -1.63 | 0.103 | |
| Osteoporosis | 1.12 (0.94,1.33) | 1.29 | 0.199 | |
| Lung Disease | 1.08 (0.95,1.24) | 1.20 | 0.230 | |
| Cancer (new) | 1.17 (0.89,1.53) | 1.15 | 0.249 | |
| Dementia or Alzheimer’s | 1.19 (1.01,1.41) | 2.04 | 0.041 | |
| Fall/Balance Concern | 1.44 (1.23,1.69) | 4.46 | <.001 | |
| Fall Event in Last Year | 1.12 (0.94,1.34) | 1.27 | 0.204 | |
| Sleep Problems in Last Month | 2+ nights a week for both | 1.17 (0.96,1.42) | 1.58 | 0.113 |
| 2+ nights a week for either | 0.88 (0.71,1.09) | -1.15 | 0.248 | |
| No Problems | Reference | |||
| Pain in Last Month | 1.44 (1.19,1.74) | 3.78 | <.001 | |
| Overall Health in Last Month | Fair/Poor (vs Good/Excellent) | 1.35 (1.15,1.58) | 3.66 | <.001 |
| Frailty Level | Frail | 1.67 (1.18,2.34) | 2.93 | 0.003 |
| Prefrail | 0.94 (0.70,1.26) | -0.43 | 0.667 | |
| Robust | Reference | |||
| Unintended Weight Loss | 1.07 (0.87,1.30) | 0.62 | 0.538 | |
| Body Mass Index (BMI) Category | Obesity (BMI ≥30) | 0.77 (0.57,1.05) | -1.65 | 0.099 |
| Overweight (BMI 25–29.9) | 1.29 (1.01,1.64) | 2.06 | 0.039 | |
| Underweight (BMI <18.5) | 0.59 (0.33,1.05) | -1.80 | 0.072 | |
| Normal (BMI 18.5–24.9) | Reference |
Mean (SD) c-index across imputations is 0.741 (0.0006).
Results of the unadjusted and adjusted logistic regression models for SDOH outcomes with self-reported dysphagia being the exposure of interest are provided in Table 3. While controlling for demographics, health characteristics, and dysphagia risk factors, dysphagia was associated with increased odds for FI (odds ratio [OR]=1.48, 95% confidence interval [CI]=1.06 to 2.07) and for being homebound (OR=1.32, 95% CI=1.13 to 1.55). Dysphagia was also associated with increased odds of no transportation access, though these results were unreliable due to low event counts. Dysphagia was not associated with technology access, receipt of meal assistance, immediate built environment, neighborhood physical disorder, or community social cohesion.
Table 3.
Associations between dysphagia and selected social determinants of health (SDOH)
| Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|
| Outcome | Event Rate N (%) | OR (95% CI) | P-value | OR (95% CI) | P-value |
| Technology Access (N=3994; 3328) | 3683 (94.8) | 0.81 (0.65, 1.02) | 0.073 | 1.18 (0.78, 1.77) | 0.437 |
| Food Insecurity (N=4041; 3361) | 180 (4.0) | 2.01 (1.62, 2.50) | <.001 | 1.48 (1.06, 2.07) | 0.023 |
| Meal Assistance (N=4027; 3354) | 296 (5.1) | 1.47 (1.16, 1.87) | 0.001 | 1.18 (0.92, 1.51) | 0.185 |
| Immediate Built Environment (N=3954; 3294) | 419 (9.6) | 1.16 (0.94, 1.42) | 0.175 | 0.92 (0.68, 1.23) | 0.556 |
| Transportation Access* (N=3983; 3315) | 3980 (99.0) | 0.52 (0.16, 1.73) | 0.285 | 1.84 (1.23, 2.75) | 0.003 |
| Being Homebound (N=4037; 3361) | 256 (4.2) | 1.97 (1.72, 2.26) | <.001 | 1.32 (1.13, 1.55) | <.001 |
| Neighborhood Physical Disorder (N=4041; 3361) | 334 (7.2) | 1.20 (0.96, 1.49) | 0.105 | 0.98 (0.77, 1.26) | 0.898 |
| Community Social Cohesion (N=4041; 3361) | 555 (12.5) | 0.74 (0.64, 0.87) | <.001 | 0.88 (0.70, 1.12) | 0.334 |
Unreliable results due to low event count for no transportation. Control variables include age, sex, race/ethnicity, marital status, education, income, insurance, metropolitan status, number of comorbid diseases, overall health, frailty, and risk factors for dysphagia determined in Table 2.
Discussion
In this cross-sectional study, we evaluated the relationships between dysphagia and relevant SDOH. In this cohort of community-dwelling Medicare beneficiaries, the prevalence of self-reported dysphagia was 10.6%. Dysphagia was associated with ethnicity, marital status, levels of education and income, and receipt of VA payment in the last month. While controlling for numerous relevant factors including medical comorbidity and frailty in our adjusted model, the presence of dysphagia was associated with a 48% increased odds for FI and being homebound by 32%. Our findings regarding the co-existence of dysphagia with FI and being homebound have important implications for older adults related to health outcomes; access to healthcare; medical adherence; and screening, assessment, and treatment.
The complications of dysphagia in older adults include malnutrition and dehydration, aspiration and non-aspiration pneumonia, and hospital readmission and institutionalization. Further, dysphagia has negative effects on QOL and psychological well-being leading to frailty, morbidity, and mortality (Ekberg et al., 2002). The co-occurrence of dysphagia with FI or being homebound may increase the vulnerability of older adults to the negative consequences of dysphagia. For example, considering that dysphagia (Carrión et al., 2015; Serra-Prat et al., 2012), FI (Grammatikopoulou et al., 2019), and being homebound (Sharkey et al., 2002) are each independently associated with malnutrition in this population, it may be that there are additive or negative synergistic effects when both dysphagia and FI or being homebound are present.
The presence of FI or being homebound may also negatively influence older adults’ ability to seek dysphagia assessment and treatment services. For example, being homebound interferes with the ability to access medical care (Leff et al., 2015), including recommended care patterns such as annual physician visits (Musich et al., 2015). Similarly, adults with FI are more likely to delay or even forgo necessary medical care (Bertoldo et al., 2022; Bhargava & Lee, 2016). While telehealth services have been advocated as a method to improve healthcare access, there is evidence that disparities exist in this sphere as well (Sachs et al., 2021; Saeed & Masters, 2021). Therefore, older adults with dysphagia and co-existing FI or who are homebound may have similar barriers that interfere with their access to healthcare services to assess and treat their swallowing difficulty.
Further, both being food insecure (Berkowitz et al., 2014) and being homebound are associated with decreased medication adherence (Musich et al., 2015). Due to their relationships with financial, social, and functional limitations, FI (Tucher et al., 2021) and being homebound (Schirghuber & Schrems, 2021) may negatively influence seniors’ adherence with dysphagia treatment recommendations, in those able to access care. For example, dietary modifications such as soft foods or thickened liquids are a commonly utilized approach for the management of dysphagia. However, such diets limit flexibility in food selections, require the use of specialized supplies and equipment, and overall increase the burden for meal preparation in terms of cost, time, and effort (Asher Wolf et al., 2016; Coutts & Solomon, 2020). Individuals with dysphagia and FI or who are homebound may lack the financial resources, social support, and functional capacity to implement and adhere to such specialized diets. Additionally, social programs that support food security in older adults (e.g., Meals on Wheels) may lack the flexibility to meet the specialized food needs of patients with dysphagia.
While we did identify an association between dysphagia and being homebound in the current study, our adjusted model did not detect relationships between dysphagia and use of meal assistance programs. This was an unexpected finding as meal assistance programs are primarily used by homebound older adults (Lloyd & Wellman, 2015). However, there was an unadjusted association between dysphagia and increased use of meal assistance programs. Although meal assistance recipients are diverse in terms of their demographics, commonly shared features include frailty and limited mobility (Frongillo et al., 2010). The present study, which controlled for comorbid conditions and frailty, may have been underpowered to detect relationships between dysphagia and the use of meal assistance programs and future studies with larger samples may be needed.
Among the 428 NHATS respondents who reported dysphagia, 49 (11.5%) were food insecure, suggesting an important role in screening for FI in older adults with dysphagia. Identifying older adults with dysphagia and, in particular, those with FI, remains an important question future studies must answer. Yet, due to its prevalence, covert nature, impact on health outcomes, and the availability of interventions, routine screening for FI in older adults has been advocated (Coutts & Solomon, 2020; Pooler et al., 2019). Sensitive and specific screening tools for FI in older adults are available such as the Hunger Vital Sign (Gunderson et al., 2017). Given our findings, this is also applicable for assessing homebound status in older adults. Tools are also available to assess an individual’s homebound status (Eghtesadi, 2019; Weiss & Milone-Nuzzo, 1999). Considering our results and previous findings suggesting that homebound individuals may not be strong advocates for themselves (Sawchuk, 2019), careful assessment of homebound status may be valuable in older adults with dysphagia. For example, identification of older adults with dysphagia who are homebound or have FI may lead to the delivery of home-based dysphagia management services such as dysphagia therapy and nutritional support. Understanding how FI or being homebound impacts the effective management of dysphagia in older adults is an important area of investigation. Although we acknowledge that the current study is cross-sectional in nature and has limited ability to identify causal relationships, the current findings may inform future longitudinal and interventional studies.
Although we are not aware of any prior research examining relationships between dysphagia and FI, some data are available to support the notion that dysphagia is linked with being homebound. Mikami and colleagues (2019) examined the relationship between the frequency of going out of the home and dry mouth, difficulty chewing, and difficulty swallowing based on questions from the Kihon Checklist in community-dwelling older adults. This study found decreased frequency of these outings was associated with both dry mouth and difficulty chewing, but not difficulty swallowing. Using the same tool, Sugiura et al. (2018) also reported a relationship between difficulty chewing and frequency of going out among mildly dependent community-dwelling older adults48. In both of these studies, difficulty swallowing was determined based on the response to questions about choking or coughing when taking tea or soup, while difficulty chewing was ascertained based on response to a query about increased difficulty chewing tough foods compared to 6 months previously. In contrast, our participants were asked whether they had difficulty chewing or swallowing in the prior month. Consistent with accepted definitions, a positive response to this question resulted in the identification of dysphagia. If these methodological differences are considered, our results align well with these prior studies, suggesting the presence of a linear correlation between dysphagia and the ability of older adults to leave their homes.
Limitations of this research include the cross-sectional design in which the exposure and outcomes were measured at the same time, making it difficult to establish causal relationships. Cross-sectional designs may also be susceptible to recall bias in which participants recall information on exposure differently based on the outcome. Dysphagia was classified by self-report, and determining evidence of objective swallowing dysfunction was not possible. However, patients with normal oropharyngeal swallowing on objective testing and with self-reported dysphagia still demonstrate signs and symptoms of aspiration, reduced dietary intake, and reduced activity (Canick et al., 2022; Dewan et al., 2021). Thus, self-reported dysphagia and its relationship to FI and homebound status have substantial implications for the health of older adults that will contribute to future research in this area.
In conclusion, our study found that 10.6% of community dwelling older individuals had self-reported dysphagia. Older adults with dysphagia had 48% and 32% increased odds for FI and being homebound, respectively. Future investigation of the relationship between dysphagia with FI and homebound status and the impacts on healthy aging is warranted. Future research is needed to better understand how best to screen, not only for dysphagia in older adults, but also concomitant FI and being homebound. Interventions to address dysphagia, nutrition, and SDOH factors are also needed.
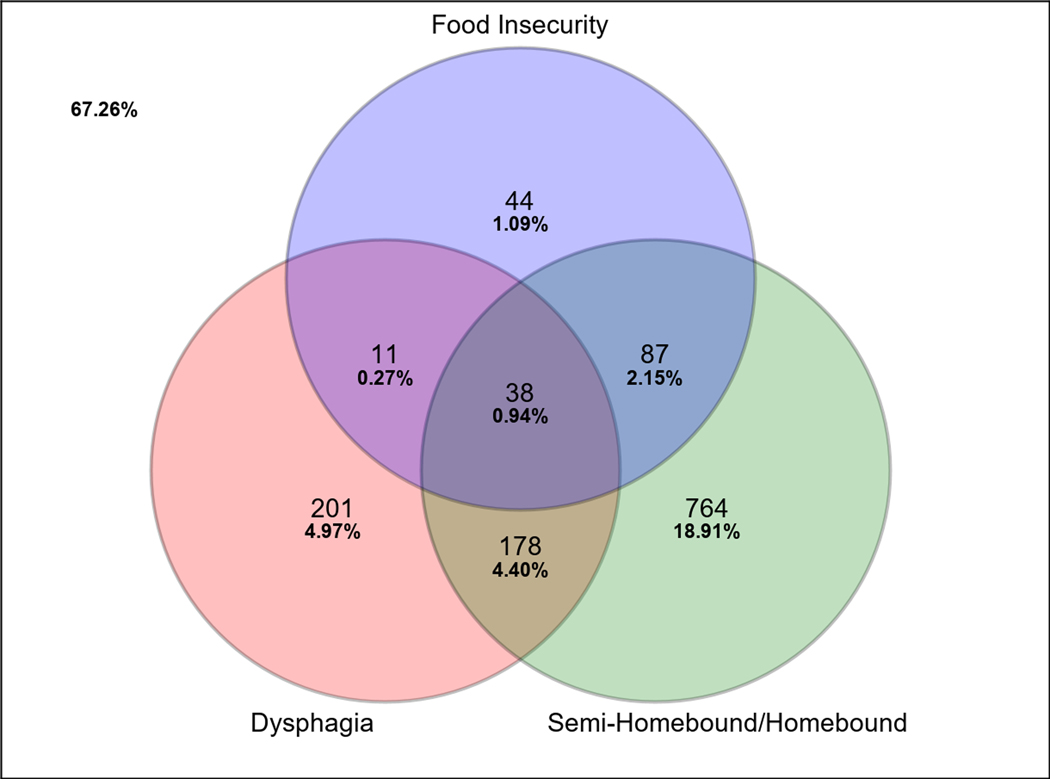
Figure 1.
Venn diagram illustrating the frequencies and co-occurrence of dysphagia, food insecurity, and being homebound in this sample of 4041 community-dwelling older adults
Funding:
This work was supported by the U.S. Department of Veterans Affairs Rehabilitation Research and Development Service Program (Grant RR&D IK2 RX002348) and the Pepper Incubation Award, Duke Claude D. Pepper Older American Independence Center (Grant NIA P30 AG028716).
HNJ has received research/grant support and honoraria from Sanofi Genzyme; is a consultant for Aspire Products LLC and receives royalties for sales of the Inspiratory Adapter 150; is one of the inventors of the Observer-Reported Communication Ability (ORCA) Measure which has been licensed to Pattern Health for commercial distribution; has received honoraria from Amicus Therapeutics; and receives royalties from Northern Speech Services. DAL is an educational consultant for Medtronic. KPS is on the speaker bureau for Abbott Nutrition Health Institute and a scientific advisor for Vivo. SMC is a consultant for Zsquare and is a Data Safety Monitoring Board Member for Syneos Health.
Footnotes
Conflicts of Interest: RN, CFP, and RDR have no conflicts of interest to report.
References
- Asher Wolf W, Huang KZ, Durban R, Iqbal ZJ, Robey BS, Khalid FJ, & Dellon ES (2016). The six-food elimination diet for eosinophilic esophagitis increases grocery shopping cost and complexity. Dysphagia, 31(6), 765–70. DOI: 10.1007/s00455-016-9739-1 [DOI] [PubMed] [Google Scholar]
- Baijens LW, Clavé P, Cras P, Ekberg O, Forster A, Kolb GF, Leners J-C, Masiero S, Mateos-Nozal J, Ortega O, Smithard DG, Speyer R, & Walshe M. (2016). European Society for Swallowing Disorders - European Union Geriatric Medicine Society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clinical Interventions in Aging, 11, 1403–28. DOI: 10.2147/CIA.S107750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz SA, Seligman HK, & Choudhry NK (2014). Treat or eat: food insecurity, cost-related medication underuse, and unmet needs. The American Journal of Medicine, 127(4):303–310.e3. DOI: 10.1016/j.amjmed.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Bertoldo J, Wolfson JA, Sundermeir SM, Edwards J, Gibson D, Agarwal S, & Labrique A. (2022). Food insecurity and delayed or forgone medical care during the COVID-19 pandemic. American Journal of Public Health, 112(5), 776–85. DOI: 10.2105/AJPH.2022.306724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava V, & Lee JS (2016). Food insecurity and health care utilization among older adults in the United States. Journal of Nutrition in Gerontology and Geriatrics, 35(3), 177–92. DOI: 10.1080/21551197.2016.1200334 [DOI] [PubMed] [Google Scholar]
- Braveman P, Egerter S, & Williams DR (2011). The social determinants of health: coming of age. Annual Review of Public Health, 32, 381–98. DOI: 10.1146/annurev-publhealth-031210-101218 [DOI] [PubMed] [Google Scholar]
- Bursac Z, Gauss CH, Williams DK, & Hosmer DW (2008). Purposeful selection of variables in logistic regression. Source Code for Biology and Medicine, 3, 17. DOI: 10.1186/1751-0473-3-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canick J, Campbell JC, Cohen SM, Jones HN, Leiman DA, Raman S, & Porter Starr KN (2022). Preoperative dysphagia risk in community-dwelling adults aged ≥50 years: prevalence and risk factors. Nutrition in Clinical Practice. DOI: 10.1002/ncp.10889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión S, Cabré M, Monteis R, Roca M, Palomera E, Serra-Prat M, Rofes L, & Clavé P. (2015). Oropharyngeal dysphagia is a prevalent risk factor for malnutrition in a cohort of older patients admitted with an acute disease to a general hospital. Clinical Nutrition, 34(3), 436–42. DOI: 10.1016/j.clnu.2014.04.014 [DOI] [PubMed] [Google Scholar]
- Cherry KE, Brown JS, Kim S, & Jazwinski SM (2016). Social factors and healthy aging: findings from the Louisiana Healthy Aging Study (LHAS). Kinesiology Review, 5(1), 50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJ (2014). The role of the built environment and assistive devices for outdoor mobility in later life. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 69(Suppl 1), S8–15. DOI: 10.1093/geronb/gbu121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SM, Porter Starr KN, Risoli T, Lee H-J, Misono S, Jones H, & Raman S. (2021). Association between dysphagia and surgical outcomes across the continuum of frailty. Journal of Nutrition in Gerontology and Geriatrics, 40(2–3), 59–79. DOI: 10.1080/21551197.2021.1929644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts KA, Solomon M. (2020). The use of diet modifications and third-party disability in adult dysphagia: The unforeseen burden of caregivers in an economically developing country. South African Journal of Communication Disorders, 67(1):e1–e8. DOI: 10.4102/sajcd.v67i1.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMatteis JM, Freedman VA, Jiao R, & Kasper JD (2020a). National Health and Aging Trends Study development of round 9 survey weights. Available from: https://www.nhats.org/sites/default/files/202101/NHATS_Round_9_Weights_Technical_Paper.pdf
- DeMatteis J, Freedman VA, Kasper JD, & Jiao R. (2020b). National Health and Aging Trends Study round 9 income imputation. Available from: https://www.nhats.org/sites/default/files/2021-01/NHATS_Round%209_Income_Imputation_090120.pdf
- Dewan K, Clarke JO, Kamal AN, Nandwani M, & Starmer HM (2021). Patient reported outcomes and objective swallowing assessments in a multidisciplinary dysphagia clinic. Laryngoscope, 131(5), 1088–94. DOI: 10.1002/lary.29194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghtesadi M. (2019). Assessing extreme elderly homebound patients with severe loss of autonomy: proposal for a practice-based periodic health examination form. Canadian Family Medicine, 65(11), 841–4. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6853345/pdf/0650841.pdf [PMC free article] [PubMed] [Google Scholar]
- Ekberg O, Hamdy S, Woisard V, Wuttge-Hannig A, & Ortega P. (2002). Social and psychological burden of dysphagia: its impact on diagnosis and treatment. Dysphagia, 17(2), 139–46. DOI: 10.1007/s00455-001-0113-5 [DOI] [PubMed] [Google Scholar]
- Farri A, Accornero A, & Burdese C. (2007). Social importance of dysphagia: its impact on diagnosis and therapy. Acta Otorhinolaryngologica Italica, 27(2), 83–6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2640007/pdf/0392-100X.27.083.pdf [PMC free article] [PubMed] [Google Scholar]
- Frongillo EA, Cantor MH, MacMillan T, Issacman TD, Sherrow R, Henry M, Wethington E, & Pillemer K. (2010). Who are the recipients of Meals-on-Wheels in New York City?: a profile of based on a representative sample of Meals-on-Wheels recipients, Part I. Care Management Journal, 11(1), 19–40. DOI: 10.1891/1521-0987.11.1.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SL, & Mawn BE (2015). Predictors of food insecurity among older adults in the United States. Public Health Nursing, 32(5), 397–407. DOI: 10.1111/phn.12173 [DOI] [PubMed] [Google Scholar]
- Grammatikopoulou MG, Gkiouras K, Theodoridis X, Tsisimiri M, Markaki AG, Chourdakis M, & Goulis DG (2009). Food insecurity increases the risk of malnutrition among community-dwelling older adults. Maturitas, 119, 8–13. DOI: 10.1016/j.maturitas.2018.10.009 [DOI] [PubMed] [Google Scholar]
- Gundersen C, Engelhard EE, Crumbaugh AS, & Seligman HK (2017). Brief assessment of food insecurity accurately identifies high-risk US adults. Public Health Nutrition, 20(8), 1367–71. DOI: 10.1017/S1368980017000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Ohnishi H, Nonaka M, Yamauchi R, Hozuki T, Hayashi T, Saitoh M, Hisahara S, Imai T, & Mori M. (2011). Relationship between dysphagia and depressive states in patients with Parkinson’s disease. Parkinsonism & Related Disorders, 17(6), 437–9. DOI: 10.1016/j.parkreldis.2011.03.006 [DOI] [PubMed] [Google Scholar]
- Holland G, Jayasekeran V, Pendleton N, Jones M, & Hamdy S. (2011). Prevalence and symptom profiling of oropharyngeal dysphagia in a community dwelling of an elderly population: a self-reporting questionnaire survey. Diseases of the Esophagus, 24(7), 476–80. DOI: 10.1111/j.1442-2050.2011.01182.x [DOI] [PubMed] [Google Scholar]
- Keeney T, & Jette AM (2019). Individual and environmental determinants of late-life community disability for persons aging with cardiovascular disease. American Journal of Physical Medicine & Rehabilitation, 98(1), 30–4. DOI: 10.1097/PHM.0000000000001011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latham K, & Clarke PJ (2018). Neighborhood disorder, perceived social cohesion, and social participation among older Americans: findings from the National Health & Aging Trends Study. Journal of Aging and Health, 30(1), 3–26. DOI: 10.1177/0898264316665933 [DOI] [PubMed] [Google Scholar]
- Lee DR, Santo EC, Lo JC, Ritterman Weintraub ML, Patton M, & Gordon NP (2018). Understanding functional and social risk characteristics of frail older adults: a cross-sectional survey study. BMC Family Practice, 19(1), 170. DOI: 10.1186/s12875-018-0851-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff B, Carlson CM, Saliba D, & Ritchie C. (2015). The invisible homebound: setting quality-of-care standards for home-based primary and palliative care. Health Affairs, 34(1), 21–9. DOI: 10.1377/hlthaff.2014.1008 [DOI] [PubMed] [Google Scholar]
- Leiman DA, Madigan K, Carlin M, Cantrell S, & Palakshappa D. (2022). Food insecurity in digestive diseases. Gastroenterology, 163(3), 547–551. DOI: 10.1053/j.gastro.2022.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd JL, & Wellman NS (2015). Older Americans Act Nutrition Programs: a community- based nutrition program helping older adults remain at home. Journal of Nutrition in Gerontology and Geriatrics, 34(2), 90–109. DOI: 10.1080/21551197.2015.1031592 [DOI] [PubMed] [Google Scholar]
- Lynch JW, Kaplan GA, & Shema SJ Cumulative impact of sustained economic hardship on physical, cognitive, psychological, and social functioning. New England Journal of Medicine, 337(26), 1889–95. DOI: 10.1056/NEJM199712253372606 [DOI] [PubMed] [Google Scholar]
- Madhavan A, LaGorio LA, Crary MA, Dahl WJ, & Carnaby GD (2016). Prevalence of and risk factors for dysphagia in the community dwelling elderly: a systematic review. Journal of Nutrition, Health & Aging, 20(8), 806–15. DOI: 10.1007/s12603-016-0712-3 [DOI] [PubMed] [Google Scholar]
- Mikami Y, Watanabe Y, Motokawa K, Shirobe M, Motohashi Y, Edahiro A, Nakajima J, Osuka Y, Inagaki H, Fujiwara Y, Shinkai S, & Awata S. (2019). Association between decrease in frequency of going out and oral function in older adults living in major urban areas. Geriatrics & Gerontology International, 19(8), 792–7. DOI: 10.1111/ggi.13715 [DOI] [PubMed] [Google Scholar]
- Musich S, Wang SS, Hawkins K, & Yeh CS. Homebound older adults: prevalence, characteristics, health care utilization and quality of care. Geriatric Nursing, 36(6), 445–50. DOI: 10.1016/j.gerinurse.2015.06.013 [DOI] [PubMed] [Google Scholar]
- Ornstein KA, Garrido MM, Bollens-Lund E, Reckrey RM, Husain M, Ferreira KB, Liu SH, Ankuda CK, Kelley AS, & Siu AL (2020). The association between income and incident homebound status among older Medicare beneficiaries. Journal of the American Geriatrics Society, 68(11), 2594–601. DOI: 10.1111/jgs.16715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein KA, Leff B, Covinsky KE, Ritchie CS, Federman AD, Roberts L, Kelley AS, Siu AL, & Szanton SL (2015). Epidemiology of the homebound population in the United States. JAMA Internal Medicine, 175(7), 1180–6. DOI: 10.1001/jamainternmed.2015.1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler JA, Hartline-Grafton H, DeBor M, Sudore RL, & Seligman HK (2019). Food insecurity: a key social determinant of health for older adults. Journal of the American Geriatrics Society, 67(3), 421–4. DOI: 10.1111/jgs.15736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs JW, Graven P, Gold JA, & Kassakian SZ Disparities in telephone and video telehealth engagement during the COVID-19 pandemic. JAMIA Open, 4(3), ooab056. DOI: 10.1093/jamiaopen/ooab056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed SA, & Masters RM (2021) Disparities in health care and the digital divide. Current Psychiatry Reports, 23(9):61. DOI: 10.1007/s11920-021-01274-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchuk P. (2019). Caring for patients at home. Canadian Family Medicine, 65(2):149. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6515498/pdf/0650149.pdf [PMC free article] [PubMed] [Google Scholar]
- Schirghuber J, & Schrems B. Homebound: a concept analysis. Nursing Forum, 56(3), 742–51. DOI: 10.1111/nuf.12586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Prat M, Palomera M, Gomez C, Sar-Shalom D, Saiz A, Montoya JG, Navajas M, Palomera E, Clavé P. (2012). Oropharyngeal dysphagia as a risk factor for malnutrition and lower respiratory tract infection in independently living older persons: a population-based prospective study. Age and Aging, 41(3), 376–81. DOI: 10.1093/ageing/afs006 [DOI] [PubMed] [Google Scholar]
- Sharkey JR, Branch LG, Zohoori N, Giuliani C, Busby-Whitehead J, & Haines PS (2002). Inadequate nutrient intakes among homebound elderly and their correlation with individual characteristics and health-related factors. The American Journal of Clinical Nutrition, 76(6), 1435–45. DOI: 10.1093/ajcn/76.6.1435 [DOI] [PubMed] [Google Scholar]
- Sugiura K, Hayashi C, & Yokoshima K. (2018). Declining oral functioning’s effects on the frequency of leaving one’s home: examining individuals living in the community who require minor nursing support. Journal of Japan Health Medicine Association, 26(4), 232–40. Available from: https://www.jstage.jst.go.jp/article/kenkouigaku/26/4/26_232/_pdf/-char/en [Google Scholar]
- Tucher EL, Keeney T, Cohen AJ, & Thomas KS (2021). Conceptualizing food insecurity among older adults: development of a summary indicator in the National Health and Aging Trends Study. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 76(10), 2063–72. DOI: 10.1093/geronb/gbaa147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Seeley RD, Subramanian SV, Li Y, & Sorensen G. (2009). Neighborhood safety, socioeconomic status, and physical activity in older adults. American Journal of Preventive Medicine, 37(3), 207–13. DOI: 10.1016/j.amepre.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RL, & Milone-Nuzzo P. (1999). A tool to assess homebound status. Home Healthcare Nurse, 17(8), 486–90. DOI: 10.1097/00004045-199908000-00003 [DOI] [PubMed] [Google Scholar]
- Woo J, Goggins W, Sham A, & Ho SC (2005). Social determinants of frailty. Gerontology, 51(6), 402–8. DOI: 10.1159/000088705 [DOI] [PubMed] [Google Scholar]
- Yang EJ, Kim MH, Lim JY, & Paik N. -J. (2013). Oropharyngeal dysphagia in a community-based elderly cohort: the Korean longitudinal study on health and aging. Journal of Korean Medical Science, 28(10), 1534–9. DOI: 10.3346/jkms.2013.28.10.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]