Abstract
Insertional mutagenesis studies of mrpB, a putative pilin-encoding open reading frame of the mrp gene cluster, which encodes mannose-resistant Proteus-like (MR/P) fimbriae of Proteus mirabilis, indicate that MrpB functions as the terminator for fimbrial assembly.
Proteus mirabilis, commonly associated with complicated urinary tract infections (UTIs), expresses several types of fimbrial structures that promote attachment to and colonization of host mucosal surfaces (8). Among them, mannose-resistant Proteus-like (MR/P) fimbria, a surface structure responsible for mannose-resistant hemagglutination, has been shown to contribute significantly to the development of experimental UTIs. First, it was shown that 63% of pyelonephritogenic strains of P. mirabilis express MR/P fimbriae as a single hemagglutinin type (9). Second, MR/P fimbriae were shown to be expressed in vivo and to elicit a strong immune response in experimental UTIs (2). Third, construction of an isogenic mrpA (encodes the major structural subunits of MR/P fimbriae) mutant demonstrated a role for this surface structure in virulence; the mrpA mutant colonized the urine, bladders, and kidneys of experimentally infected CBA mice in numbers significantly smaller than those of the wild-type strain (3). Finally, our recent studies on the expression of MR/P fimbriae at the transcriptional level have shown that an invertible element which regulates transcription in a manner similar to that for Escherichia coli type 1 fimbria is 100% turned on in vivo (in the urine of infected mice) versus at most 50% in vitro (static culture) (12). This strong selective pressure for the expression of MR/P fimbriae in vivo demonstrates its critical contribution to the development of UTIs.
In this study, insertional mutagenesis with a kanamycin resistance cassette was applied to disrupt mrpB. The mutation was targeted to the chromosome by allelic exchange to construct an isogenic mrpB mutant in wild-type P. mirabilis HI4320, an isolate cultured from the urine of an elderly woman with catheter-associated bacteriuria (10). Southern analysis confirmed the disruption of the mrpB gene in the mutant. Immunogold labeling of the mutant with antiserum against whole MR/P fimbriae showed that it produced MR/P fimbriae that were significantly longer than those produced by the wild-type strain. An episomal copy of the mrpB gene successfully complemented the mutation, restoring the synthesis of short fimbriae.
Construction of an mrpB mutant.
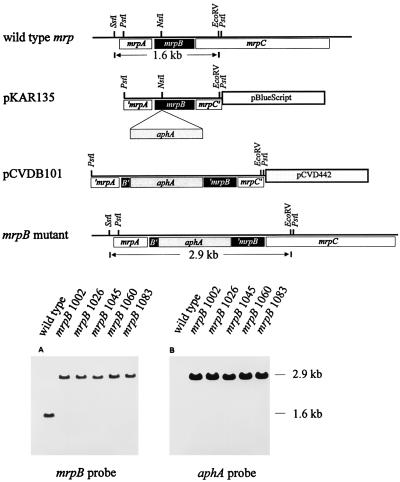
A 1,450-bp PstI DNA fragment containing mrpB, the 3′ end of mrpA, and the 5′ end of mrpC was subcloned from cosmid clone pMRP101 (4) into the PstI site of pBluescript (SK+) and designated pKAR135 (Fig. 1). Then, a 1,298-bp kanamycin resistance cassette (from pUC4κ; Pharmacia Biotech Inc., Piscataway, N.J.) was inserted into the NsiI site within mrpB. The disrupted mrpB along with its flanking sequence (650 bp upstream and 800 bp downstream) was transferred to λpir-dependent, positive-selection suicide vector pCVD442 (Ampr) (5) and electroporated into P. mirabilis HI4320. After electroporation, the bacterial suspension was inoculated onto Luria-Bertani (LB) agar plates containing kanamycin (50 μg/ml) for isolation of transconjugants that had undergone either a single-crossover or double-crossover event. If a single-crossover event were to occur, it would result in a cointegrate, which would be both kanamycin and ampicillin resistant since the vector is integrated into the chromosome. If a double-crossover event were to occur, it should result in an mrpB mutant, which would be kanamycin resistant but ampicillin susceptible; reversion to the wild type would be selected against by antibiotic pressure. Among 100 kanamycin-resistant colonies tested for ampicillin resistance on LB agar plates containing ampicillin (100 μg/ml), there were five ampicillin-susceptible transconjugants (mrpB1002, mrpB1026, mrpB1045, mrpB1060, and mrpB1083). These five clones were considered potential mrpB mutants and subjected to Southern blot analysis.
FIG. 1.
Construction and Southern blot analysis of the mrpB mutant. A 1,450-bp PstI DNA fragment containing mrpB, the 3′ end of mrpA, and the 5′ end of mrpC was subcloned into the PstI site of pBluescript (SK+) and designated pKAR135. Then, a 1,298-bp kanamycin resistance cassette was inserted into the NsiI site within mrpB, resulting in pKAR135::aphA (not shown in the figure). The disrupted mrpB along with its flanking sequence was transferred from pKAR135::aphA to the suicide vector pCVD442; this construct was designated pCVDB101. Then, pCVDB101 was electroporated into wild-type HI4320, and potential double-crossover mutants were selected (see text for details) and subjected to Southern blot analysis. Chromosomal preparations of wild-type HI4320 and the five mrpB mutants were digested with EcoRV and SstI, separated on a 0.8% agarose gel, transferred to a QIABRANE Nylon Plus membrane, and hybridized with either the mrpB probe (the 1,400-bp PstI-EcoRV fragment of pKAR135) (A) or the aphA probe (the 1,298-bp kanamycin resistance cassette) (B).
Molecular characterization of the mrpB mutant.
Chromosomal DNA was prepared from wild-type strain HI4320 and the five mrpB mutants as described by Marmur (7), digested with EcoRV and SstI, separated on a 0.8% agarose gel, and transferred to a QIABRANE Nylon Plus membrane (Qiagen Inc., Chatsworth, Calif.). The membrane was hybridized sequentially with two probes: first with the mrpB probe (Fig. 1A) and then with the aphA kanamycin resistance cassette probe (Fig. 1B). The results were identical for all five transconjugants and demonstrated disruption of mrpB. When the membrane was probed with mrpB, a 1.6-kb fragment and a 2.9-kb fragment were labeled in the wild type and the mutants, respectively, indicating that there was a 1.3-kb (size of the kanamycin resistance cassette) fragment inserted within this region in the mutants. When the membrane was probed with aphA, only the 2.9-kb fragment of the mutants was labeled, demonstrating that the kanamycin resistance cassette was inserted into a fragment whose size was identical to that of the fragment which reacted with the mrpB probe. Therefore, the Southern analysis confirmed that the insertion mutation within mrpB had indeed been introduced into the chromosome.
Fimbrial phenotypes.
The mrpB mutants were examined for fimbrial biogenesis by immunogold electron microscopy. Bacteria were grown under conditions optimal for the production of MR/P fimbriae: they were passaged statically three times for 48 h each in LB broth containing kanamycin (50 μg/ml) at 37°C. One drop of the static bacterial culture was placed on a Formvar-coated grid (Electron Microscopy Sciences, Fort Washington, Pa.) for 5 min. Excess liquid was wiped off, and the grid was air dried. Phosphate-buffered saline (PBS) (containing, per liter, 8 g of NaCl, 0.2 g of KCl, 1.44 g of Na2HPO4, and 0.24 g of KH2PO4 [pH 7.2]) containing 1% bovine serum albumin (BSA) was used to block the grid. The grid was incubated for 30 min with a drop of a 1:100 dilution of rabbit polyclonal antiserum that had been raised against purified MR/P fimbriae (6), washed three times with a drop of PBS containing 1% BSA, incubated for another 30 min with a drop of a 1:25 dilution of goat anti-mouse immunoglobulin G conjugated with 30-nm-diameter gold beads (AuroProbe EM protein GAR protein G30; Amersham Corp., Arlington Heights, Ill.), washed three times with a drop of PBS containing 1% BSA and three times with a drop of distilled water, negatively stained with 1% sodium phosphotungstic acid (pH6.8), and examined by transmission electron microscopy.
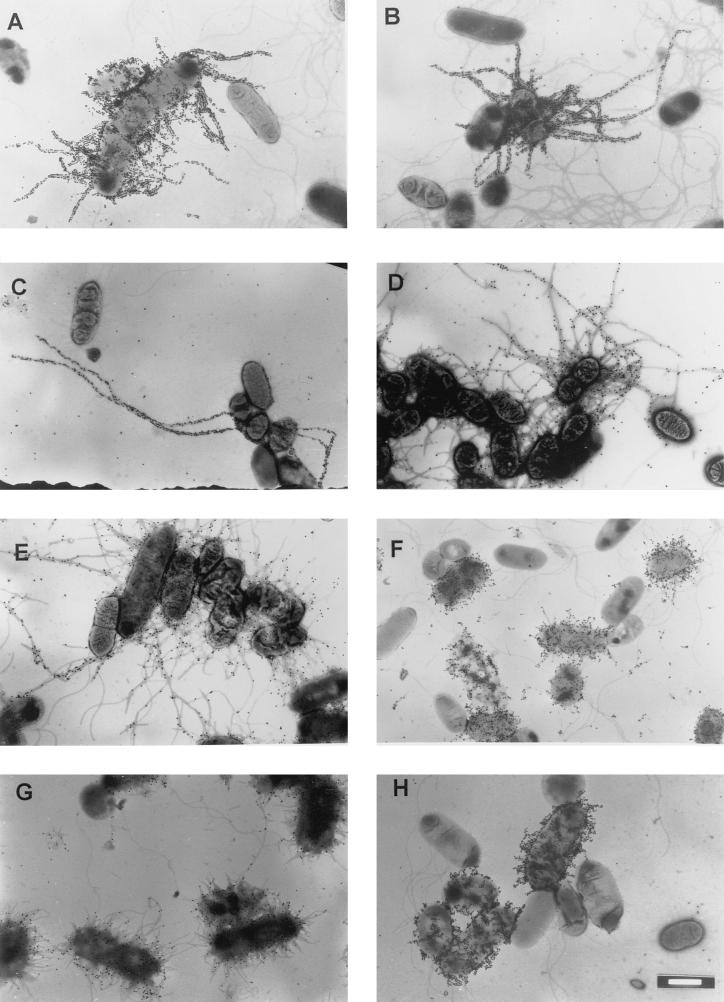
The distinctive feature of the MR/P fimbriae produced by the MrpB mutant is that they are significantly longer (mean length = 1.80 μm after three passages [P < 0.001]) than those produced by the wild-type strain (mean length = 0.29 μm) (Table 1, Fig. 2). The percentage of bacterial cells that were MR/P fimbriated on the third passage of the MrpB mutant (5%) was significantly lower (P < 0.0001 by Fisher’s exact test) than the corresponding percentage of wild-type strain cells (48%) (Table 1); no unusual numbers of free fimbriae were observed in culture supernatants of the mutant. This raised some concern that the observation of long fimbriae was artifactual because so few cells were fimbriated. However, when the MrpB mutant was passaged six times under static-culture conditions, it was found that 53% of the bacteria expressed the elongated fimbriae. This observation demonstrated that production of elongated fimbriae in the MrpB mutant was not a rare event. While it is possible that a secondary mutation in passaged cells could also have resulted in this phenotype, we view this possibility as unlikely.
TABLE 1.
Phenotypes of P. mirabilis HI4320 and mrpB mutants
| P. mirabilis construct | Plasmid used for transformation | Comments | Hemagglu- tinationa | Mean fimbria length (μm)b | No. of bacteria
with immunogold-labeled fimbriae/total no. of bacteria (%) after:
|
|
|---|---|---|---|---|---|---|
| 3rd passagec | 6th passaged | |||||
| Parent | None | Wild-type mrpB in strain HI4320 | ++++ | 0.29 ± 0.21h | 10/21 (48) | NDf |
| mrpB::aphA | None | mrpB insertionally inactivated with a Kanr cassette; no significant polar effects evident | +/−e | 1.80 ± 0.98g | 13/290 (5) | 17/32 (53) |
| mrpB::aphA | pXL4901 | mrpB mutant complemented with mrpAB under control of native promoter | ++++ | 0.85 ± 0.27g | 17/18 (94) | ND |
| mrpB::aphA | pXL8601 | mrpB mutant complemented with mrpB under control of lac promoter | ++ | 0.16 ± 0.10g | 10/309 (3) | ND |
++++, strong and rapid clumping of erythrocytes in the bacterial suspension; ++, unambiguous but less strong clumping of erythrocytes; +/−, some bacterial suspensions show weak hemagglutination; other preparations are negative.
Mean length of 10 randomly selected fimbriae on each of four different bacterial cells (n = 40) cultured statically for three passages.
Bacteria were cultured in LB broth statically (without aeration) and subcultured three times for 3 days at each passage.
Bacteria were cultured in LB broth statically and subcultured six times for 3 days at each passage.
See text for a discussion of the self-aggregation phenotype.
ND, not determined.
P < 0.001 for this value compared to the wild-type value by using the t test.
The lengths of fimbriae of the wild-type strain passaged six times appear quantitatively identical to those of fimbriae from three-passage wild-type cultures.
FIG. 2.
Immunogold labeling of MR/P fimbriae of P. mirabilis. Bacteria were reacted first with antiserum against MR/P fimbria and then with a goat anti-rabbit immunoglobulin G secondary antibody conjugated to 30-nm-diameter gold particles. (A to E) P. mirabilis HI4320 mrpB::aphA (MrpB mutant) after three (A to C) and six (D and E) passages of static culture; (F) P. mirabilis HI4320 (parent strain); (G) MrpB mutant complemented with mrpAB; (H) MrpB mutant complemented with mrpB. See Table 1 for phenotypes of constructs. White bar, 1 μm.
Assay for polar effects.
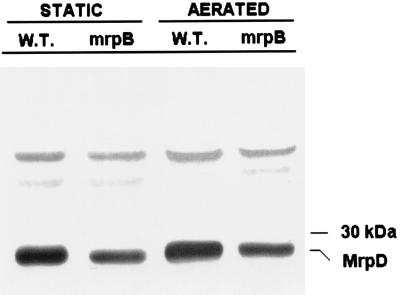
To determine whether the phenotype of the MrpB mutant was due to the loss of functional MrpB or whether there were polar effects of the insertional inactivation on genes downstream of mrpB, we assayed for synthesis of MrpD, the chaperone protein that is encoded by two genes downstream of mrpB. Protein was isolated from the periplasmic spaces of the parent strain and the MrpB mutant by osmotic shock as described previously (11). Protein (5 μg) was electrophoresed on a sodium dodecyl sulfate–12.5% polyacrylamide gel and subjected to Western blotting (Fig. 3) using polyclonal rabbit antiserum raised to a MrpD-maltose binding protein fusion which had been purified on an amylose column (data not shown). MrpD was synthesized in significant amounts by both the parent strain and the MrpB mutant (under static- and aerated-growth conditions), demonstrating that the insertion mutation of mrpB did not disrupt transcription and translation of downstream genes. It should be noted, however, that the mutant appears to synthesize slightly less MrpD than the parent strain.
FIG. 3.
Western blot of osmotic shock fluid from P. mirabilis HI4320 and its mrpB mutant developed with anti-MrpD serum. Protein (5 μg) from osmotically shocked bacteria was electrophoresed on a sodium dodecyl sulfate–12.5% polyacrylamide gel and subjected to Western blot analysis using polyclonal rabbit antiserum raised against MrpD. W.T., wild-type parental strain P. mirabilis HI4320; mrpB, MrpB mutant; STATIC, cultures grown in broth without shaking; AERATED, cultures grown in broth with shaking (200 rpm).
Complementation.
To confirm that the phenotype of the MrpB mutant is due to the loss of functional MrpB but not due to polar effects of the mutation on downstream mrp genes or the presence of a mutation elsewhere in the genome, we complemented the mutant with two separate constructs, one carrying mrpAB and one carrying mrpB alone. First, the mrpAB construct driven by the authentic mrp promoter (2.1-kb AflIII-ClaI fragment of pMRP101 [4]) was cloned into pBluescript (SK+) (new construct designated pXL4901) and electroporated into the MrpB mutant. The mrpA gene was included in this construct because we reasoned that the high copy number of mrpB may cause bacteria to produce short MR/P fimbriae (see below). This complemented mrpB mutant was shown by immunoelectron microscopy to produce MR/P fimbriae that were significantly shorter than those produced by the MrpB mutant (P < 0.001) (Fig. 2G, Table 1), suggesting that the insertional mutation in the chromosome had no significant polar effect on the expression of downstream mrp genes. To specifically demonstrate that mrpB alone was sufficient to complement the mutant, mrpB was cloned and placed under the control of the lac promoter (the 1.0-kb PvuII-ClaI fragment of pMRP101 [4] cloned into EcoRV-ClaI-digested pBluescript (SK+) [new construct designated pXL8601]) and electroporated into the MrpB mutant (P. mirabilis is lac negative). When examined by immunogold electron microscopy (Fig. 2H), these complemented mutants were found to be heavily fimbriated, but the fimbriae were not only not elongated, they were actually significantly shorter (mean length = 0.16 μm; P < 0.001) than the wild-type fimbriae (Table 1). This finding was predicted and is consistent with MrpB acting as terminator. Overexpression of mrpB would result in more-frequent insertion of MrpB into a growing fimbrial shaft and would be more likely to result in premature termination of fimbrial biogenesis, resulting in shortened fimbriae that retained binding activity. It should be noted that the copy number of pBluescript (SK+), the vector used in these complementation studies, may have some effect on fimbriation. Despite the fact that the native promoter was used in one construct, the gene dosage would still be much higher than the single chromosomal copy found in the wild-type strain.
Hemagglutination.
P. mirabilis HI4320, the mrpB mutant, and the complemented mutants were also characterized in terms of mannose-resistant hemagglutination. Bacteria grown under conditions optimal for MR/P fimbrial production, as described earlier, were collected by centrifugation (4,000 × g, 10 min, 4°C). Bacterial pellets were resuspended in PBS to approximately 109 CFU/ml. Twofold serial dilutions of the bacterial suspension were mixed with equal volumes of a 3% (vol/vol) erythrocyte suspension (in 0.85% saline) containing 50 mM mannose in a round-bottomed 96-well microtiter plate, which was then set at room temperature for 30 min. Nonagglutinated erythrocytes settled as a tight button, whereas agglutinated erythrocytes settled at the bottom as an enlarged mat. The mrpB mutant was weak for mannose-resistant hemagglutination compared to wild-type HI4320. Since the significantly longer MR/P fimbriae produced by this P. mirabilis mutant are likely to be more susceptible to mechanical shearing caused by suspending cell aggregates in PBS buffer, it is not surprising that we did not always detect significant hemagglutination in these preparations.
When the MrpB mutant was complemented with cloned mrpAB, hemagglutination was fully restored. That hemagglutination power was slightly reduced in the mutant complemented with mrpB alone, and this reduction may have resulted from the adhesin being presented only a short distance from the bacterial surface, making it more difficult to span the distance from the bacterium to the receptor on the erythrocyte surface. The level of hemagglutination, however, was still significant (Table 1).
In a separate study of the MR/P hemagglutinin, MrpH, when expressed in E. coli DH5α, conferred on bacteria both the ability to cause mannose-resistant hemagglutination and the ability to aggregate in liquid cultures (data not shown). Therefore, we addressed the ability of the MrpB mutant to cause mannose-resistant hemagglutination in this manner. When we expressed the mrp fimbrial gene cluster carrying the mrpB insertional mutation in E. coli DH5α by cloning the entire gene cluster (with a mutant mrpB) into a high-copy-number vector [pBluescript (SK+)], the bacteria retained the ability to aggregate in liquid culture, a characteristic of MrpH expression. This demonstrated that the insertional mutation did not have a polar effect on the expression of the last gene of the operon, mrpH. This argument is valid, of course, only if the mrp gene cluster is an operon. It appears from the predicted transcriptional organization (4) that this is the case; however, this has not been confirmed experimentally.
Amino acid sequence similarity of MrpB and PapH.
In agreement with our conclusion from the above studies that MrpB functions as a terminator for MR/P fimbrial assembly is the finding that MrpB has 29% amino acid sequence identity with PapH (Fig. 4) and is the most similar homolog (P = 1.2 × 10−17). Mutagenesis studies of the pap operon by Baga et al. (1) have shown that PapH plays a role in anchoring the fimbria to the cell and in modulating pilus length. The functional homology between MrpB and PapH is further supported by their significant sequence homology. An alternate interpretation of the MrpB mutant phenotype, however, could highlight a role for MrpB in optimizing the initiation of fimbrial assembly. A mutant with such a protein would also have the phenotype of inefficient initiation, resulting in fewer but longer fimbriae. Complementation with mrpB in the presence of mrpA would also result in more-numerous but shorter fimbriae compared to those of the wild-type strain. Further studies are required to test this hypothesis.
FIG. 4.
Amino acid sequence alignment of MrpB and PapH. The primary amino acid (single-letter code) sequences of P. mirabilis MrpB and E. coli PapH (accession no., P07111 [1]) were aligned. For 194 positions aligned, there were 57 exact matches (:), 17 conservative replacements (.), and 120 mismatches. MrpB and PapH had 29% amino acid sequence identity and 38% similarity (exact matches plus conservative replacements).
Acknowledgments
This work is supported in part by Public Health Service grant DK47920 from the National Institutes of Health.
REFERENCES
- 1.Baga M, Norgren M, Normark S. Biogenesis of E. coliPap pili: PapH, a minor pilin subunit involved in cell anchoring and length modulation. Cell. 1987;49:241–251. doi: 10.1016/0092-8674(87)90565-4. [DOI] [PubMed] [Google Scholar]
- 2.Bahrani F K, Johnson D E, Robbins D, Mobley H L T. Proteus mirabilisflagella and MR/P fimbriae: isolation, purification, N-terminal analysis, and serum antibody response following experimental urinary tract infection. Infect Immun. 1991;59:3574–3580. doi: 10.1128/iai.59.10.3574-3580.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahrani F K, Massad G, Lockatell C V, Johnson D E, Russell R G, Warren J W, Mobley H L T. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect Immun. 1994;62:3363–3371. doi: 10.1128/iai.62.8.3363-3371.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahrani F K, Mobley H L T. Proteus mirabilisMR/P fimbriae: molecular cloning, expression, and nucleotide sequence of the major fimbrial subunit gene. J Bacteriol. 1993;175:457–464. doi: 10.1128/jb.175.2.457-464.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coliby using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Zhao H, Geymonat L, Bahrani F, Johnson D E, Mobley H L T. Proteus mirabilis mannose-resistant, Proteus-like fimbriae: MrpG is located at the fimbrial tip and is required for fimbrial assembly. Infect Immun. 1997;65:1327–1334. doi: 10.1128/iai.65.4.1327-1334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 8.Mobley H L T, Belas R. Swarming and pathogenicity of Proteus mirabilisin the urinary tract. Trends Microbiol. 1995;3:280–284. doi: 10.1016/s0966-842x(00)88945-3. [DOI] [PubMed] [Google Scholar]
- 9.Mobley H L T, Chippendale G R. Hemagglutinin, urease, and hemolysin production by Proteus mirabilisfrom clinical sources. J Infect Dis. 1990;161:525–530. doi: 10.1093/infdis/161.3.525. [DOI] [PubMed] [Google Scholar]
- 10.Mobley H L T, Warren J W. Urease-positive bacteriuria and obstruction of long-term urinary catheters. J Clin Microbiol. 1987;25:2216–2217. doi: 10.1128/jcm.25.11.2216-2217.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neu H C, Heppel L A. The release of enzymes from Escherichia coliby osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965;240:3685–3692. [PubMed] [Google Scholar]
- 12.Zhao H, Li X, Johnson D E, Blomfield I, Mobley H L T. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilisinfecting the urinary tract. Mol Microbiol. 1997;23:1009–1019. doi: 10.1046/j.1365-2958.1997.2791645.x. [DOI] [PubMed] [Google Scholar]