This comprehensive set of clinical investigations may help to distinguish patients with fibromyalgia syndrome and small fiber neuropathy in clinical practice.
Keywords: Fibromyalgia syndrome, Small fiber neuropathy, Clinical phenotype, Pain pattern, Differential diagnosis
Abstract
Introduction:
Fibromyalgia syndrome (FMS) and small fiber neuropathy (SFN) are distinct pain conditions that share commonalities and may be challenging as for differential diagnosis.
Objective:
To comprehensively investigate clinical characteristics of women with FMS and SFN to determine clinically applicable parameters for differentiation.
Methods:
We retrospectively analyzed medical records of 158 women with FMS and 53 with SFN focusing on pain-specific medical and family history, accompanying symptoms, additional diseases, and treatment. We investigated data obtained using standardized pain, depression, and anxiety questionnaires. We further analyzed test results and findings obtained in standardized small fiber tests.
Results:
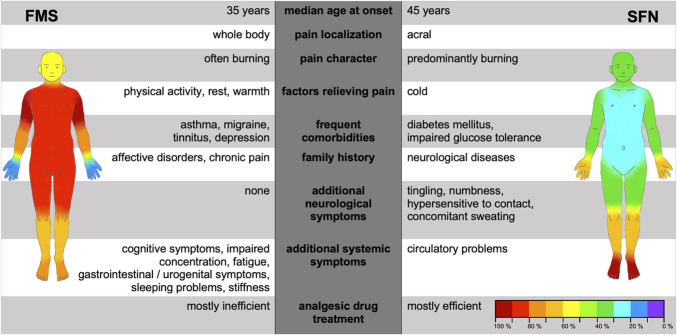
FMS patients were on average ten years younger at symptom onset, described higher pain intensities requiring frequent change of pharmaceutics, and reported generalized pain compared to SFN. Pain in FMS was accompanied by irritable bowel or sleep disturbances, and in SFN by paresthesias, numbness, and impaired glucose metabolism (P < 0.01 each). Family history was informative for chronic pain and affective disorders in FMS (P < 0.001) and for neurological disorders in SFN patients (P < 0.001). Small fiber pathology in terms of skin denervation and/or thermal sensory threshold elevation was present in 110/158 (69.7 %) FMS patients and 39/53 (73.6 %) SFN patients. FMS patients mainly showed proximally reduced skin innervation and higher corneal nerve branch densities (p<0.001) whereas SFN patients were characterized by reduced cold detection and prolonged electrical A-delta conduction latencies (P < 0.05).
Conclusions:
Our data show that FMS and SFN differ substantially. Detailed pain, drug and family history, investigating blood glucose metabolism, and applying differential small fiber tests may help to improve diagnostic differentiation and targeted therapy.
1. Introduction
Small fiber pathology is defined as clinical symptoms of small nerve fiber impairment accompanied by signs of small fiber damage on functional or conduction and/or morphological level.10 Damage to the small caliber nerve fibers as a hallmark of small fiber pathology is also present in a subgroup of patients with fibromyalgia syndrome (FMS)31,38,49 giving rise to the question, whether FMS equals small fiber neuropathy (SFN).47 There is multilevel evidence for the distinction between FMS and SFN. The traditional clinical description of FMS is deeply located chronic widespread pain with additional symptoms such as depression and fatigue.15,24,55 In SFN, superficial acral burning pain is predominant, accompanied by sensory disturbance and autonomic dysfunction.2 Electrophysiologically evoked potentials investigating A-delta and C nerve fibers and microneurography have provided data supporting a distinction between FMS and SFN in some studies10,11,38,49 while not in others.50,52 Morphologically, loss of skin nociceptors is a confirmed finding in subgroups of FMS patients, which results in distinct innervation patterns.7,9,11,20,46,52 However, clinical investigations so far failed to determine defined differences in FMS subgroups with and without small fiber pathology.12 In contrast, some studies reported no small fiber pathology in FMS patients.14,50,51 On a functional level, elevated thermal perception thresholds were reported in FMS.11,32
The question remains, if clinically applicable characteristics can be found that may help distinguishing FMS from SFN. So far, one study assessed the intergroup difference of patients with FMS with and without small fiber pathology using questionnaire data and reported minor differences.25 We retrospectively studied an extensive data set of women with FMS and SFN who were recruited in 2 previous studies10,11 asking for potentially distinguishing factors to be used in clinical practice.
2. Patients and methods
2.1. Patients
We retrospectively analyzed clinical data of 158 women with FMS11 and 53 women with SFN,10 who were monocentrically recruited during 2 individual studies at our Department of Neurology, University of Würzburg, Germany. The respective studies were approved by the Ethics Committee of the University of Würzburg (#121/14 and #135/15), and all participants gave written informed consent before study inclusion. For study inclusion, current diagnostic criteria for FMS54,55 and SFN8 were applied. The following inclusion criteria were further observed for both patient groups: adult patients and no hints for polyneuropathy in the neurological examination and nerve conduction studies. Exclusion criteria for both cohorts were as follows: pain of other origin, renal insufficiency, previously diagnosed diabetes mellitus, untreated thyroid dysfunction, acute infection, malignancy within the last 5 years, epilepsy, drug or alcohol abuse, eye diseases or surgery, usage of hard contact lenses, cardiac pacemaker, and pending compensation claims. In the SFN cohort, patients with B12 hypovitaminosis were additionally excluded.10 In the FMS cohort, severe psychiatric disorder currently requiring treatment was another exclusion criterion.11 All patients were interviewed in a standardized manner and neurologically investigated by a neurologist (D.E., N.Ü.). The SFN cohort consists of patients seen as regular inpatients or outpatients at our department. Patients with FMS were recruited for study participation from all over Germany.
2.2. Pain characterization
Individual pain characteristics were determined by spontaneously reported descriptors of the patients covering pain phenotype (character, intensity, location, radiation, onset, relieving, and aggravating factors) and symptoms accompanying pain. Intensities were reflected on a numeric rating scale (NRS) with 0 = no pain and 10 = worst pain imaginable. To assess the potential influence of disease duration on symptoms and signs, we have performed a subgroup analysis comparing 33 patients with FMS and 32 patients with SFN with a disease duration of ≤5 years. All patients filled in the following standardized pain questionnaires: Neuropathic Pain Symptom Inventory (NPSI),5 Graded Chronic Pain Scale (GCPS),53 and Pain Catastrophizing Scale (PCS).29,41 Pain chronicity was rated on the Mainz Pain Staging System (MPSS).16 For depressive symptoms, the “Allgemeine Depressionsskala” (ADS) was used.34 We further studied analgesic medication and nonpharmacological treatments applied.
2.3. General medical assessment
We compared data obtained on patients' comorbidities. Family history was recorded in a systematic manner asking patients about neurological diagnoses, FMS or SFN diagnosis, respectively, or similar symptoms in family members, such as parents, grandparents, siblings, own children, as well as the siblings of parents and grandparents. Laboratory data that were cross-compared comprised the oral glucose tolerance test (oGTT), glycosylated hemoglobin (HbA1c), thyroid stimulating hormone (TSH), vitamin B12, and blood count (erythrocytes, leukocytes, thrombocytes, hematocrit, and hemoglobin).
2.4. Small nerve fiber assessment
We analyzed data on small nerve fiber morphology, function, and electrical conduction collected as follows: (1) intraepidermal nerve fiber density (IENFD) quantified on 6-mm skin punch biopsies taken from the lower leg and upper thigh.45 In both studies, skin biopsies were taken according to a standardized protocol45 and were assessed following published counting rules.21 After fixation and immunoreaction with an antibody against protein gene product-9.5 (Ultraclone RA95101 PGP9.5 1:800; Wellow, Isle of Wight, Great Britain), imaging was performed using a fluorescence microscope (Zeiss Axiophot 2, Jena, Germany). IENFD <5.4 fibers/mm was the distal limit and <8.5 fibers/mm the proximal limit. (2) Corneal nerve fiber length (NFL), density (NFD), and branching (NFB) determined by corneal confocal microscopy (CCM).42 (3) Individual sensory profiles established by quantitative sensory testing (QST) at the dorsal foot.35 (4) Latencies and peak-to-peak amplitudes (PPA), when recording pain-related evoked potential (PREP) at the feet.49 Normative values were used as detailed in Ref. 10 and listed in the respective table. Pain-related evoked potential was performed according to a standardized protocol18,22,49 using 2 superficial and concentric stimulation electrodes on the dorsum of the feet which induce a pinprick sensation. The recording of the potentials was performed with a needle electrode at Cz and 2 reference electrodes at the earlobes (A1–A2) according to international 10 to 20 system.19 Twenty triple pulses with an intensity twice as strong as the individual perception threshold were applied. Ten curves each were averaged and compared for the extraction of first positive peak (N1), following negative peak (P1) and peak-to-peak amplitude.
2.5. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics software version 27 (IMB Deutschland GmbH, Ehningen, Germany). A significance level of P < 0.05 was defined. The Shapiro–Wilk test was used to test for normal distribution. Normally distributed data were analyzed with the Student t test, whereas nonnormally distributed data using the nonparametric Mann–Whitney U test. Categorical data were assessed using Fisher exact test and the χ2 test. Krita (Version 5.1.0; Stichting Krita Foundation, Deventer, Netherlands) was used for graphical data visualization.
3. Results
3.1. Epidemiological characterization of study cohorts
Table 1 gives a synopsis of the main epidemiological characteristics of the study cohorts. At symptom onset, patients with FMS were on average 10 years younger than patients with SFN (FMS: median 35.0, 4–65 years; SFN: median 47.0, 12–67 years; P < 0.001). Time until diagnosis was 3 times longer in patients with FMS compared with patients with SFN (FMS: median 8.0, 0–46 years; SFN: median 2.8, 0–20 years; P < 0.001). We found that etiology was potentially genetic in 11 of 53 (20.8%) patients with SFN, potentially metabolic in 17 of 53 patients (32.1%), and potentially autoimmune in 6 of 53 patients (11.3%). In 19 of 53 patients with SFN (35.9%), etiology remained idiopathic.
Table 1.
Epidemiological characteristics of study cohorts.
| FMS | SFN | P | |
|---|---|---|---|
| No. of patients | 158 | 53 | NA |
| Median age in years [range] | 51.5 [21.6–74.8] | 53.4 [22.4–73.2] | n.s. |
| Median age at symptom onset [range] | 35.0 [4–65] (n = 158) | 47.0 [12–67] (n = 52) | P < 0.001 |
| Median age at diagnosis [range] | 45.5 [19.6–67.3] | 53.2 [20.4–73.2] | P < 0.001 |
| Median disease duration in years [range] | 15.8 [0.0–56.0] | 4.0 [0–20.0] | P < 0.001 |
| Median time in years between symptom onset and diagnosis [range] | 8.0 [0–46] | 2.8 [0–20] | P < 0.001 |
FMS, fibromyalgia syndrome; NA, not applicable; n.s., not significant; SFN, small fiber neuropathy.
3.2. Pain in fibromyalgia syndrome is generalized and variable, while mainly focal and constant in small fiber neuropathy
Table 2 shows pain characteristics of patients with FMS and SFN. During pain interviews, the main discriminators between the 2 entities were burning (FMS: 66/158 [41.8%], SFN: 45/53 [84.9%], P < 0.001) or stabbing pain (FMS: 39/158 [24.7%], SFN: 35/53 [66.0%], P < 0.001). Patients with FMS further described pain like muscle soreness (40/158, 25.3%). Pain localization also distinguished well between FMS and SFN with widespread pain being predominant in FMS and acral pain in patients with SFN (Fig. 1). In FMS, physical activity, rest, and warmth alleviated pain, whereas cold and stress evoked pain (Table 2). In contrast, patients with SFN reported cold, warmth, and touch as both pain relieving and triggering factors (Table 2). Furthermore, patients with FMS reported a higher number of aggravating factors compared with patients with SFN (FMS: 2.3 [0–6], SFN: 1.6 [0–5], P < 0.001). When using pain questionnaires, the NPSI pressure score (FMS: median 0.5 [0.0–1.0], SFN: median 0.3 [0–0.9], P < 0.001), evoked pain score (FMS: median 0.4 [0–0.9], SFN: median 0.3 [0–0.9], P < 0.001), and GCPS pain intensity (FMS: median 66.7 [26.7–90.0], SFN: median 56.7 [13.3–86.7], P < 0.001) discriminated best between FMS and SFN (Table 3). Interview data are summarized in Figure 2.
Table 2.
Pain characteristics of patients with fibromyalgia syndrome and small fiber neuropathy (elicitation by interview).
| FMS | SFN | P | |
|---|---|---|---|
| Median pain intensity on NRS [range] | |||
| During interview | 5.0 [0–9] (n = 156) | 4.0 [0–9] (n = 53) | <0.01 |
| After pain medication | 2.0 [0–6] (n = 126) | 1.0 [0–1] (n = 40) | <0.001 |
| Pain character | |||
| Burning | 66/158 (41.8%) | 45/53 (84.9%) | <0.001 |
| Stabbing | 39/158 (24.7%) | 35/53 (66.0%) | <0.001 |
| Tearing | 16/158 (10.1%) | 8/53 (15.1%) | n.s. |
| Pain localization | |||
| Head | 91/158 (57.6%) | 16/52 (30.8%) | <0.01 |
| Neck | 141/158 (89.2%) | 18/52 (34.6%) | <0.001 |
| Shoulders/upper arm | 151/158 (95.6%) | 18/52 (34.0%) | <0.001 |
| Elbow/lower arm | 112/158 (70.9%) | 18/52 (34.0%) | <0.001 |
| Hands/fingers | 23/158 (14.6%) | 34/52 (64.2%) | <0.001 |
| Trunk | 123/158 (77.8%) | 7/53 (13.2%) | <0.001 |
| Upper back | 131/158 (82.9%) | 17/53 (32.1%) | <0.001 |
| Lower back | 137/158 (86.7%) | 22/53 (41.5%) | <0.001 |
| Hips | 124/158 (78.5%) | 16/51 (31.4%) | <0.001 |
| Thighs | 130/158 (82.3%) | 20/53 (37.7%) | <0.001 |
| Knees/lower legs | 102/158 (64.6%) | 37/53 (69.8%) | n.s. |
| Feet/toes | 115/158 (72.8%) | 52/53 (98.1%) | <0.001 |
| Pain triggers | |||
| Heat | 4/158 (2.5%) | 18/53 (34.0%) | <0.001 |
| Cold | 106/158 (67.1%) | 12/53 (22.6%) | <0.001 |
| Stress | 101/158 (63.9%) | 6/53 (11.3%) | <0.001 |
| Humidity | 28/158 (17.7%) | 1/53 (1.9%) | <0.01 |
| Time of day | 0/158 (0.0%) | 3/53 (5.7%) | <0.05 |
| Weather | 32/158 (20.3%) | 1/53 (1.9%) | <0.01 |
| Touch | 4/158 (2.5%) | 20/53 (37.7%) | <0.001 |
| Median number of pain aggravating factors [range] | 2.0 [0–6] | 2.0 [0–5] | <0.001 |
| Pain relieving factors | |||
| Physical activity | 112/158 (70.9%) | 12/53 (22.6%) | <0.001 |
| Resting | 73/158 (46.2%) | 10/53 (18.9%) | <0.001 |
| Cold | 3/158 (1.9%) | 7/53 (13.2%) | <0.01 |
| Heat | 129/158 (81.6%) | 9/53 (17.0%) | <0.001 |
| Touch | 1/158 (0.6%) | 5/53 (9.4%) | <0.01 |
| Median number of pain relieving factors [range] | 2.0 [0–4] | 1.0 [0–3] | <0.001 |
FMS, fibromyalgia syndrome; NRS, numeric rating scale; n.s., not significant; SFN, small fiber neuropathy.
Figure 1.
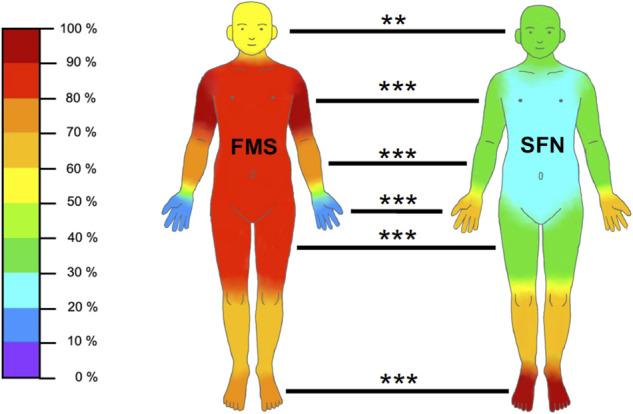
Proportion of patients who reported pain in distinct body areas. The graph depicts the frequency of pain reported in distinct body areas in relation to the FMS (n = 158) and SFN (n = 53) patient groups. For exact data, please see Table 2. FMS, fibromyalgia syndrome; SFN, small fiber neuropathy. **P < 0.01, ***P < 0.001.
Table 3.
Pain characteristics of patients with fibromyalgia syndrome and small fiber neuropathy (elicitation by questionnaires and Mainz Pain Staging System).
| FMS | SFN | P | |
|---|---|---|---|
| NPSI | |||
| Sum score: mean [range] | 0.4 [0.1–0.9] | 0.4 [0.0–0.7] | <0.05 |
| Burning score: median [range] | 0.5 [0–10.0] | 0.4 [0.0–0.9] | n.s. |
| Pressure score: median [range] | 0.5 [0–10.0] | 0.3 [0.0–0.9] | <0.001 |
| Attack score: median [range] | 0.4 [0.0–1.4] | 0.4 [0–10.0] | n.s. |
| Evoked pain score: median [range] | 0.4 [0.0–0.9] | 0.3 [0.0–0.9] | <0.001 |
| Paresthesia/dysesthesia score: median [range] | 0.4 [0.0–1.0] | 0.6 [0–10.0] | n.s. |
| Discriminative score: mean [range] | 54.3 [23.4–95.2] | 51.2 [28.6–79.3] | n.s. |
| GCPS: median [range] | |||
| Pain intensity | 66.7 [26.7–90.0] | 56.7 [13.3–86.7] | <0.001 |
| Disability | 60.0 [10.0–86.7] | 50.0 [3.3–100.0] | <0.05 |
| Grade | 2.0 [1–4] | 2.0 [0–4] | n.s. |
| ADS median [range] | 23.0 [3–51] | 17.0 [2–38] | <0.001 |
| PCS median [range] | 22.2 [0–49] | 21.2 [3–41] | n.s. |
| Classification according to MPSS | |||
| Median [range] | 3.0 [2–3] | 3.0 [1–3] | <0.05 |
ADS, allgemeine depressionsskala; FMS, fibromyalgia syndrome; GCPS, Graded Chronic Pain Scale; MPSS, Mainz Pain Staging System; NPSI, Neuropathic Pain Symptom Inventory; n.s., not significant; PCS, Pain Catastrophizing Scale; SFN, small fiber neuropathy.
Figure 2.
Clinical aspects distinguishing FMS and SFN. The plot summarizes the most important opposing aspects that can be collected during interview. The figurines showing the pain localization are taken from Figure 1. The exact data can be found in Tables 1, 2, 4, 5 and 7. FMS, fibromyalgia syndrome; SFN, small fiber neuropathy.
3.3. Patients with fibromyalgia syndrome report sleep disturbance and depressed mood, whereas patients with small fiber neuropathy mainly suffer from sensoalgesic symptoms
We evaluated patients' comorbidities and further symptoms, an overview is given in Table 4. The average number of additional symptoms spontaneously reported by the patients was higher in patients with FMS than in patients with SFN (FMS: median 8.0 [0–28], SFN: median 4.0 [0–14], P < 0.001). Patients with FMS rarely reported paresthesias, whereas patients with SFN often described tingling (FMS: 26/158 [16.5%], SFN: 36/53 [67.9%], P < 0.001), numbness (FMS: 19/158 [12.0%], SFN: 15/53 [28.3%], P < 0.01), or hypersensitivity to touch (FMS: 1/158 [0.6%], SFN: 7/53 [13.2%], P < 0.001). Patients with FMS more frequently suffered from gastrointestinal and urogenital symptoms than patients with SFN (Table 4). Patients with FMS also more frequently described sleep problems (Table 4), fatigue (FMS: 139/158 [88.0%], SFN: 3/53 [5.7%], P < 0.001), or apathy (FMS: 24/158 [15.2%], SFN: 2/53 [3.8%], P < 0.01). They reported cognitive impairment (FMS: 62/158 [39.2%], SFN: 0/53 [0%], P < 0.001) or problems of attention (FMS: 114/158 [72.2%], SFN: 1/53 [1.9%], P < 0.001). Furthermore, patients with FMS more prevalently reported depressed mood (FMS: 29/158 [18.4%], SFN: 1/53 [1.9%], P < 0.01) than patients with SFN.
Table 4.
Additional symptoms and comorbidities in patients with fibromyalgia syndrome and small fiber neuropathy.
| FMS | SFN | P | |
|---|---|---|---|
| Comorbidities | |||
| Bronchial asthma | 20/158 (12.7%) | 1/53 (1.9%) | <0.05 |
| Migraine with aura | 12/158 (7.6%) | 3/53 (5.7%) | n.s. |
| Migraine without aura | 27/158 (17.1%) | 1/53 (1.9%) | <0.01 |
| Tinnitus | 18/158 (11.4%) | 1/53 (1.9%) | <0.05 |
| Depression | 74/158 (46.8%) | 6/53 (11.3%) | <0.001 |
| Diabetes type 2 | 0/158 (0.0%) | 3/53 (5.7%) | <0.05 |
| Sicca syndrome | 8/158 (5.1%) | 3/53 (5.7%) | n.s. |
| Borreliosis | 9/158 (5.7%) | 3/53 (5.7%) | n.s. |
| Hypothyreosis | 20/158 (12.7%) | 7/53 (13.2%) | n.s. |
| Hyperthyreosis | 1/158 (0.6%) | 1/53 (1.9%) | n.s. |
| Hashimoto disease | 13/158 (8.2%) | 7/53 (13.2%) | n.s. |
| Neurological symptoms | |||
| Numbness | 19/158 (12.0%) | 15/53 (28.3%) | <0.01 |
| Tingling | 26/158 (16.5%) | 36/53 (67.9%) | <0.001 |
| Paresthesias | 24/158 (15.2%) | 6/53 (11.3%) | n.s. |
| Hypersensitivity to touch | 1/158 (0.6%) | 7/53 (13.2%) | <0.001 |
| Hypohidrosis | 8/158 (5.1%) | 6/53 (11.3%) | n.s. |
| Hyperhidrosis | 59/158 (37.3%) | 28/53 (52.8%) | n.s. |
| Conspicuous sweating (hypohidrosis or hyperhidrosis) | 66/158 (41.8%) | 34/53 (64.2%) | <0.01 |
| GI and urogenital symptoms | |||
| Irritable bladder | 25/158 (15.8%) | 1/53 (1.9%) | <0.01 |
| Obstipation | 25/158 (15.8%) | 1/53 (1.9%) | <0.01 |
| Diarrhea | 24/158 (15.2%) | 5/53 (9.4%) | n.s. |
| Irritable bowel | 69/158 (43.7%) | 1/53 (1.9%) | <0.001 |
| Nausea | 1/158 (4.4%) | 5/53 (9.4%) | n.s. |
| Sleep problems | |||
| Unrefreshed sleep | 81/158 (51.3%) | 0/52 (0.0%) | <0.001 |
| Sleep disturbance | 100/158 (63.3%) | 11/53 (20.8%) | <0.001 |
| Difficulties in falling asleep | 29/158 (18.4%) | 2/53 (3.8%) | <0.01 |
| Mental symptoms | |||
| Fatigue | 139/158 (88.0%) | 3/53 (5.7%) | <0.001 |
| Apathy | 24/158 (15.2%) | 2/53 (3.8%) | <0.01 |
| Asthenia | 30/158 (19.0%) | 6/53 (11.3%) | n.s. |
| Agitation | 16/158 (10.1%) | 1/53 (1.9%) | n.s. |
| Irritability | 13/158 (8.2%) | 1/53 (1.9%) | n.s. |
| Depressed mood | 29/158 (18.4%) | 1/53 (1.9%) | <0.01 |
| Cognitive symptoms | 62/158 (39.2%) | 0/53 (0.0%) | <0.001 |
| Concentration problems | 114/158 (72.2%) | 1/53 (1.9%) | <0.001 |
| Other symptoms | |||
| Limb stiffness | 95/158 (60.1%) | 3/53 (5.7%) | <0.001 |
| Joint swelling | 8/158 (5.1%) | 9/53 (17.0%) | <0.05 |
| Vertigo | 19/158 (12.0%) | 8/53 (15.1%) | n.s. |
| Circulatory problems | 12/158 (7.6%) | 11/53 (20.8%) | <0.05 |
| Palpitations | 14/158 (8.9%) | 3/53 (5.7%) | n.s. |
| Respiratory distress | 13/158 (8.2%) | 0/53 (0.0%) | <0.05 |
| Restless legs | 12/158 (7.6%) | 2/53 (3.8%) | n.s. |
| Total number of additional and spontaneously reported symptoms: median [range] | 8.0 [0–28] | 4.0 [0–14] | <0.001 |
FMS, fibromyalgia syndrome; GI, gastrointestinal; n.s., not significant; SFN, small fiber neuropathy.
3.4. Family history is indicative of chronic pain in fibromyalgia syndrome while of neurological disorders in small fiber neuropathy
Mental disorders (FMS: 30/158 [19.0%], SFN: 3/53 [5.7%], P < 0.05) and chronic pain (FMS: 78/158 [49.4%], SFN: 17/53 [32.1%], P < 0.05) were mostly present in the family history of patients with FMS. In contrast, patients with FMS had fewer relatives suffering from neurological diseases than patients with SFN (FMS: 26/158 [16.5%], SFN: 22/53 [41.5%], P < 0.001). Detailed data and reported diseases are listed in Table 5.
Table 5.
Family history of patients with fibromyalgia syndrome and small fiber neuropathy.
| FMS | SFN | P | |
|---|---|---|---|
| Chronic pain (eg, migraine, joint/back pain, FMS, and rheumatoid arthritis) | 78/158 (49.4%) | 17/53 (32.1%) | <0.05 |
| Neurological diseases (eg, multiple sclerosis, epilepsy, Parkinson disease, polyneuropathy, and dementia) | 26/158 (16.5%) | 22/53 (41.5%) | <0.001 |
| Mental disorders (eg, depression, bipolar disorder, schizophrenia, drug or alcohol abuse, and psychosis) | 30/158 (19.0%) | 3/53 (5.7%) | <0.05 |
FMS, fibromyalgia syndrome; SFN, small fiber neuropathy.
3.5. Glucose metabolism is often impaired in small fiber neuropathy but mostly normal in patients with fibromyalgia syndrome
Table 6 shows the results of the blood tests performed. Patients with FMS had lower HbA1c levels compared with patients with SFN (FMS: median 5.4% [4.7–6.4], SFN: median 5.5% [3.6–7.7], P < 0.05). However, data may be biased because diagnosed diabetes mellitus before study inclusion was an exclusion criterion. HbA1c was ≤6.4% in all patients with FMS. In contrast, 3 of 51 (5.9%) patients with SFN had an HbA1c >6.4%,indicating diabetes mellitus (P < 0.05). Although fasting blood glucose levels revealed no difference between the 2 cohorts, abnormalities were evident in the oGTT: after 1 hour (FMS: median 138.0 [68–246] mg/dL, SFN: median 172.0 [89–333] mg/dL, P < 0.01) and 2 hours (FMS: median 120.0 [65–217] mg/dL, SFN: median 123.0 [79–284] mg/dL, P < 0.05), patients with FMS were characterized by lower blood glucose levels than patients with SFN and less frequently had pathological results (2h oGTT >140 mg/dL: FMS: 23/157 [14.6%], SFN: 14/47 [29.8%]). As for TSH and vitamin B12 levels, patients in both cohorts showed normal values.
Table 6.
Blood tests in patients with fibromyalgia syndrome and small fiber neuropathy.
| FMS | SFN | P | |
|---|---|---|---|
| Median [range] | |||
| HbA1c (%) | 5.4 [4.7–6.4] | 5.5 [3.6–7.7] | <0.05 |
| Fasting blood sugar levels (mg/dL) | 95.5 [56–128] | 97.0 [74–144] | n.s. |
| oGTT 1 h (mg/dL) | 138.0 [68–246] | 172.0 [89–333] | <0.01 |
| oGTT 2 h (mg/dL) | 120.0 [65–217] | 123.0 [79–284] | <0.05 |
| TSH (mU/L) | 1.8 [0.0–10.8] | 1.6 [0.0–9.2] | n.s. |
| Vitamin B12 (pg/mL) | 449.5 [183–2000] | 470.5 [215–2000] | n.s. |
| Pathological test results | |||
| HbA1c indicating prediabetes (5.7–6.4%) | 32/157 (20.4%) | 16/50 (32.0%) | n.s. |
| HbA1c indicating diabetes (>6.4%) | 0/157 (0.0%) | 3/51 (5.9%) | <0.05 |
| Pathological oGTT (>140 mg/dL after 2 h) | 23/157 (14.6%) | 14/47 (29.8%) | <0.05 |
| Pathological TSH (<0.4/> 4.0 mU/L) | 20/157 (12.7%) | 8/50 (16.0%) | n.s. |
| Reduced vitamin B12 (<200 pg/mL) | 2/142 (1.4%) | 0/50 (0%) | n.s. |
FMS, fibromyalgia syndrome; HbA1c, glycated hemoglobin A1c; n.s., not significant; oGTT, oral glucose tolerance test; SFN, small fiber neuropathy; TSH, thyroid-stimulating hormone.
3.6. Analgesic treatment attempts are more frequent in patients with fibromyalgia syndrome history than in small fiber neuropathy
We further recorded the pharmacological and nonpharmacological therapeutic approaches that patients had undertaken to treat FMS and SFN symptoms (Table 7). Patients with FMS reported more frequent therapy attempts than patients with SFN. This was reflected by the number of different pharmacological therapies (FMS: median 4.0 [0–19], SFN: median 3.0 [0–10], P < 0.001), medical interventions such as injections or surgery (FMS: median 0.0 [0–6], SFN: median 0.0 [0–1], P < 0.01), as well as nonpharmacological therapies (FMS: median 2.0 [0–17], SFN: median 0.0 [0–7], P < 0.001). Frequently used medication is listed in Table 7. Nonpharmacological therapies were predominantly applied by patients with FMS, whereas patients with SFN rather used food supplements in addition to pharmaceuticals. Psychotherapy was more frequently applied in patients with FMS than in patients with SFN (FMS: 21/158 [13.3%], SFN: 1/53 [1.9%], P < 0.05).
Table 7.
Therapy approaches in fibromyalgia syndrome and small fiber neuropathy.
| FMS | SFN | P | |
|---|---|---|---|
| Median number of medications [range] | 4.0 [0–19] | 3.0 [0–10] | <0.001 |
| Median number of medical interventions [range] | 0.0 [0–6] | 0.0 [0–1] | <0.01 |
| Median number of nonpharmaceutical therapies [range] | 2.0 [0–17] | 0.0 [0–7] | <0.001 |
| Median number of rehabilitations [range] | 0.0 [0–7] | 0.0 [0–2] | <0.01 |
| Multimodal treatment | 29/157 (18.5%) | 2/53 (3.8%) | <0.01 |
| Medication: median [range] | |||
| Nonopioids (NSAID/metamizole dipyrone/acetaminophen) | 2.0 [0–5] | 0.0 [0–3] | <0.001 |
| Opioids | 0.0 [0–3] | 0.0 [0–2] | n.s. |
| Anticonvulsants | 0.0 [0–3] | 1.0 [0–4] | <0.001 |
| SSRI | 0.0 [0–2] | 0.0 [0–1] | <0.01 |
| SSNRI | 0.0 [0–2] | 0.0 [0–1] | n.s. |
| Tricyclic antidepressants | 1.0 [0–3] | 0.0 [0–3] | <0.01 |
| Muscle relaxer | 0.0 [0–2] | 0.0 [0–1] | <0.05 |
| Topical agents | 0.0 [0–1] | 0.0 [0–2] | n.s. |
| Nonpharmaceutical approaches: median [range] | |||
| Food supplements | 0.0 [0–5] | 0.0 [0–4] | <0.05 |
| Active methods | 0.0 [0–3] | 0.0 [0–1] | <0.001 |
| Passive methods | 0.0 [0–4] | 0.0 [0–1] | <0.001 |
| Acupuncture | 0.0 [0–2] | 0.0 [0–1] | <0.05 |
| Temperature methods | 0.0 [0–2] | 0.0 [0–1] | <0.001 |
| Electricity methods | 0.0 [0–2] | 0.0 [0–1] | <0.05 |
| Relaxation methods | 0.0 [0–3] | 0.0 [0–1] | <0.001 |
| Asian relaxation methods (ie, Tai Chi, Yoga, Qigong) | 0.0 [0–2] | 0.0 [0–1] | <0.05 |
| Psychotherapy | |||
| In past | 38/158 (24.1%) | 2/53 (3.8%) | <0.01 |
| Currently | 21/158 (13.3%) | 1/53 (1.9%) | <0.05 |
FMS, fibromyalgia syndrome; n.s., not significant; NSAID, nonsteroidal anti-inflammatory drugs; SFN, small fiber neuropathy; SSNRI, selective serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor.
3.7. Small fiber pathology mainly manifests as proximal skin denervation in fibromyalgia syndrome and prolonged electrical A-delta conductance in small fiber neuropathy
Table 8 lists the results of small fiber tests. Table 9 compares the frequency of pathological test results. Table 10 summarizes the main differences in small fiber tests. Neurological examination revealed sensory abnormalities in thermal hypoesthesia or allodynia in 31 of 53 (58.5%) patients with SFN. The most striking result was that mere reduction of proximal IENFD was a phenomenon twice frequently observed in patients with FMS (48/157, 31%) than in patients with SFN (8/53, 15%, P < 0.05). In contrast, distal IENFD did not differ between groups. When comparing individual IENFD with our laboratory normative values, 18 of 158 (11%) patients with FMS had reduced distal IENFD compared with 10 of 53 (19%) patients with SFN (P > 0.05).
Table 8.
Small fiber examinations.
| FMS | SFN | P | |
|---|---|---|---|
| Skin biopsy | |||
| Median IENFD lower leg (fibers/mm) [range] | 6.3 [0.0 to 14.4] | 5.4 [0.0 to 16.3] | n.s. |
| Median IENFD upper thigh (fibers/mm) [range] | 8.4 [1.2 to 20.0] | 8.8 [1.5 to 16.5] | n.s. |
| QST: median [range] | |||
| CDT (°C) | −2.2 [−22.0 to −0.9] | −3.9 [-22.0 to −0.8] | <0.001 |
| WDT (°C) | 6.7 [1.8 to 18.0] | 5.4 [1.0 to 17.6] | n.s. |
| TSL (°C) | 10.5 [1.5 to 40.0] | 11.1 [4.0 to 40.0] | n.s. |
| PHS (x/3) | 0.0 [0 to 3] | 0.0 [0 to 3] | n.s. |
| CPT (°C) | 16.1 [10.0 to 30.6] | 11.1 [10.0 to 29.4] | <0.05 |
| HPT (°C) | 45.2 [35.8 to 50.0] | 46.0 [37.2 to 50.0] | n.s. |
| MDT (mN) | 2.1 [0.2 to 724.1] | 4.9 [0.2 to 207.9] | <0.001 |
| MPT (mN) | 59.7 [5.7 to 724.1] | 27.9 [5.7 to 724.1] | <0.01 |
| MPS (rating) | 1.8 [0.0 to 72.7] | 1.3 [0.0 to 20.3] | n.s. |
| DMA (rating) | 0.0 [0.0 to 70.6] | 0.0 [0.0 to 5.3] | n.s. |
| WUR (ratio) | 2.0 [0.0 to 42.0] | 2.0 [1.0 to 5.0] | n.s. |
| VDT (x/8) | 7.0 [3 to 8] | 6.5 [5 to 8] | n.s. |
| PPT (kPa) | 368.0 [196 to 1030] | 441.0 [235 to 840] | <0.01 |
| CCM | |||
| Median NFD (fibers/mm2) [range] | 23.0 [5.2 to 38.5] | 23.0 [6.3 to 36.5] | n.s. |
| Median NBD (fibers/mm2) [range] | 66.6 [9.7 to 181.5] | 46.4 [10.4 to 102.1] | <0.001 |
| Median NFL (mm/mm2) [range] | 13.3 [5.7 to 21.8] | 13.1 [7.2 to 19.9] | n.s. |
| PREP (average left/right side): median [range] | |||
| Foot N1 latency (ms) | 168.2 [97.7 to 232.3] | 206.7 [0.0 to 285.3] | <0.001 |
| Foot P1 latency (ms) | 211.5 [118.9 to 310.6] | 272.8 [0.0 to 332.8] | <0.001 |
| Foot PPA (µV) | 11.7 [1.2 to 39.8] | 13.5 [0.0 to 26.9] | <0.05 |
CCM, corneal confocal microscopy; CDT, cold detection threshold; CPT, cold pain threshold; DMA, dynamic mechanical allodynia; FMS, fibromyalgia syndrome; HPT, heat pain threshold; IENFD, intraepidermal nerve fiber density; MDT, mechanical detection threshold; MPT, mechanical pain threshold; MPS, mechanical pain sensitivity; N1, first negative peak latency; NBD, nerve branch density; NFD, nerve fiber density; NFL, nerve fiber length; NRS, numeric rating scale; n.s., not significant; P1, subsequent positive peak latency; PHS, paradoxical heat sensations; PPA, peak-to-peak amplitudes; PPT, pressure pain threshold; PREP, pain-related evoked potentials; QST, quantitative sensory testing; SFN, small fiber neuropathy; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind-up ratio.
Table 9.
Data of pathological small fiber tests.
| FMS | SFN | P | |
|---|---|---|---|
| Skin biopsy | |||
| IENFD distally reduced (ie, <5.4 fibers/mm) | 18/158 (11.4%) | 10/53 (18.9%) | n.s. |
| IENFD proximally reduced (ie, <8.5 fibers/mm) | 48/157 (30.6%) | 8/53 (15.1%) | <0.05 |
| IENFD generally reduced (ie, distal <5.4 fibers/mm AND proximal <8.5 fibers/mm) | 38/158 (24.1%) | 13/53 (24.5%) | n.s. |
| Pathological IENFD in at least one localization | 104/157 (66.2%) | 31/53 (58.5%) | n.s. |
| QST pathological16 | |||
| CDT | 6/157 (3.8%) | 10/53 (18.9%) | <0.01 |
| WDT | 13/157 (8.3%) | 10/53 (18.9%) | <0.05 |
| TSL | 18/157 (11.5%) | 15/53 (28.3%) | <0.01 |
| QST (ie, CDT, WDT, or TSL pathological) | 24/157 (15.3%) | 19/53 (35.8%) | <0.01 |
| PHS | 32/157 (20.4%) | 18/53 (34.0%) | n.s. |
| CPT | 0/157 (0%) | 2/53 (3.8%) | n.s. |
| HPT | 16/156 (10.3%) | 7/53 (13.2%) | n.s. |
| MDT | 13/157 (8.3%) | 10/53 (18.9%) | <0.05 |
| MPT | 25/157 (15.9%) | 16/53 (30.2%) | <0.05 |
| MPS | 34/156 (21.8%) | 12/52 (23.1%) | n.s. |
| DMA | 14/157 (8.9%) | 2/52 (3.8%) | n.s. |
| WUR | 6/157 (3.8%) | 4/52 (7.7%) | n.s. |
| VDT | 18/157 (11.5%) | 3/53 (5.7%) | n.s. |
| PPT | 23/157 (14.6%) | 4/53 (7.5%) | n.s. |
| CCM | |||
| Pathological NFD (ie, <19.3/mm2) | 34/133 (25.6%) | 11/50 (22.0%) | n.s. |
| Pathological NBD (ie, <36.3/mm2) | 15/133 (11.3%) | 18/50 (36.0%) | <0.001 |
| Pathological NFL (ie, <11.1 mm/mm2) | 33/133 (24.8%) | 14/50 (28.0%) | n.s. |
| At least one pathological CCM parameter | 47/133 (35.3%) | 22/51 (43.1%) | n.s. |
| PREP | |||
| Pathological N1 (ie, >224.89 ms) | 3/123 (2.4%) | 14/39 (35.9%) | <0.001 |
| Pathological P1 (ie, >285.46 ms) | 2/123 (1.6%) | 15/39 (38.5%) | <0.001 |
| Pathological PPA (ie, <10.0 µV) | 51/123 (41.5%) | 7/38 (18.4%) | <0.05 |
| At least one pathological PREP parameter | 52/123 (42.3%) | 21/39 (53.8%) | n.s. |
CCM, corneal confocal microscopy; CDT, cold detection threshold; CPT, cold pain threshold; DMA, dynamic mechanical allodynia; FMS, fibromyalgia syndrome; HbA1c, glycated hemoglobin A1c; HPT, heat pain threshold; IENFD, intraepidermal nerve fiber density; MDT, mechanical detection threshold; MPT, mechanical pain threshold; MPS, mechanical pain sensitivity; N1, first negative peak latency; NBD, nerve branch density; NFD, nerve fiber density; NFL, nerve fiber length; n.s., not significant; oGTT, oral glucose tolerance test; P1, subsequent positive peak latency; NRS, numeric rating scale; PHS, paradoxical heat sensations; PPA, peak-to-peak amplitudes; PPT, pressure pain threshold; PREP, pain-related evoked potentials; QST, quantitative sensory testing; SFN, small fiber neuropathy; TSH, thyroid-stimulating hormone; TSL, thermal sensory limen; VDT, vibration detection threshold; WDT, warm detection threshold; WUR, wind-up ratio.
Table 10.
Differences between fibromyalgia syndrome and small fiber neuropathy in small fiber tests.
| FMS | SFN | |
|---|---|---|
| Skin innervation | Proximal denervation | Distal denervation |
| Corneal innervation | Mostly normal | Reduced NBD |
| Sensory profiles compared with healthy controls | CPT, MPS, PTT ↑ MDT, MPT↓ |
CDT↑, MDT ↓ more often pathological |
| A-delta conductance | PPA ↓ | P1 ↑, N1 ↑ |
CDT, cold detection threshold; CPT, cold pain threshold; FMS, fibromyalgia syndrome; MDT, mechanical detection threshold; MPT, mechanical pain threshold; MPS, mechanical pain sensitivity; N1, first negative peak latency; NBD, nerve branch density; P1, subsequent positive peak latency; PPA, peak-to-peak amplitudes; PPT, pressure pain threshold; SFN, small fiber neuropathy.
In QST, we first assessed patients' individual data comparing results with published normative values.27 QST showed small fiber impairment in 24 of 157 (15.3%) patients with FMS compared with 19 of 53 (35.8%) patients with SFN (P < 0.01). Direct comparison of the 2 cohorts revealed diversity for the cold detection threshold (CDT), cold pain threshold (CPT), mechanical detection threshold (MDT), mechanical pain threshold (MPT), and pressure pain threshold (PPT).
The evaluation of CCM showed no differences in corneal NFD and NFL between the 2 cohorts, also in comparison with our laboratory normative values (NFD <19.3 fibers/mm2: FMS 34/133 [25%], SFN 11/50 [20%]; NFL <11.1 mm/mm2: FMS 33/133 [25%], SFN 14/50 [28%]). However, NBD was pathologically reduced in 15 of 133 (11%) patients with FMS and in 18 of 50 (36%) patients with SFN (P < 0.001).
When recording PREP, 52 of 123 (42%) patients with FMS and 21 of 39 (54%) patients with SFN had pathological findings. Comparing data with our laboratory normative values, N1 latencies were prolonged in 14 of 39 (36%) patients with SFN (FMS: 3/123 [2%], P < 0.001). In 15 of 39 (39%) patients with SFN, the P1 latency was longer than normal (FMS: 2/123 [2%], P < 0.001). Peak-to-peak amplitude was pathologically reduced more in patients with FMS than in patients with SFN (FMS: 51/123 [41.5%], SFN: 7/38 [18.4%], P < 0.05).
3.8. Influence of disease duration
In contrast to data analysis of the entire study groups, pain intensity without analgesic treatment and the number of analgesics used were comparable in both patient groups when assessed for ≤5 years of disease duration (Table 11). Also, distal skin innervation was higher in patients with FMS than in patients with SFN with a short disease duration (P < 0.05).
Table 11.
Subgroup comparison for short disease duration of ≤5 years.
| FMS (n = 33) | SFN (n = 32) | P | |
|---|---|---|---|
| Median pain intensity during interview (NRS) [range] | 5.0 [1–9] | 4.0 [1–9] | n.s. |
| Median pain intensity after medication (NRS) [range] | 2.0 [0–4] | 1.0 [0–1] | <0.001 |
| Median number of additional symptoms [range] | 7.0 [1–28] | 4.0 [0–11] | <0.001 |
| Median number of pain aggravating factors [range] | 2.0 [1–6] | 1.0 [0–3] | <0.01 |
| Median number of pain relieving factors [range] | 2.0 [1–4] | 1.0 [0–3] | <0.001 |
| Median number of medications [range] | 4.0 [1–8] | 3.0 [0–10] | n.s. |
| Median number of nonpharmaceutical therapy attempts [range] | 3.0 [0–15] | 0.0 [0–4] | <0.001 |
| Psychotherapy in past | 7/33 (21.2%) | 0/32 (0%) | <0.01 |
| Median IENFD lower leg (fibers/mm) [range] | 6.4 [0–14.4] | 5.4 [0–11.8] | <0.05 |
| Median IENFD upper thigh (fibers/mm) [range] | 8.2 [1.3–16.4] | 9.8 [1.5–16.5] | n.s. |
FMS, fibromyalgia syndrome; IENFD, intraepidermal nerve fiber density; NRS, numeric rating scale; n.s., not significant; SFN, small fiber neuropathy.
4. Discussion
Since the description of small fiber pathology in FMS patient subgroups,11,49 there is an ongoing controversy weather FMS equals SFN.47 The distinction is crucial because prognosis and treatment options differ substantially between both entities. We pioneer a direct comparative approach and report clinical characteristics that may be useful in differential diagnosis.
We confirm that also patients with FMS may have small nerve fiber impairment as reported before.17,38,49 However, it is the patients with SFN who rather suffer from a neuropathic pain phenotype with mainly acral pain accompanied by additional sensory symptoms together with a family history of neurological diseases. Thirty-one of 53 (58.5%) women with SFN showed sensory abnormalities indicating peripheral deafferentiation. A similar distribution was reported previously8 and emphasizes the importance to equally consider the results of neurological examination when making the diagnosis of SFN. Patients with FMS were characterized by generalized musculoskeletal pain regularly accompanied by sleep disturbance, fatigue, and concentration problems along with a family history of chronic pain syndromes. Depression and depressed mood occurred more frequently in patients with FMS than in patients with SFN (Table 4).
While nonpharmacological treatment is recommended first line in FMS in national39 and international guidelines,26,44 tricyclic antidepressants, anticonvulsants, and serotonin norepinephrine reuptake inhibitors are used first line against pain in idiopathic SFN treatment.13 Although patients with SFN and FMS received analgesic medication mostly in accordance with national and international guidelines, it was the patients with FMS who reported numerous insufficient analgesic treatment attempts, whereas patients with SFN mostly experienced pain relief upon antineuropathic pain treatment. Patients with FMS reported a higher number of analgesics used than patients with SFN. However, the number of drug therapy attempts was comparable between groups in patients with short disease duration, ie, ≤5 years. It is of note that standardized pain questionnaires such as the NPSI and the GCPS were of minor use in distinguishing FMS from SFN. A previous questionnaire survey for small fiber neuropathy in patients with FMS also gave similar results in patient subgroups.25 Our study underscores that specifically designed pain and neuropathic symptom questionnaires are needed to help differentiating FMS from SFN. For this, our data collection may provide a valuable base.
We report a higher prevalence of impaired glucose metabolism in patients with SFN compared to patients with FMS, which is in line with previous data.40 Although data are conflicting about the pathophysiological influence of mere prediabetes,43 we believe that thorough search for potential impairment in glucose metabolism is crucial in the clinical management of patients with FMS and SFN. These data are also of immense importance regarding the underlying pathomechanism in both entities. We suspect nociceptive hyperexcitability because of sensitization and degeneration of sensory neurons.38
Multilevel investigation of small fiber pathology revealed that patients with FMS mostly show proximal skin denervation, whereas reduction of lower leg IENFD was most common in patients with SFN.10,11 This is an intriguing finding also reported by others52 and remains of unclear pathophysiology. Regarding the proximal denervation in FMS found by us and others,33,52 we speculate that an impairment of sensory neurons in the dorsal root ganglia may be present. Neuropathies normally show a distal-to-proximal spread; however, predominant proximal denervation was also shown in patients with Sjögren syndrome,4 celiac disease,6 or autoimmune hepatitis.28 Although merely speculative, impairment of ganglionic sensory neurons may play a role in FMS, such that further investigations are needed. It is further of note that in patients with SFN, distal skin denervation was associated with prolonged latencies of electrically evoked A-delta potentials.
As for sensory profiles, QST was normal in almost all patients of both groups when compared with control values. This is interesting because several studies have reported elevated thermal perception thresholds in patients with FMS compared with healthy controls.3,23 The main reason for this discrepancy may be the diversity in the number of subjects investigated keeping in mind that large-enough sample size is necessary to obtain robust QST data.27 Normal QST profiles in patients with SFN were already reported by several previous studies.10,37,48 Interestingly, intergroup comparison revealed single parameters that might be of value to distinguish FMS from SFN (Table 9).
While our finding of higher NBD on CCM in patients with FMS compared with patients with SFN remains unclear as for its pathophysiological relevance, longer N1 and P1 latencies in patients with SFN is consistent with previous data.30 These findings may reflect axonopathy as in analogy to data obtained in diabetic neuropathy via laser-evoked potentials.1,36 Potential influences of disease duration on our data need to be taken into account because intergroup differences varied when assessing short or long periods.
Our study has some limitations. The study cohort consisted of women; hence, our data cannot be transferred to men. The FMS patient group was 3 times larger than the SFN group and group sizes were overall small. However, given the homogeneity in data acquisition during the original monocentric studies, we believe this is of minor influence. Because of the retrospective nature of data collected in 2 independent studies on patients seen at our Department, matching was not possible. Furthermore, the fact that the SFN cohort consisted of patients seen at our department and agreeing to participate in our study, whereas the FMS patient cohort was recruited for study participation needs to be taken into account when interpreting our results. Although inclusion criteria differed naturally investigating patients with 2 different diagnoses,10,11 exclusion criteria were also not identical between both initial studies: B12 hypovitaminosis was an exclusion criterion in patients with SFN. Hence, the finding that vitamin B12 levels did not distinguish between patients with FMS and SFN may be biased by the fact that patients with SFN with already diagnosed vitamin B12 deficiency were not enrolled. Similarly, patients with FMS with severe depression currently requiring treatment were not included such that data on the frequency of depression in the 2 cohorts may be biased. Furthermore, our data on the prevalence of impaired glucose metabolism may be biased because previously diagnosed diabetes mellitus was an exclusion criterion.
Still, we performed the first head-to-head comparison of a rich set of monocentrically collected clinical data between patients with FMS and SFN and provide clinical guidance directly applicable in daily practice.
Disclosures
The authors have no conflict of interest to declare.
Acknowledgements
Expert technical help by Daniela Urlaub and Danilo Prtvar, MSc, is gratefully acknowledged. The authors thank Prof. Claudia Sommer, Dr. Johanna Frank, and Dr. Alexander Klitsch for help during patient recruitment. The original studies were supported by the Else Kröner-Fresenius-Stiftung (N.Ü., 2014_A129) and the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG: UE171/3-1). L.K. was funded by the Interdisciplinary Center for Clinical Research (Z-2/CSP_22). N.Ü. was supported by DFG (UE171/15-1).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Sarah Jänsch, Email: sarah.jaensch95@gmail.com.
Dimitar Evdokimov, Email: d_evdokimov@yahoo.de.
Nadine Egenolf, Email: nadine.egenolf@web.de.
Caren Meyer zu Altenschildesche, Email: caren@mza-net.de.
Luisa Kreß, Email: kress_l@ukw.de.
References
- [1].Agostino R, Cruccu G, Romaniello A, Innocenti P, Inghilleri M, Manfredi M. Dysfunction of small myelinated afferents in diabetic polyneuropathy, as assessed by laser evoked potentials. Clin Neurophysiol 2000;111:270–6. [DOI] [PubMed] [Google Scholar]
- [2].Basantsova NY, Starshinova AA, Dori A, Zinchenko YS, Yablonskiy PK, Shoenfeld Y. Small-fiber neuropathy definition, diagnosis, and treatment. Neurol Sci 2019;40:1343–50. [DOI] [PubMed] [Google Scholar]
- [3].Berwick RJ, Siew S, Andersson DA, Marshall A, Goebel A. A systematic review into the influence of temperature on fibromyalgia pain: meteorological studies and quantitative sensory testing. J Pain 2021;22:473–86. [DOI] [PubMed] [Google Scholar]
- [4].Birnbaum J, Duncan T, Owoyemi K, Wang KC, Carrino J, Chhabra A. Use of a novel high-resolution magnetic resonance neurography protocol to detect abnormal dorsal root Ganglia in Sjogren patients with neuropathic pain: case series of 10 patients and review of the literature. Medicine (Baltimore) 2014;93:121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, Rostaing S, Lanteri-Minet M, Collin E, Grisart J, Boureau F. Development and validation of the neuropathic pain symptom inventory. PAIN 2004;108:248–57. [DOI] [PubMed] [Google Scholar]
- [6].Brannagan TH, III, Hays AP, Chin SS, Sander HW, Chin RL, Magda P, Green PH, Latov N. Small-fiber neuropathy/neuronopathy associated with celiac disease: skin biopsy findings. Arch Neurol 2005;62:1574–8. [DOI] [PubMed] [Google Scholar]
- [7].Caro XJ, Winter EF. Evidence of abnormal epidermal nerve fiber density in fibromyalgia: clinical and immunologic implications. Arthritis Rheumatol 2014;66:1945–54. [DOI] [PubMed] [Google Scholar]
- [8].Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, Broglio L, Granieri E, Lauria G. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008;131:1912–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Doppler K, Rittner HL, Deckart M, Sommer C. Reduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgia. PAIN 2015;156:2319–25. [DOI] [PubMed] [Google Scholar]
- [10].Egenolf N, Zu Altenschildesche CM, Kress L, Eggermann K, Namer B, Gross F, Klitsch A, Malzacher T, Kampik D, Malik RA, Kurth I, Sommer C, Üçeyler N. Diagnosing small fiber neuropathy in clinical practice: a deep phenotyping study. Ther Adv Neurol Disord 2021;14:17562864211004318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Evdokimov D, Frank J, Klitsch A, Unterecker S, Warrings B, Serra J, Papagianni A, Saffer N, Meyer Zu Altenschildesche C, Kampik D, Malik RA, Sommer C, Üçeyler N. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol 2019;86:504–16. [DOI] [PubMed] [Google Scholar]
- [12].Fasolino A, Di Stefano G, Leone C, Galosi E, Gioia C, Lucchino B, Terracciano A, Di Franco M, Cruccu G, Truini A. Small-fibre pathology has no impact on somatosensory system function in patients with fibromyalgia. PAIN 2020;161:2385–93. [DOI] [PubMed] [Google Scholar]
- [13].Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpaa M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015;14:162–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Galosi E, Truini A, Di Stefano G. A systematic review and meta-analysis of the prevalence of small fibre impairment in patients with fibromyalgia. Diagnostics (Basel) 2022;12:1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Galvez-Sanchez CM, Reyes Del Paso GA. Diagnostic criteria for fibromyalgia: critical review and future perspectives. J Clin Med 2020;9:1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gerbershagen U. Organized treatment of pain. Determination of status. Internist (Berl) 1986;27:459–69. [PubMed] [Google Scholar]
- [17].Giannoccaro MP, Donadio V, Incensi A, Avoni P, Liguori R. Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve 2014;49:757–9. [DOI] [PubMed] [Google Scholar]
- [18].Katsarava Z, Ayzenberg I, Sack F, Limmroth V, Diener HC, Kaube H. A novel method of eliciting pain-related potentials by transcutaneous electrical stimulation. Headache 2006;46:1511–7. [DOI] [PubMed] [Google Scholar]
- [19].Klem GH, Luders HO, Jasper HH, Elger C. The ten-twenty electrode system of the international federation. The international federation of clinical neurophysiology. Electroencephalogr Clin Neurophysiol Suppl 1999;52:3–6. [PubMed] [Google Scholar]
- [20].Kosmidis ML, Koutsogeorgopoulou L, Alexopoulos H, Mamali I, Vlachoyiannopoulos PG, Voulgarelis M, Moutsopoulos HM, Tzioufas AG, Dalakas MC. Reduction of Intraepidermal Nerve Fiber Density (IENFD) in the skin biopsies of patients with fibromyalgia: a controlled study. J Neurol Sci 2014;347:143–7. [DOI] [PubMed] [Google Scholar]
- [21].Lauria G, Cornblath DR, Johansson O, McArthur JC, Mellgren SI, Nolano M, Rosenberg N, Sommer C; European Federation of Neurological Societies. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol 2005;12:747–58. [DOI] [PubMed] [Google Scholar]
- [22].Lefaucheur JP, Ahdab R, Ayache SS, Lefaucheur-Menard I, Rouie D, Tebbal D, Neves DO, Ciampi de Andrade D. Pain-related evoked potentials: a comparative study between electrical stimulation using a concentric planar electrode and laser stimulation using a CO2 laser. Neurophysiol Clin 2012;42:199–206. [DOI] [PubMed] [Google Scholar]
- [23].Leone C, Galosi E, Esposito N, Falco P, Fasolino A, Di Pietro G, Di Stefano G, Camerota F, Vollert J, Truini A. Small-fibre damage is associated with distinct sensory phenotypes in patients with fibromyalgia and small-fibre neuropathy. Eur J Pain 2023;27:163–73. [DOI] [PubMed] [Google Scholar]
- [24].Lichtenstein A, Tiosano S, Amital H. The complexities of fibromyalgia and its comorbidities. Curr Opin Rheumatol 2018;30:94–100. [DOI] [PubMed] [Google Scholar]
- [25].Lodahl M, Treister R, Oaklander AL. Specific symptoms may discriminate between fibromyalgia patients with vs without objective test evidence of small-fiber polyneuropathy. Pain Rep 2018;3:e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Hauser W, Fluss E, Choy E, Kosek E, Amris K, Branco J, Dincer F, Leino-Arjas P, Longley K, McCarthy GM, Makri S, Perrot S, Sarzi-Puttini P, Taylor A, Jones GT. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis 2017;76:318–28. [DOI] [PubMed] [Google Scholar]
- [27].Magerl W, Krumova EK, Baron R, Tolle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. PAIN 2010;151:598–605. [DOI] [PubMed] [Google Scholar]
- [28].Martinez AR, Nunes MB, Nucci A, Franca MC, Jr. Sensory neuronopathy and autoimmune diseases. Autoimmune Dis 2012;2012:873587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Meyer K, Sprott H, Mannion AF. Cross-cultural adaptation, reliability, and validity of the German version of the Pain Catastrophizing Scale. J Psychosom Res 2008;64:469–78. [DOI] [PubMed] [Google Scholar]
- [30].Mueller D, Obermann M, Koeppen S, Kavuk I, Yoon MS, Sack F, Diener HC, Kaube H, Katsarava Z. Electrically evoked nociceptive potentials for early detection of diabetic small-fiber neuropathy. Eur J Neurol 2010;17:834–41. [DOI] [PubMed] [Google Scholar]
- [31].Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. PAIN 2013;154:2310–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Palmer S, Bailey J, Brown C, Jones A, McCabe CS. Sensory function and pain experience in arthritis, complex regional pain syndrome, fibromyalgia syndrome, and pain-free volunteers: a cross-sectional study. Clin J Pain 2019;35:894–900. [DOI] [PubMed] [Google Scholar]
- [33].Quitadamo SG, Vecchio E, Delussi M, Libro G, Clemente L, Lombardi R, Modena D, Giannotta M, Iannone F, de Tommaso M. Outcome of small fibre pathology in fibromyalgia: a real life longitudinal observational study. Clin Exp Rheumatol 2023;41:1216–24. [DOI] [PubMed] [Google Scholar]
- [34].Radloff LS. The CES-D: a self-report symptom scale to detect depression. Appl Psychol Meas 1977;3:385–401. [Google Scholar]
- [35].Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain 2006;10:77–88. [DOI] [PubMed] [Google Scholar]
- [36].Rossi P, Morano S, Serrao M, Gabriele A, Di Mario U, Morocutti C, Pozzessere G. Pre-perceptual pain sensory responses (N1 component) in type 1 diabetes mellitus. Neuroreport 2002;13:1009–12. [DOI] [PubMed] [Google Scholar]
- [37].Scherens A, Maier C, Haussleiter IS, Schwenkreis P, Vlckova-Moravcova E, Baron R, Sommer C. Painful or painless lower limb dysesthesias are highly predictive of peripheral neuropathy: comparison of different diagnostic modalities. Eur J Pain 2009;13:711–8. [DOI] [PubMed] [Google Scholar]
- [38].Serra J, Collado A, Sola R, Antonelli F, Torres X, Salgueiro M, Quiles C, Bostock H. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol 2014;75:196–208. [DOI] [PubMed] [Google Scholar]
- [39].Sommer C, Alten R, Bar KJ, Bernateck M, Bruckle W, Friedel E, Henningsen P, Petzke F, Tolle T, Uceyler N, Winkelmann A, Hauser W. Drug therapy of fibromyalgia syndrome: updated guidelines 2017 and overview of systematic review articles. Schmerz 2017;31:274–84. [DOI] [PubMed] [Google Scholar]
- [40].Strand N, Wie C, Peck J, Maita M, Singh N, Dumbroff J, Tieppo Francio V, Murphy M, Chang K, Dickerson DM, Maloney J. Small fiber neuropathy. Curr Pain Headache Rep 2022;26:429–38. [DOI] [PubMed] [Google Scholar]
- [41].Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- [42].Tavakoli M, Marshall A, Pitceathly R, Fadavi H, Gow D, Roberts ME, Efron N, Boulton AJ, Malik RA. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol 2010;223:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Thaisetthawatkul P, Lyden E, Americo Fernandes J, Jr, Herrmann DN. Prediabetes, diabetes, metabolic syndrome, and small fiber neuropathy. Muscle Nerve 2020;61:475–9. [DOI] [PubMed] [Google Scholar]
- [44].Thorpe J, Shum B, Moore RA, Wiffen PJ, Gilron I. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst Rev 2018;2:CD010585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Üçeyler N, Kafke W, Riediger N, He L, Necula G, Toyka KV, Sommer C. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology 2010;74:1806–13. [DOI] [PubMed] [Google Scholar]
- [46].Üçeyler N, Sommer C. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. PAIN 2013;154:2569. [DOI] [PubMed] [Google Scholar]
- [47].Üçeyler N, Sommer C. Reply: small fibre neuropathy, fibromyalgia and dorsal root ganglia sodium channels. Brain 2013;136:e247. [DOI] [PubMed] [Google Scholar]
- [48].Üçeyler N, Vollert J, Broll B, Riediger N, Langjahr M, Saffer N, Schubert AL, Siedler G, Sommer C. Sensory profiles and skin innervation of patients with painful and painless neuropathies. PAIN 2018;159:1867–76. [DOI] [PubMed] [Google Scholar]
- [49].Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, Casanova-Molla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013;136:1857–67. [DOI] [PubMed] [Google Scholar]
- [50].Van Assche DCF, Plaghki L, Masquelier E, Hatem SM. Fibromyalgia syndrome-A laser-evoked potentials study unsupportive of small nerve fibre involvement. Eur J Pain 2020;24:448–56. [DOI] [PubMed] [Google Scholar]
- [51].Vecchio E, Lombardi R, Paolini M, Libro G, Delussi M, Ricci K, Quitadamo SG, Gentile E, Girolamo F, Iannone F, Lauria G, de Tommaso M. Peripheral and central nervous system correlates in fibromyalgia. Eur J Pain 2020;24:1537–47. [DOI] [PubMed] [Google Scholar]
- [52].Vecchio E, Quitadamo SG, Ricci K, Libro G, Delussi M, Lombardi R, Lauria G, de Tommaso M. Laser evoked potentials in fibromyalgia with peripheral small fiber involvement. Clin Neurophysiol 2022;135:96–106. [DOI] [PubMed] [Google Scholar]
- [53].Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. PAIN 1992;50:133–49. [DOI] [PubMed] [Google Scholar]
- [54].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–10. [DOI] [PubMed] [Google Scholar]
- [55].Wolfe F Smythe HA Yunus MB Bennett RM Bombardier C Goldenberg DL Tugwell P Campbell SM Abeles M Clark P Fam AG Farber SJ Fiechtner JJ Franklin CM Gatter RA Hamaty GD Lessard J Lichtbroun AS Masi AT McCain GA Reynolds WJ Romano TJ Russell IJ Sheon RP.. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum 1990;33:160–72. [DOI] [PubMed] [Google Scholar]