Abstract
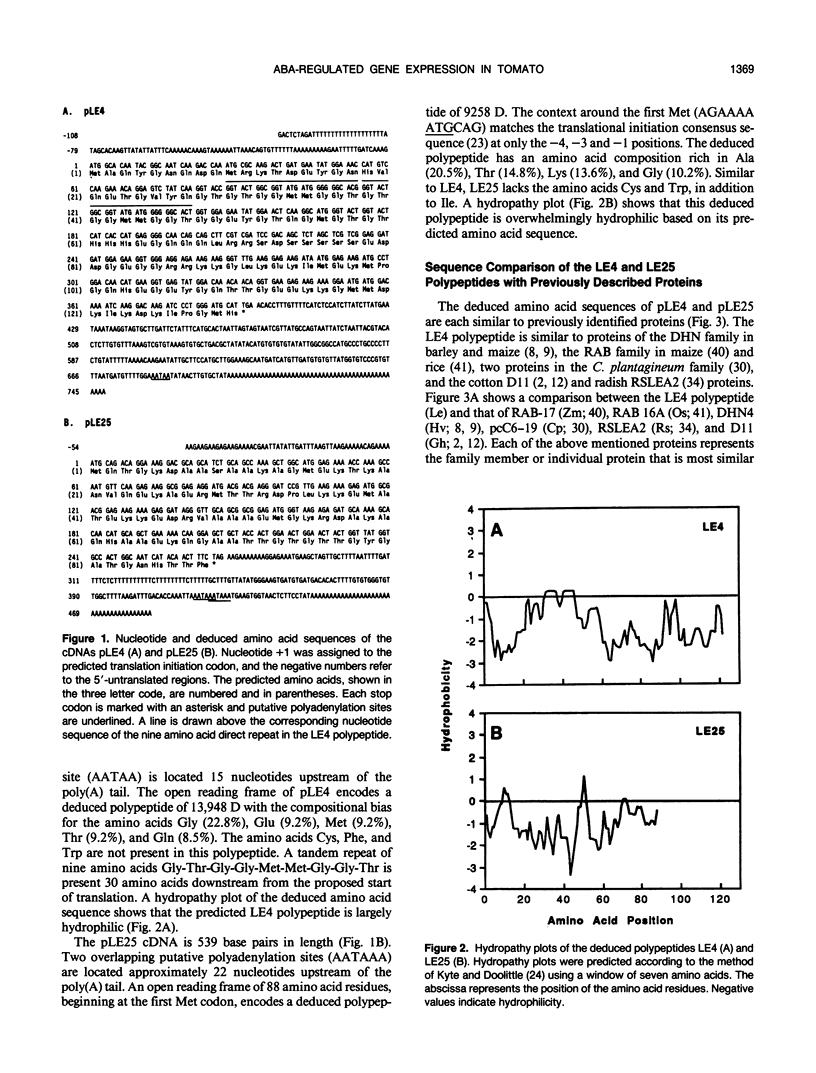
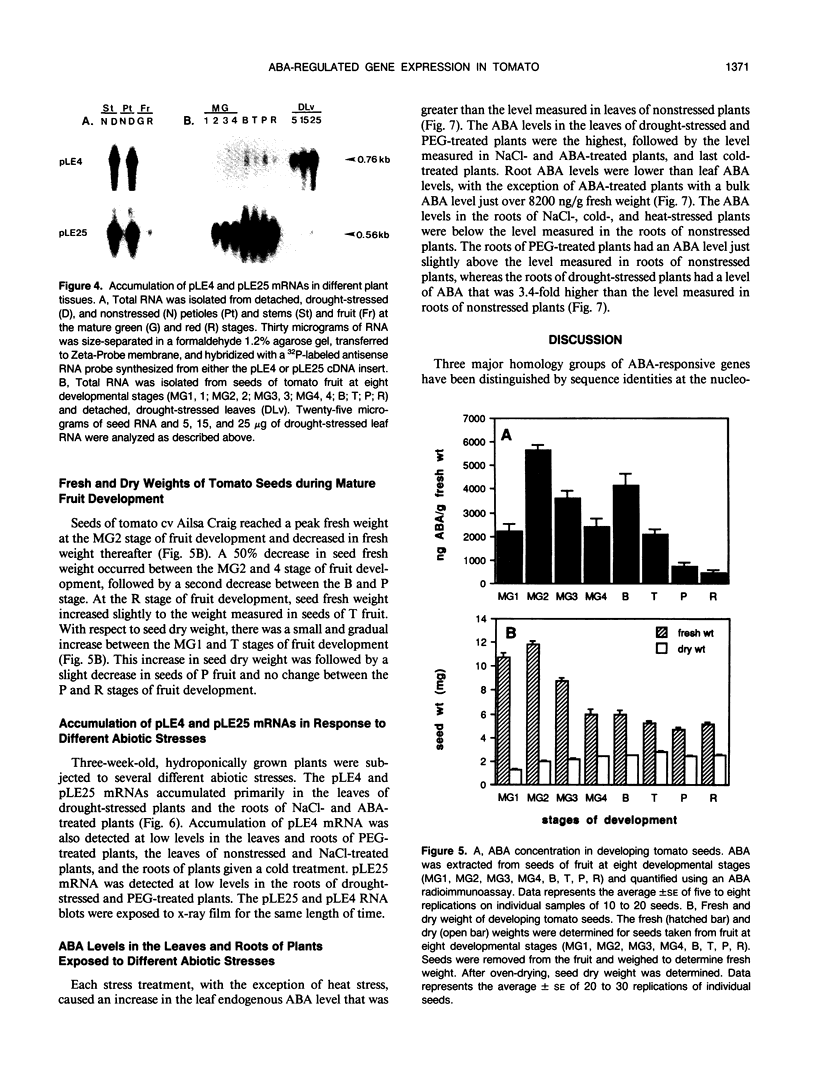
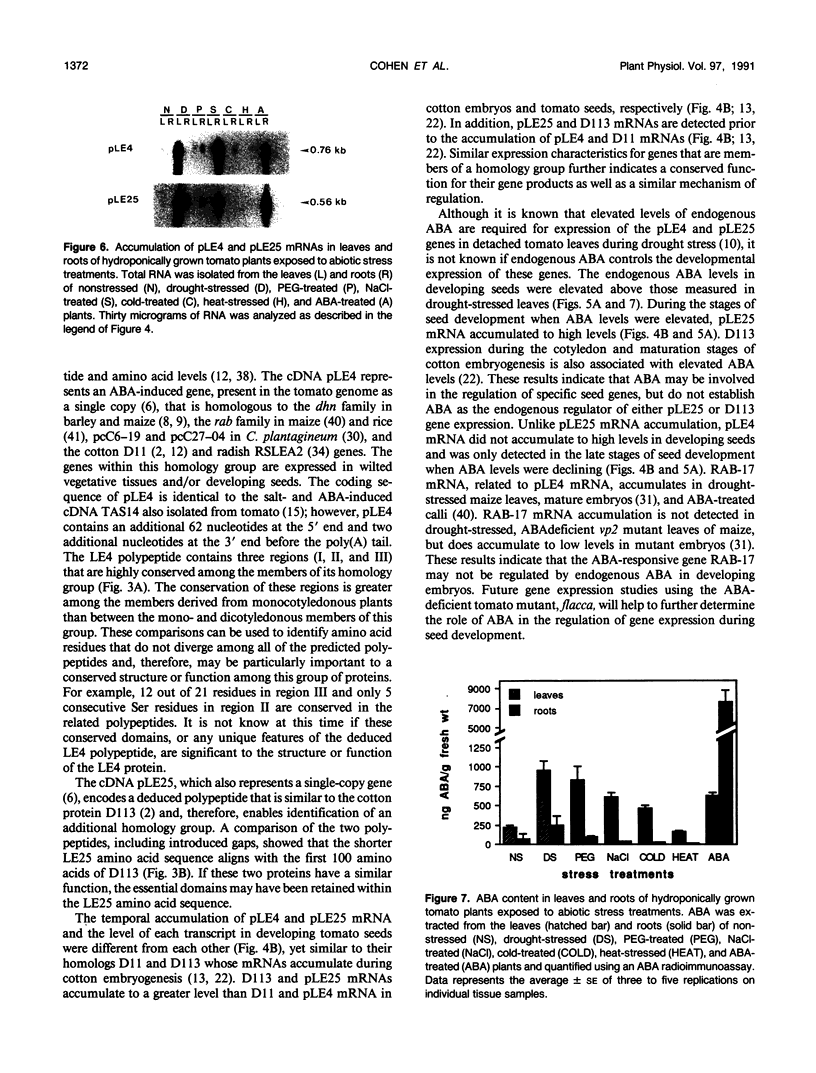
The cDNAs, pLE4 and pLE25, represent mRNAs that accumulate in response to water deficit and elevated levels of endogenous abscisic acid in detached leaves of drought-stressed tomato (Lycopersicon esculentum Mill., cv Ailsa Craig) (A Cohen, EA Bray [1990] Planta 182: 27-33). DNA sequence analysis of pLE4 and pLE25 showed that the deduced polypeptides were 13.9 and 9.3 kilodaltons, respectively. Each polypeptide was hydrophilic, cysteine- and tryptophan-free, and found to be similar to previously identified proteins that accumulate during the late stages of embryogenesis. pLE4 and pLE25 mRNA accumulated in a similar organ-specific pattern in response to specific abiotic stresses. Yet, expression patterns of the corresponding genes in response to developmental cues were not similar. pLE25 mRNA accumulated to much higher levels in developing seeds than in drought-stressed vegetative organs. pLE4 mRNA accumulated predominantly in drought-stressed leaves. The similarities and differences in the accumulation characteristics of these two mRNAs indicates that more than one mechanism exists for the regulation of their corresponding genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray E. A., Beachy R. N. Regulation by ABA of beta-Conglycinin Expression in Cultured Developing Soybean Cotyledons. Plant Physiol. 1985 Nov;79(3):746–750. doi: 10.1104/pp.79.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray E. A. Drought- and ABA-Induced Changes in Polypeptide and mRNA Accumulation in Tomato Leaves. Plant Physiol. 1988 Dec;88(4):1210–1214. doi: 10.1104/pp.88.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes B., Dekeyser R., Villarroel R., Van den Bulcke M., Bauw G., Van Montagu M., Caplan A. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell. 1990 Jan;2(1):19–27. doi: 10.1105/tpc.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close T. J., Kortt A. A., Chandler P. M. A cDNA-based comparison of dehydration-induced proteins (dehydrins) in barley and corn. Plant Mol Biol. 1989 Jul;13(1):95–108. doi: 10.1007/BF00027338. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galau G. A., Bijaisoradat N., Hughes D. W. Accumulation kinetics of cotton late embryogenesis-abundant mRNAs and storage protein mRNAs: coordinate regulation during embryogenesis and the role of abscisic acid. Dev Biol. 1987 Sep;123(1):198–212. doi: 10.1016/0012-1606(87)90442-8. [DOI] [PubMed] [Google Scholar]
- Godoy J. A., Pardo J. M., Pintor-Toro J. A. A tomato cDNA inducible by salt stress and abscisic acid: nucleotide sequence and expression pattern. Plant Mol Biol. 1990 Nov;15(5):695–705. doi: 10.1007/BF00016120. [DOI] [PubMed] [Google Scholar]
- Guerrero F. D., Jones J. T., Mullet J. E. Turgor-responsive gene transcription and RNA levels increase rapidly when pea shoots are wilted. Sequence and expression of three inducible genes. Plant Mol Biol. 1990 Jul;15(1):11–26. doi: 10.1007/BF00017720. [DOI] [PubMed] [Google Scholar]
- Gómez J., Sánchez-Martínez D., Stiefel V., Rigau J., Puigdomènech P., Pagès M. A gene induced by the plant hormone abscisic acid in response to water stress encodes a glycine-rich protein. Nature. 1988 Jul 21;334(6179):262–264. doi: 10.1038/334262a0. [DOI] [PubMed] [Google Scholar]
- Hahn M., Walbot V. Effects of cold-treatment on protein synthesis and mRNA levels in rice leaves. Plant Physiol. 1989 Nov;91(3):930–938. doi: 10.1104/pp.91.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hughes D. W., Galau G. A. Temporally modular gene expression during cotyledon development. Genes Dev. 1989 Mar;3(3):358–369. doi: 10.1101/gad.3.3.358. [DOI] [PubMed] [Google Scholar]
- Irifune M., Ogino S., Harada T., Matsunaga T., Sakai K. [The desensitization therapy in children with nasal allergy to house dust]. Nihon Jibiinkoka Gakkai Kaiho. 1989 Mar;92(3):395–401. doi: 10.3950/jibiinkoka.92.395. [DOI] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Larosa P. C., Hasegawa P. M., Rhodes D., Clithero J. M., Watad A. E., Bressan R. A. Abscisic Acid Stimulated Osmotic Adjustment and Its Involvement in Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1987 Sep;85(1):174–181. doi: 10.1104/pp.85.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitcham E. J., Gross K. C., Ng T. J. Tomato Fruit Cell Wall Synthesis during Development and Senescence : In Vivo Radiolabeling of Wall Fractions Using [C]Sucrose. Plant Physiol. 1989 Feb;89(2):477–481. doi: 10.1104/pp.89.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. S., Poole R. J., Dhindsa R. S. Abscisic Acid-regulated gene expression in relation to freezing tolerance in alfalfa. Plant Physiol. 1988 Jun;87(2):468–473. doi: 10.1104/pp.87.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra S. S., Wolfraim L., Poole R. J., Dhindsa R. S. Molecular cloning and relationship to freezing tolerance of cold-acclimation-specific genes of alfalfa. Plant Physiol. 1989 Jan;89(1):375–380. doi: 10.1104/pp.89.1.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatkowski D., Schneider K., Salamini F., Bartels D. Characterization of Five Abscisic Acid-Responsive cDNA Clones Isolated from the Desiccation-Tolerant Plant Craterostigma plantagineum and Their Relationship to Other Water-Stress Genes. Plant Physiol. 1990 Dec;94(4):1682–1688. doi: 10.1104/pp.94.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pla M., Goday A., Vilardell J., Gómez J., Pagès M. Differential regulation of ABA-induced 23-25 kDa proteins in embryo and vegetative tissues of the viviparous mutants of maize. Plant Mol Biol. 1989 Oct;13(4):385–394. doi: 10.1007/BF00015550. [DOI] [PubMed] [Google Scholar]
- Raynal M., Gaubier P., Grellet F., Delseny M. Nucleotide sequence of a radish cDNA clone coding for a late embryogenesis abundant (LEA) protein. Nucleic Acids Res. 1990 Oct 25;18(20):6132–6132. doi: 10.1093/nar/18.20.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skriver K., Mundy J. Gene expression in response to abscisic acid and osmotic stress. Plant Cell. 1990 Jun;2(6):503–512. doi: 10.1105/tpc.2.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. R., Voetberg G. Relationship between Stress-Induced ABA and Proline Accumulations and ABA-Induced Proline Accumulation in Excised Barley Leaves. Plant Physiol. 1985 Sep;79(1):24–27. doi: 10.1104/pp.79.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilardell J., Goday A., Freire M. A., Torrent M., Martínez M. C., Torné J. M., Pagès M. Gene sequence, developmental expression, and protein phosphorylation of RAB-17 in maize. Plant Mol Biol. 1990 Mar;14(3):423–432. doi: 10.1007/BF00028778. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K., Mundy J., Chua N. H. Four tightly linked rab genes are differentially expressed in rice. Plant Mol Biol. 1990 Jan;14(1):29–39. doi: 10.1007/BF00015652. [DOI] [PubMed] [Google Scholar]




