Abstract
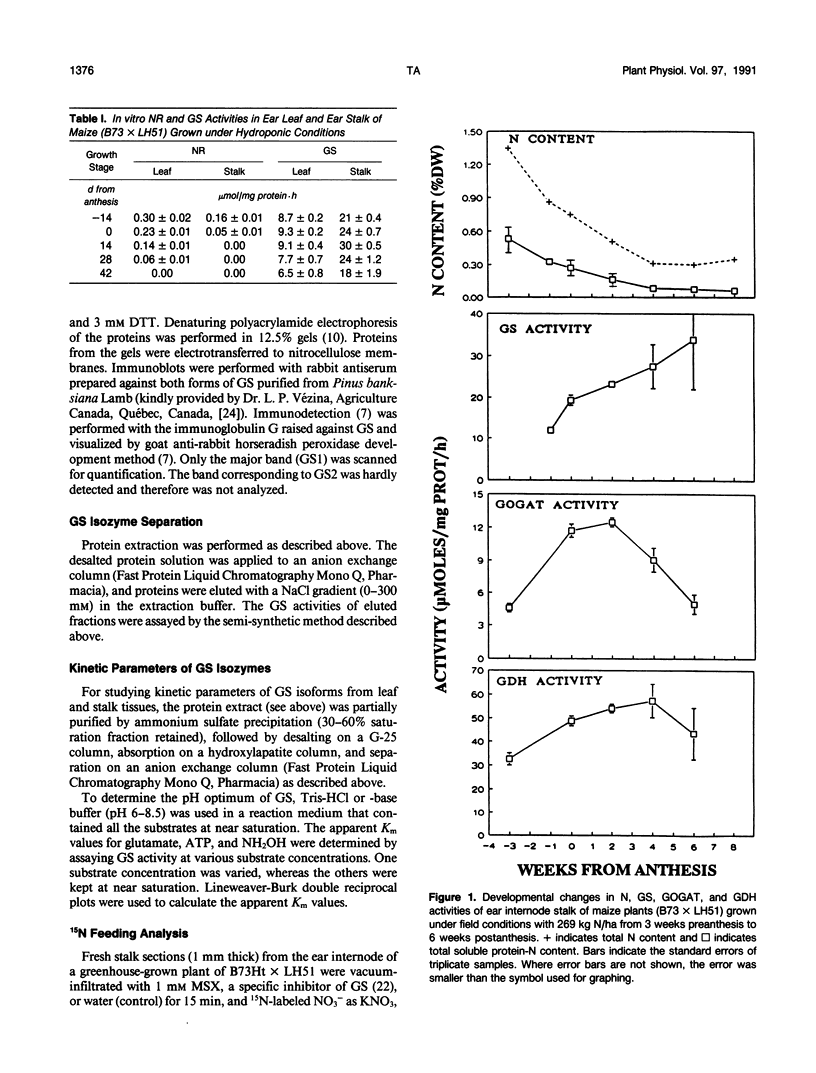
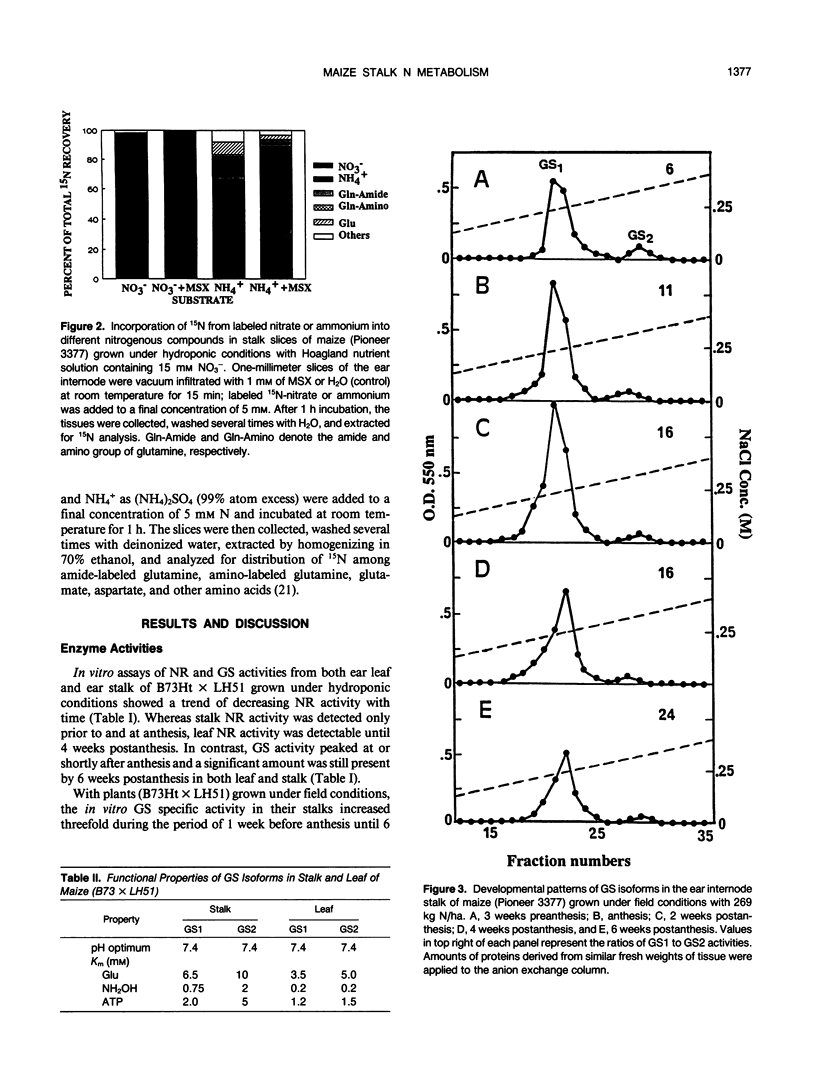
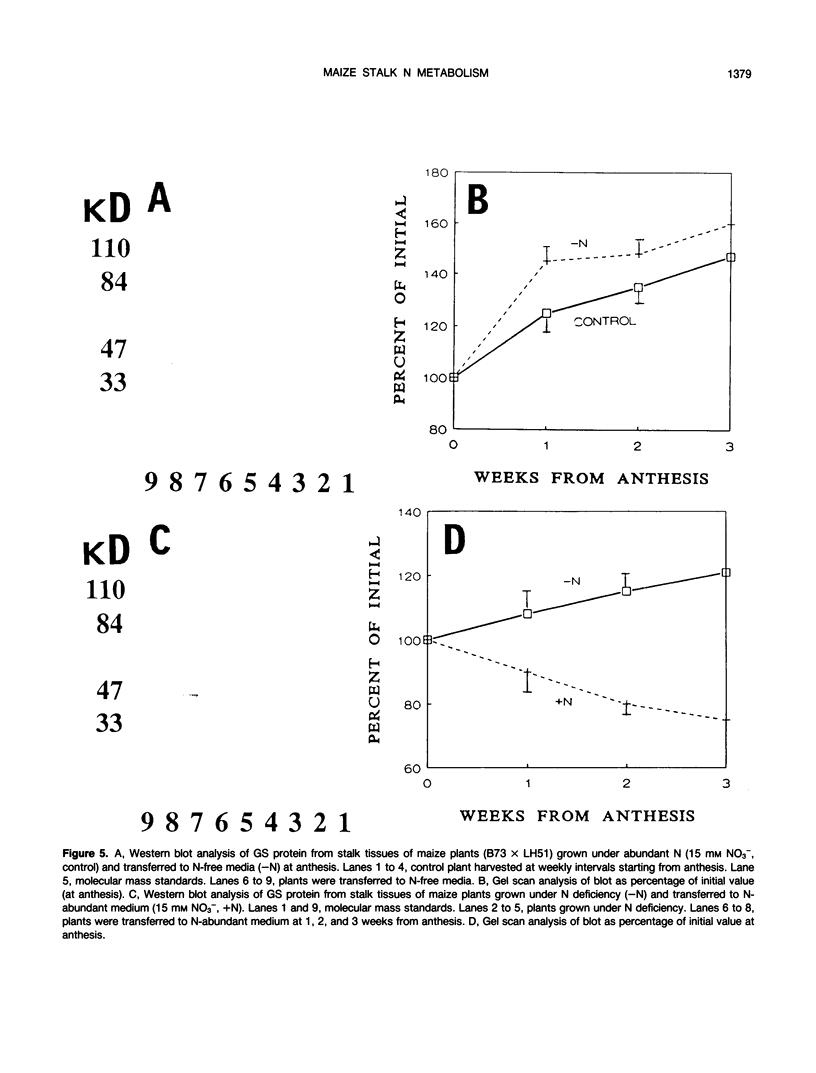
During ear development in maize (Zea mays L.), nitrogenous compounds are translocated from vegetative organs to the kernels. At anthesis, the stalk contains approximately 40% of the total plant N, and contributes 45% of the N remobilized to the ear. Therefore, the stalk has an important function as a temporary reservoir for N. Little is known of the metabolism of maize stalks, and this paper describes initial studies of enzymes of N metabolism. High in vitro activity of glutamine synthetase (GS) in maize stalk samples throughout ear development contrasted with a peak in activity of glutamate synthase soon after anthesis and negligible nitrate reductase. With fresh sections of stalk tissue collected at anthesis, 15N-feeding experiments confirmed high GS and low nitrate reductase activities. Two isoforms of GS were separated from extracts from stalk tissue: GS1, the cytoplasmic form, increased to maximum levels at 2 weeks postanthesis and remained fairly high thereafter; whereas the plastidic form, GS2, declined progressively during kernel development. Western blot analysis confirmed the presence of constantly high levels of GS protein after anthesis. The levels of GS proteins decreased after transfer of N-starved, hydroponically grown plants to N-rich conditions in order to restrict remobilization of N. In contrast, transfer of plants grown under abundant N conditions to N-free medium, which encourages N remobilization, resulted in a relative increase in GS protein. Because glutamine is the major form of N transported in maize, the results indicate that GS, specifically the GS1 isoform, has a central role in the remobilization on nitrogenous compounds from the stalk to the ear.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Below F. E., Christensen L. E., Reed A. J., Hageman R. H. Availability of reduced N and carbohydrates for ear development of maize. Plant Physiol. 1981 Nov;68(5):1186–1190. doi: 10.1104/pp.68.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Christensen L. E., Below F. E., Hageman R. H. The effects of ear removal on senescence and metabolism of maize. Plant Physiol. 1981 Nov;68(5):1180–1185. doi: 10.1104/pp.68.5.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. W., Walker E. L., Coruzzi G. M. Cell-specific expression in transgenic plants reveals nonoverlapping roles for chloroplast and cytosolic glutamine synthetase. Proc Natl Acad Sci U S A. 1990 May;87(9):3459–3463. doi: 10.1073/pnas.87.9.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami N., Watanabe A. Senescence-specific increase in cytosolic glutamine synthetase and its mRNA in radish cotyledons. Plant Physiol. 1988 Dec;88(4):1430–1434. doi: 10.1104/pp.88.4.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Muhitch M. J. Purification and Characterization of Two Forms of Glutamine Synthetase from the Pedicel Region of Maize (Zea mays L.) Kernels. Plant Physiol. 1989 Nov;91(3):868–875. doi: 10.1104/pp.91.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl R. L., Harper J. E., Hageman R. H. Improvements of the nitrite color development in assays of nitrate reductase by phenazine methosulfate and zinc acetate. Plant Physiol. 1974 Jun;53(6):825–828. doi: 10.1104/pp.53.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey R., Beevers L. Enzymology of Glutamine Metabolism Related to Senescence and Seed Development in the Pea (Pisum sativum L.). Plant Physiol. 1978 Apr;61(4):494–500. doi: 10.1104/pp.61.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank J. C., Below F. E., Lambert R. J., Hageman R. H. Interaction of carbon and nitrogen metabolism in the productivity of maize. Plant Physiol. 1982 Oct;70(4):1185–1190. doi: 10.1104/pp.70.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta T. C., Faris M. A., Macdowall F. D. Pathways of Nitrogen Metabolism in Nodules of Alfalfa (Medicago sativa L.). Plant Physiol. 1986 Apr;80(4):1002–1005. doi: 10.1104/pp.80.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta T. C., Joy K. Separation of amino acid and amide nitrogen from plant extracts for 15N analysis. Anal Biochem. 1986 May 1;154(2):564–569. doi: 10.1016/0003-2697(86)90030-8. [DOI] [PubMed] [Google Scholar]
- Vézina L. P., Margolis H. A. Purification and Properties of Glutamine Synthetase in Leaves and Roots of Pinus banksiana Lamb. Plant Physiol. 1990 Oct;94(2):657–664. doi: 10.1104/pp.94.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]