Abstract
GRPR is a type of seven-transmembrane G-protein coupled receptor that belongs to the bombesin protein receptor family. It is highly expressed in various cancers, including prostate cancer, breast cancer, lung cancer, gastrointestinal cancer, and so on. As a result, molecular imaging studies have been conducted using radiolabeled GRPR ligands for tumor diagnosis, as well as monitoring of recurrence and metastasis. In this paper, we provided a comprehensive overview of relevant literature from the past two decades, with a specific focus on the advancements made in radiolabeled GRPR ligands for imaging prostate cancer and breast cancer.
GRPR
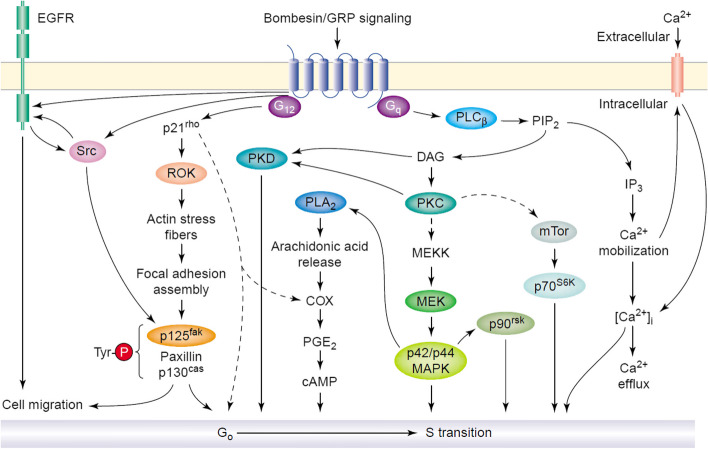
The Gastrin Releasing Peptide Receptor (GRPR) is a G-protein-coupled receptor that belongs to the bombesin protein receptor family [1]. The natural ligand for GRPR is gastrin releasing peptide (GRP). The C-terminal peptide sequence of GRP shares high similarity with the amphibious peptide bombesin, a 14-amino acid peptide that exhibits high affinity for GRPR and the neuromedin B receptor. Both GRP and bombesin bind strongly to GRPR in the nanomolar range. When these peptides bind to GRPR, they initiate downstream signaling cascades and activate various physiological and biological effects, including cell proliferation, differentiation, and mitosis [2–4]. Currently, all GRPRs are characterized as G-protein-coupled receptors with seven transmembrane domains. The primary signaling cascade involves the activation of phospholipase C (PLC), resulting in intracellular calcium changes, the production of diacylglycerol, and the activation of protein kinase C (PKCs) [2, 5–8]. These effects are primarily achieved through coupling with heterotrimeric G proteins of the Gq/11 and G12/13 families [5, 9, 10]. Other intracellular mediators activated by GRPRs include mitogen-activated protein kinases, adhesion kinases, phosphatidylinositol 3-kinases, and, in certain cases, cyclic AMP response element binding proteins (Fig. 1) [11–13]. GRPR is involved in various physiological mechanisms within the human body. For instance, it regulates gastrointestinal movement and gastric emptying, as well as induces smooth muscle contraction [14]. Endogenous gastrin release is stimulated by activating sensory neurons in the gastric mucosa [15, 16]. Furthermore, GRPR plays a role in the regulation of trypsin release [17] and is involved in immune responses [18, 19]. In addition to these functions, GRPR is implicated in certain brain functions such as the regulation of circadian rhythm [20, 21], memory [22], and the modulation of stress, fear, and anxiety [23–25].
Fig. 1.
Signal transduction pathways activated by engagement of the bombesin/GRP receptor, a paradigm of mitogenic GPCR. The binding of the ligand (e.g. bombesin) to the cognate GPCR (e.g. the bombesin/GRP preferring receptor) induces activation of the heterotrimeric G proteins of the Gq and G12 subfamilies. Signaling through Gq/G11 leads to PLC activation, hydrolysis of PIP2, generation of IP3 and DAG, and activation of subsequent phosphorylation cascades leading to the activation of ERKs, p70S6K, and PKD. These pathways are representative of studies in Swiss 3T3 fibroblasts, SCLC cell lines, and pancreatic cancer cells. In other cell types, activation of tyrosine phosphorylation pathways including Src, EGFR, and/or Pyk-2 promote Ras-mediated ERK activation via the SOS–Grb2 complex. Signaling through the G12 subfamily (comprising Gα12 and Gα13) transduces GPCR signals into Rho activation, actin remodeling, assembly of focal adhesions, and tyrosine phosphorylation of the focal adhesion-associated proteins FAK, CAS, and paxillin, and complex formation between FAK and Src. These pathways are implicated in both cell proliferation and cell migration. With permission, from Ref. [12]
Expression of GRPR in different cancers
GRPR has garnered significant interest in the fields of oncology and nuclear medicine due to its high-density expression in various cancers, including prostate cancer (PC) [26–28], breast cancer (BC) [29–32], small cell lung cancer (SCLC) [33], gastrinoma, gastrointestinal stromal tumors [34, 35], and other cancers [36]. Notably, apart from the pancreas and gastrointestinal tract, there is minimal physiological expression of GRPR in other tissues [37]. In the following section, we make a concise overview of GRPR expression in several common tumors.
The expression of the GRPR gene was assessed in fresh frozen specimens from 12 cases of PC and 6 cases of benign prostatic hyperplasia using Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and in situ hybridization techniques. The findings revealed that GRPR was expressed in both PC and benign prostatic hyperplasia tissues. The concentration of GRPR mRNA in cancerous tissue varied widely, ranging from very high to undetectable levels (approximately 30% of cases), whereas the concentration of GRPR mRNA in normal tissue remained consistently low [38]. The radioactive ligand 125I-Tyr4-bombesin was utilized to examine the expression of GRPR, and the positive expression rate of GRPR in invasive PC (30/30) and in prostatic intraepithelial proliferative lesions (26/26) was found 100%. In hyperplastic prostate glands, GRPR was only detected in a few instances, and its density was substantially lower in glandular tissues, primarily localized in certain interstitial tissues [26]. Among the 80 PC specimens tested using 125I-Tyr4-bombesin receptor binding assays, 63% exhibited high-affinity and low-volume binding sites for bombesin/GRP, with 12 receptor-positive samples displaying two types of binding sites. Concurrently, 91% of PC samples analyzed via RT-PCR expressed GRPR mRNA [39]. GRPRs were also confirmed in various PC cell lines [40, 41]. Furthermore, when assessing GRPR levels in 80 cases of primary PC using receptor binding tests, 68% demonstrated a high affinity to GRPR. Subsequent analysis of these samples through RT-PCR revealed high levels of GRPR mRNA in 91% of cases, while the detectable GRPR mRNA level in non-neoplastic prostate tissue remained significantly low. These observations strongly suggested that GRPR may serve as a molecular marker for precancerous changes in PC [42]. Given the overexpression of GRPR in PC and its high labeling efficiency, GRPR-targeted radioligands have been extensively investigated and applied in PC diagnosis and treatment [27].
GRPR is predominantly expressed in the majority of BCs, while it is not detected in normal breast epithelial cells [36]. Among BC cell lines, GRPR was found in 38% of cases, whereas long-term cultured normal breast epithelial cells did not exhibit GRPRs [43]. In a study conducted by Halmos et al., the binding of 125I-Tyr4-bombesin to cell membranes isolated from 100 human BC cases revealed that 33% of individuals expressed GRPRs [44]. M. Gugger et al. utilized in vitro receptor autoradiography to evaluate the presence of GRPRs in human breast tissues, including both non-tumor and tumor samples, and found GRPR expression in 63% of cases diagnosed with invasive ductal carcinoma and 65% of cases diagnosed with ductal carcinoma in situ. Notably, the expression of GRPRs was predominantly observed within the neoplastic breast epithelial cells, exhibiting a high density but an uneven distribution [29]. Furthermore, a comprehensive study employing in vitro autoradiography to examine the expression of GRPR subtypes reported that 72% (41 out of 57) of BCs expressed GRPRs [36].
The expression of GRPR in lung cancer has been extensively studied. Several investigations have reported the overexpression of GRPR in non-small cell lung cancer (NSCLC) and its potential role in promoting tumor growth. In a study by Paola et al., the occurrence frequency, relative quantitative expression, activation signaling, and impact on cell growth of GRPR were examined in 13 different human lung cancer cell lines. The findings revealed that GRPR could stimulate the breakdown of inositol phosphate, induce changes in intracellular calcium levels, increase MAPK phosphorylation, promote cell growth, trigger trans-activation of EGFR in specific lung cancer cell lines, and influence cell signaling and growth. Additionally, the activation of GRPR was found to enhance the survival of lung cancer cells exposed to tyrosine kinase inhibitors (TKIs) [36]. Approximately two-thirds of SCLC tumors produce gastrin-releasing peptide precursor (pro-GRP), which establishes the theoretical basis for pro-GRP as a specific tumor marker for SCLC. Subsequent experiments have demonstrated that pro-GRP can not only be utilized in the early diagnosis of SCLC but also aid in assessing treatment effectiveness and detecting tumor recurrence in a timely manner [45]. Furthermore, high expression of GRPR has been observed in several other types of cancer, although further details on these findings are beyond the scope of this discussion.
PET/CT
Positron emission tomography/computed tomography (PET/CT) represents a novel imaging modality that synergistically combines two advanced techniques: functional metabolic imaging (PET) and anatomical structural imaging (CT). By administering a small amount of positron-emitting radiotracer into the human body and utilizing specialized detectors, PET/CT facilitates the assessment of positron annihilation distribution in various organs. Concurrently, computer tomography enables precise localization of uptake of the PET emitter, thereby offering comprehensive visualization of the physiological and metabolic functions of major human organs. This integration harnesses the respective advantages of PET and CT, maximizing their potential [46, 47]. Considered an advanced imaging technology within the realm of nuclear medicine, PET/CT serves as a powerful tool to capture changes in disease-related physiological functions [48–50]. By merging PET's functional imaging capabilities with CT's anatomical information, PET/CT provides molecular-level insights into tissue cell metabolism, function, blood flow, cell proliferation, and receptor distribution. It has as extensive applications in oncology, cardiovascular diseases, neurology, and other fields, providing vital diagnostic information for physiological and pathological conditions. Consequently, PET/CT has become an indispensable imaging modality in clinical practice [51, 52].
Application of radionuclide labeled GRPR agonist/antagonist in PET imaging of various cancers
Nowadays, a lot of radionuclide-labeled GRPR agonists/antagonists have been developed and applied in tumor imaging and treatment. Over the past two decades, extensive studies were focused on various types of cancer, with particular emphasis on PC and BC, as these two cancers have the highest incidence rates among men and women, respectively, in western countries. Furthermore, PC and BC are associated with substantial morbidity and mortality, especially during the metastatic stage [53]. Hence, there is a critical need for non-invasive and reliable methods to diagnose and stage these cancers. Biopsies, which are often inconclusive, can cause patient discomfort, anxiety, and increased medical costs [54, 55]. Conventional imaging techniques, including MRI, CT, ultrasound, and even established nuclear medicine procedures like 18F-FDG-PET, have limited diagnostic value due to their lack of specificity. Consequently, receptor-targeted imaging has emerged as an appealing alternative for achieving highly specific and sensitive diagnosis of primary and metastatic diseases. The high expression of GRPR in pathological lesions offers great promise for application of receptor-targeted imaging in PC and BC. Other types of cancer are rarely reported or only briefly described.
Prostate cancer (PC)
The bombesin protein [56–58], an amphibian counterpart of mammalian gastrin-releasing peptide, has been extensively utilized in the development of molecular probes for GRPR imaging. Specifically, the fragment peptide BBN (7–14) has gained significant popularity. Initially, radiolabeled bombesin analogues were created to target GRPR-positive tumors in vivo, primarily due to their rapid and extensive internalization into cancer cells [59, 60]. At that time, internalization was considered crucial for prolonging retention and potentially improving diagnostic sensitivity and therapeutic effectiveness. However, mounting evidence suggests that radiolabeled GRPR antagonists exhibit surprisingly superior capabilities in visualizing GRPR-positive tumors in vivo [61, 62]. Notably, GRPR antagonists also offer an advantage in terms of biological safety. In comparison to agonists, antagonists do not induce pharmacological effects following receptor binding, resulting in better tolerance after intravenous administration.
Rosalba Mansi et al. conducted a comparative analysis between the newly developed 111In/68Ga-labeled bombesin antagonist RM1 and the GRPR-targeting agonist 111In-AMBA. The IC50 value of natIn-RM1 was 14 ± 3.4 nM. nat/111In-RM1 was found to bind to the GRPR with a Kd of 8.5 ± 2.7 nM compared with a Kd of 0.6 ± 0.3 nM of 111In-AMBA. A higher Bmax value was observed for 111In-RM1 (2.4 ± 0.2 nM) compared with 111In-AMBA (0.7 ± 0.1 nM). Additionally, the researchers investigated the biodistribution and imaging in PC-3 tumor-bearing nude mice. The efficacy of the antagonists was assessed by examining their impact on calcium release and receptor internalization, which were monitored through immunofluorescence microscopy. Despite exhibiting relatively low affinity for GRPR, the antagonist 111In/68Ga-RM1 demonstrated superior targeting capabilities compared to 111In-AMBA. These findings suggested that radiolabeled GRPR antagonists hold greater potential than radiolabeled agonists for in vivo imaging and targeted radiotherapy of GRPR-positive tumors [62].
Rosalba Mansi et al. introduced a novel compound, RM2, labeled with radioactive metals such as 111In and 68Ga. The researchers synthesized RM2 and conducted in vitro evaluations using PC-3 cells. The IC50 values were 7.7 ± 3.3 nM for RM2 and 9.3 ± 3.3 nM for natIn-RM2, The Kd value for 111In-RM2 was 2.9 ± 0.4 nM while the Bmax value was 1.1 ± 0.05 nM. The efficacy of the antagonists was evaluated through immunofluorescence-based internalization and calcium mobilization tests. Furthermore, the in vivo distribution of 111In-RM2 and 68Ga-RM2, as well as PET imaging of 68Ga-RM2 were performed in mice bearing PC-3 and LNCaP tumors. The findings demonstrated that RM2 and 111In-RM2 exhibited high affinity and selectivity as ligands for GRPRs. Immunofluorescence-based internalization and calcium mobilization experiments confirmed the absence of agonist effects. Both 68Ga-RM2 and 111In-RM2 displayed substantial tumor-specific uptake, particularly in the pancreas. Tumor uptake remained high, while the clearance rate in the pancreas and other abdominal organs was relatively rapid. Pharmacokinetic and imaging studies indicated that 111In-RM2 and 68Ga-RM2 were suitable candidates for clinical SPECT and PET investigations [63].
The elevated expression of GRPR in PC compared to benign prostatic hyperplasia presents a promising target for PC staging and monitoring. Based on the assumption of increased metabolic activity of cancer cells, metabolic-based tracers are also employed for PC imaging. R.P.J. Schroeder et al. conducted a study comparing GRPR-based targeting using 68Ga-labeled bombesin analogue AMBA and metabolism-based targeting using 18F-methylcholine (18F-FCH) in nude mice implanted with human prostate VCaP xenografts. Uptake was 6.7 ± 1.4%ID/g (N = 8) for 68Ga-AMBA, and only 1.6 ± 0.5%ID/g (N = 8) for 18F-FCH. This difference was highly significant (p < 0.001). Similarly, for PC-3 tumors, uptake was 9.2 ± 1.1%ID/g (N = 3) for 68 Ga-AMBA and 1.2 ± 0.3%ID/g (N = 3) for 18F-FCH. Dynamic PET images were reconstructed and quantitatively analyzed. The study revealed that 68Ga-AMBA effectively visualized all tumors, whereas 18F-FCH exhibited significantly lower contrast due to its inferior tumor-to-background ratio. PET quantitative analysis demonstrated rapid tumor uptake and high retention rates for both tracers. Similar results were observed in PC-3 tumor-bearing mice. The biodistribution data aligned with the PET findings, showing higher tumor uptake of 68Ga-AMBA compared to 18F-FCH in VCaP tumors. Apart from GRPR-expressing organs, the uptake of 68Ga-AMBA in other organs was lower than that of 18F-FCH. In the same PC tumor-bearing mice, the tumor uptake of 68Ga-AMBA was higher than that of 18F-FCH, while the overall background activity was lower. These results suggested that peptide receptor-based targeting using the bombesin analogue AMBA was superior to choline-based metabolic targeting in radionuclide imaging of PC [64].
Several studies have utilized different chelating agents to label the C-terminal eight amino acids of bombesin (7–14) with 64Cu. These analogues have demonstrated GRPR-specific PET imaging capabilities in small animal tumor models, but they came with various advantages and disadvantages. 64Cu-labeled compounds may be superior to 68Ga-labeled compounds in the future because of the longer half-life of 64Cu (12.7 h), which would allow for a longer time span for PET imaging. In the research conducted by Kimberly A. Lears et al., a chelating agent called SarAr was employed to conjugate with bombesin. They synthesized SarAr and conjugated SarAr with bombesin(7–14) via solid-phase synthesis method, obtaining SarAr-SA-Aoc-bombesin(7–14) and SarAr-SA-Aoc-GSG-bombesin(7–14). A competitive binding assay was performed using PC-3 cells and 125I-Tyr4-bombesin to determine the half-maximal inhibitory concentration (IC50). These peptide conjugates were labeled with 64Cu, and their internalization in PC-3 cells in vitro and the uptake in PC-3 xenografts in mice were evaluated. The results of the competitive binding assay indicated that both SarAr-SA-Aoc-bombesin(7–14) and SarAr-SA-Aoc-GSG-bombesin(7–14) exhibited high affinity for GRPR, with IC50 values of 3.5 nM and 4.5 nM, respectively. Both peptides were successfully labeled with 64Cu and displayed similar levels of internalization in PC-3 cells. In vivo, these radiolabeled peptides demonstrated tumor-specific uptake and exhibited improved imaging performance compared to previously reported 64Cu-labeled bombesin analogues. 64Cu-SarAr-Aoc-GSG-bombesin(7–14) exhibited faster blood clearance and lower uptake in tumor and normal tissues compared to 64Cu-SarAr-SA-Aoc-bombesin(7–14), resulting in similar tumor-to-blood ratios for both analogues. Both 64Cu-SarAr-Aoc-bombesin(7–14) and 64Cu-SarAr-SA-Aoc-GSG-bombesin(7–14) possessed high affinity for GRPR-expressing cells and had potential for PC PET imaging (Fig. 2) [65].
Fig. 2.
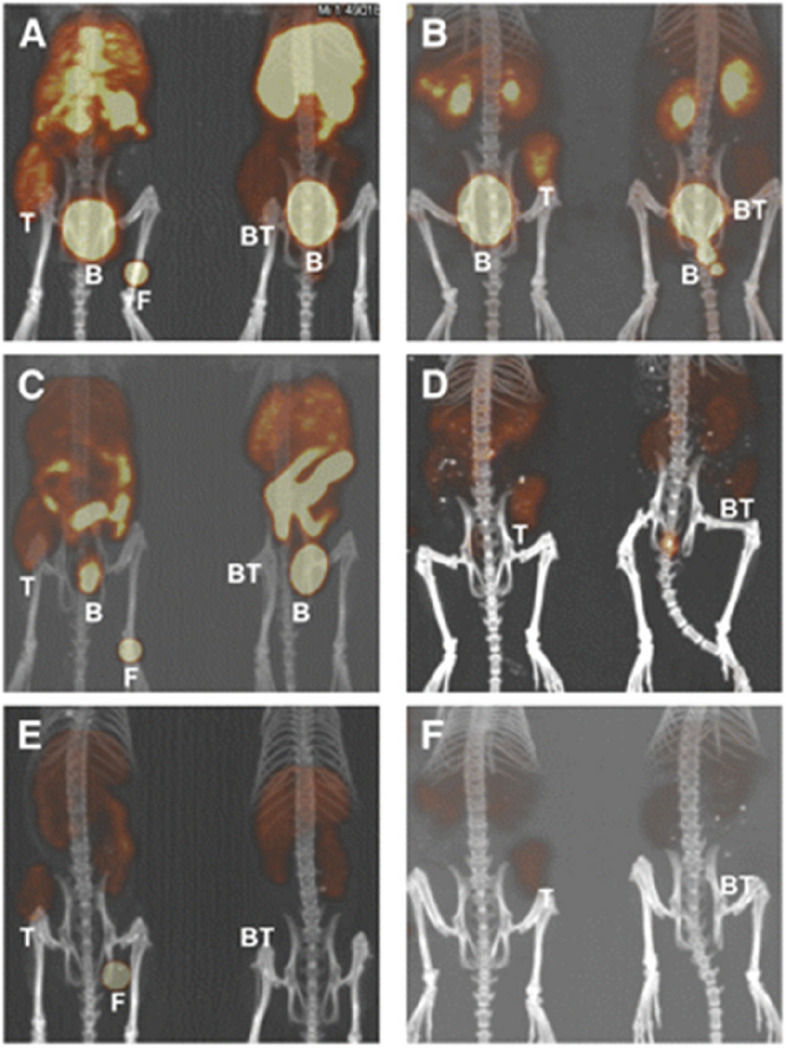
Coronal views of maximum-intensity projections of small-animal PET images with coregistered CT image of mice bearing PC-3 xenografts in rear flank at 1 (A and B), 4 (C and D), and 24 h (E and F). Mice were injected intravenously with 64Cu-SarAr-SA-Aoc bombesin(7–14) (A, C, and E; tumors on the left flank) and 64Cu-SarAr-SA-Aoc-GSG bombesin(7–14) (B, D, and F; tumors on the right flank). Mice on the left of each frame were not injected with a blocking agent, whereas mice on right received 100 µg of Tyr4-bombesin as an inhibitor. Fiducial markers (F) are also shown in some images. B = bladder; BT = blocked tumors; T = nonblocked tumors. This study was originally published in JNM. Lears, K.A., et al., In vitro and in vivo evaluation of 64Cu-labeled SarAr-bombesin analogs in gastrin-releasing peptide receptor-expressing prostate cancer. J Nucl Med, 2011. 52(3): p. 470–7. © SNMMI
Chatalic K.L. et al. conducted a comparative study on three novel GRPR-targeting tracers: Al18F-JMV5132, 68Ga-JMV5132, and 68Ga-JMV4168. GRPR antagonist JMV594 [66] was coupled with NODA-MPAA, and labeled with Al18F. JMV5132 was labeled with both 68Ga and 18F, while JMV4168 was labeled with 68Ga. The inhibitory concentration of JMV4168, JMV5132, natGa-JMV4168, natGa-JMV5132 and AlnatF-JMV5132 to GRPR were determined using a competitive binding assay. IC50 values were 6.8 nM for JMV5132, 13.2 nM for JMV4168, 3.0 nM for natGa-JMV5132, 3.2 nM for natGa-JMV4168, and 10.0 nM for AlnatF-JMV5132. The tumor targeting ability of these compounds was evaluated in mice with subcutaneously transplanted PC-3 tumors. Small animal PET/CT images were acquired, and the biodistribution of the tracers was determined through in vitro measurements. The study revealed that Al18F-JMV5132 could be accomplished within 20 min. In mice with PC-3 tumors, all tracers exhibited rapid clearance from the blood. 68Ga-JMV4168 was predominantly cleared via the kidneys, while 68Ga-JMV5132 and Al18F-JMV5132 showed partial clearance from the liver and gallbladder. Small animal PET/CT imaging clearly visualized PC-3 tumors, with Al18F-JMV5132 displaying the highest resolution. The study demonstrated that Al18F-JMV5132, 68Ga-JMV5132, and 68Ga-JMV4168 exhibited specific accumulation in GRPR-positive PC-3 tumors. These novel PET tracers hold promise as potential candidates for future clinical imaging applications [67].
Theodosia Maina et al. introduced 68Ga-SB3, as an alternative to 99mTc-labeled tetraamine using the chelating agent DOTA for PET imaging with the radioactive metal 68Ga. The researchers conducted competitive binding experiments of SB3 and natGa-SB3 using [125I-Tyr4] BBN on PC-3 cell membranes. Blood samples were collected from mice after injecting the 67Ga-SB3 surrogate, and the degradation products were analyzed using high-performance liquid chromatography (HPLC). Biodistribution studies were performed in severe combined immunodeficient mice with PC-3 xenograft tumors after injecting 67Ga-SB3. Additionally, 17 patients with BC (8 cases) and PC (9 cases) were injected with 68Ga-SB3 and PET/CT fusion images were obtained. The results demonstrated that SB3 and natGa-SB3 exhibited high affinity for human GRPR, and 67Ga-SB3 showed good in vivo stability. 67Ga-SB3 displayed higher retention in PC-3 xenografts but faster clearance from the GRPR-rich pancreas. Among the patients, no adverse reactions were observed following administration of 68Ga-SB3, and 50% of the BC patients (4 out of 8) and 55% of the PC patients (5 out of 9) exhibited pathological uptake of 68Ga-SB3 on PET/CT imaging. In PC-3 tumor-bearing mice, 67Ga-SB3 demonstrated favorable pharmacokinetics, while 68Ga-SB3 PET/CT imaging revealed approximately 50% of lesions in patients with advanced PC and BC. The researchers anticipated that 68Ga-SB3 might provide even better outcomes for patients with BC or PC [68].
In order to investigate the safety and efficacy of 68Ga-labeled GRPR antagonist SB3 in PET/CT imaging of primary PC, Ingrid L. Bakker et al. conducted a study focusing on the biological distribution, dosimetry, pathology, and GRPR expression. The study included 10 PC patients scheduled for prostatectomy, who underwent PET/CT imaging within 2 weeks prior to the surgery. The tumor location and Gleason score of prostate tissue were evaluated, and GRPR expression was determined through in vitro autoradiography. The findings demonstrated that 68Ga-SB3 was well tolerated, with no significant changes in vital signs and laboratory results. PET/CT imaging using 68Ga-SB3 revealed lesions in 8 out of 10 cases. Pathological analysis identified a total of 16 tumor lesions, of which 14 were detected by PET/CT, resulting in a sensitivity of 88%. Additionally, 68Ga-SB3 PET/CT imaging showed two large prostate intraepithelial neoplasia uptakes, with a specificity of 88%. Autoradiography of tumor lesions displayed varied expression levels of GRPR, with 4 cases showing negative expression. Notably, two patients who tested negative on PET/CT were found to have GRPR-negative tumors. Among autoradiography-positive tumors, the level of GRPR expression correlated significantly with the uptake of the tracer on PET/CT. Regarding dosimetry, the highest absorbed dose was observed in the pancreas, which exhibited physiological GRPR expression. Following were the bladder wall and kidneys. Based on these results, the researchers concluded that 68Ga-SB3 PET/CT was a safe and promising imaging method for early detection of PC [69].
Bogdan Mitran et al. have developed a PET imaging agent using 55Co-labeled RM26, a GRPR antagonist, to visualize tumors expressing GRPR. They found that the tumor-to-background ratio increased significantly over time to 24 h after injection, due to high uptake and long-term retention in the tumor, as well as rapid clearance from the blood and organs that express GRPR, highlighting the importance of radionuclide half-life in highly sensitive molecular imaging. 55Co-NOTA-AMBA provided better imaging contrast than its 68Ga-labeled counterpart because it can be imaged at 24 h after injection. Second-day imaging with long-lived radionuclides could detect lower abdominal lymph node involvement, which required the highest possible sensitivity and was the ultimate goal of PC imaging. For second-day imaging, positron-emitting metals with a half-life of 10–20 h are the best choice. Positron-emitting nuclides that may be used for this purpose include 64Cu (T1/2 = 12.7 h), 86Y (T1/2 = 14.7 h) and 55Co (T1/2 = 17.5 h). Among them, the positron abundance of 55Co (76% β+) is higher than that of 64Cu, and the ratio of annihilation photons to co-emitted gamma is higher than 86Y, which provides better image quality. The favorable biodistribution profile of Co-labeled NOTA-PEG2-RM26 enabled its use in high-contrast preclinical PET/CT imaging (using 55Co) and SPECT/CT imaging (using 57Co) (Fig. 3) [70].
Fig. 3.
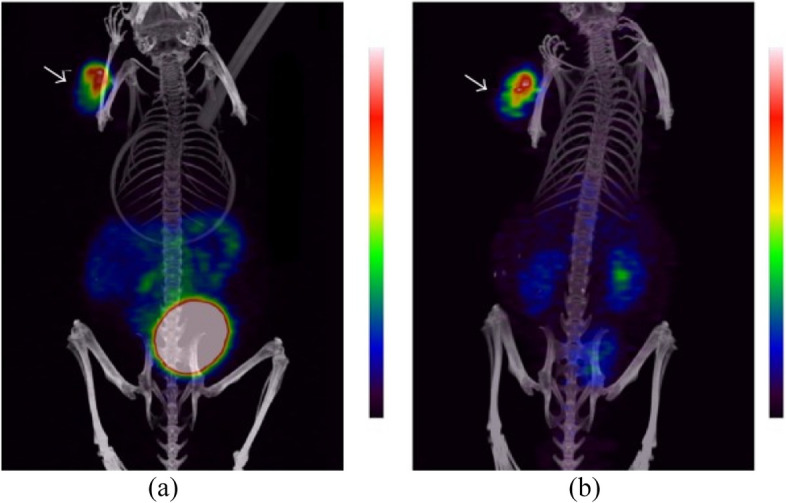
Coronal MIP preclinical PET/CT images showing tracer distribution in PC-3 xenografted NOD-SCID mice. The animals were injected with 0.18 nmol of 55Co-NOTA-PEG2-RM26 (approx. 3 MBq) and scanned at (a) 3 h and (b) 24 h pi. The tumor is shown by the arrow. With permission, from Ref. [70]
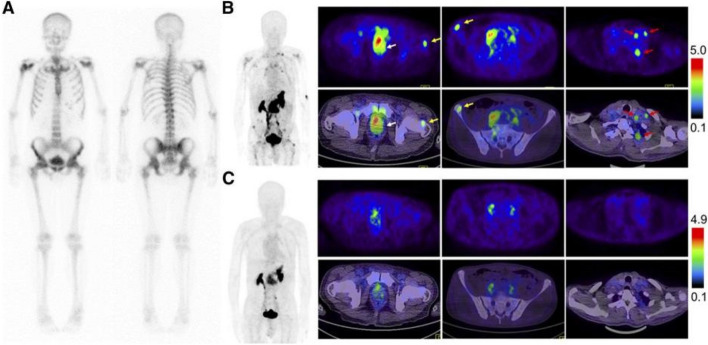
Zhang et al. conducted a study to evaluate the safety, biodistribution, radiation dosimetry, and clinical diagnostic value of the GRPR antagonist PET tracer 68Ga-RM26 in patients with PC. The study also compared 68Ga-RM26 with the GRPR agonist 68Ga-BBN. Safety verification and dosimetry calculations of 68Ga-RM26 were performed in 5 healthy volunteers. A total of 28 PC patients (17 newly diagnosed and 11 after treatment) participated in the study and provided written informed consent. PET/CT scans were conducted on all cancer patients for 15–30 min following intravenous injection of 68Ga-RM26. Among them, 22 patients (11 newly diagnosed and 11 after treatment) were also examined using 68Ga-BBN PET/CT within one week as a control. Additionally, 99mTc-MDP bone scans were performed as another control after 2 weeks. Tumor specimens were subjected to GRPR immunohistochemical staining. The results showed that all subjects tolerated 68Ga-RM26 well, with no reported adverse symptoms during the procedure or during the 2-week follow-up. In 17 newly diagnosed PC patients, 68Ga-RM26 PET/CT showed 15 positive tumors. Among the 11 patients who underwent prostatectomy or brachytherapy, 68Ga-RM26 PET/CT detected 8 metastatic lymph nodes in 3 cases and 21 bone metastases in 8 cases. Compared to 68Ga-RM26 PET/CT, GRPR agonist 68Ga-BBN PET/CT detected fewer primary lesions, lymph node metastases, and showed lower tracer accumulation. The study demonstrated that 68Ga-RM26, a GRPR antagonist, was safe and effective. 68Ga-RM26 PET/CT hold great value in the diagnosis of PC and PC metastasis. Furthermore, 68Ga-RM26 outperforms GRPR agonist as an imaging tracer for evaluating GRPR expression in PC (Fig. 4) [71].
Fig. 4.
Comparison of 99mTc-MDP bone scintigraphy (A), 68Ga-RM26 PET/CT (B), and 68Ga-BBN PET/CT (C) in a 73-y-old man diagnosed as having PC (white arrow) with lymph node involvement (red arrow) and bone metastasis (yellow arrow) before prostatectomy. 68Ga-RM26 PET/CT detected primary tumors, multiple lymph node involvement, and bone metastasis lesion, whereas those lesions did not significantly show up on 99mTc-MDP bone scintigraphy and showed extremely mild uptake on 68 Ga-BBN PET/CT. This study was originally published in JNM. Zhang, J., et al., PET Using a GRPR Antagonist (68)Ga-RM26 in Healthy Volunteers and Prostate Cancer Patients. J Nucl Med, 2018. 59(6): p. 922–928. © SNMMI
The high pancreatic uptake observed with GRPR targeted radiopharmaceuticals, particularly in targeted radioligand therapy, has been a significant challenge. Wang et al. conducted a study to address this issue by exploring the complex of TacsBOMB2, TacsBOMB3, TacsBOMB4, TacsBOMB5, and TacsBOMB6 derived from the effective GRPR antagonist sequence [Leu13GRPRThz14]bombesin(7–14), and compared them with 68Ga-RM2. PET imaging of PC-3 transplanted tumors demonstrated that 68Ga-TacsBOMB2, 68Ga-TacsBOMB3, 68Ga-TacsBOMB5, 68Ga-TacsBOMB6, and 68Ga-RM2 exhibited clear visualization of the tumors, with 68Ga-TacsBOMB5 displaying the highest tumor uptake rate. Importantly, it was observed that the pancreatic uptake of 68Ga-TacsBOMB2, 68Ga-TacsBOMB3, 68Ga-TacsBOMB5, and 68Ga-TacsBOMB6 was significantly lower than that of 68Ga-RM2. Among the tested derivatives of 68Ga-bombesin(7–14), 68Ga-TacsBOMB5 demonstrated the highest tumor uptake rate and the greatest contrast between the tumor and background, indicating its potential for clinical imaging of tumors expressing GRPR [72].
Based on the results of a single-center phase II clinical study by Schollhammer et al., the significant potential of the combination of 68Ga-RM2 PET/CT and 68Ga-PSMA-617 PET/CT in evaluating different aspects of PC biology was compared. 68Ga-PSMA-617 PET/CT is helpful to show the lesions with high ISUP (International Society of Urological Pathology) score and great clinical significance. When the score of ISUP was low, the uptake rate of 68Ga-RM2 was higher than that of 68Ga-PSMA-617. However, when the score of ISUP was higher, the uptake was similar to that of 68Ga-PSMA-617. Importantly, nearly 20% of lesions can only be seen on GRPR- PET (13%) or PSMA (prostate-specific membrane antigen)-PET (6%), which revealed the complementarity of these imaging procedures. The combination of PSMA-PET and GRPR-PET can better classify the lesions in the prostate [73], Compared with PSMA, GRPR has high sensitivity and specificity in patients with prostate cancer. GRPR can play an important complementary role in PSMA-negative tumors and tumors with heterogeneous expression of cell surface receptors. These GRPR ligands have shown reliable detection of various types of PC in patients, representing significant progress in the clinical diagnosis of PC. Here, we list a table (see Table at the end of this article) that briefly summarizes radiopharmaceuticals that target GRPR in PC.
Breast cancer (BC)
Mammography is a well-established technique for primary BC detection, but it has certain limitations (even under the best conditions of photography and diagnosis, about 5% of BC is false negative due to various reasons. Another major limitation of breast X-ray examination is the differentiation of benign and malignant lesions, which is the same as other system lesions. Breast lesions also have the problem of "different shadow of the same disease, different diseases with the same shadow") that can be addressed through the use of nuclear imaging. Currently available radiopharmaceuticals have limited sensitivity in detecting small primary lesions, necessitating the development of new radiopharmaceuticals for improved detection of primary BC, metastasis, recurrence, and treatment monitoring. As the studies on GRPR progresses, scientists are gradually advancing the application of radionuclide-labeled GRPR agonists/antagonists for the diagnosis and treatment of BC.
In a study conducted by Jesse J. Parry et al., the potential of PET imaging using 64Cu-labeled BN analogues was assessed for BC. The binding and internalization of a series of BN analogues, containing linkers with varying carbon lengths (4, 5, 6, 8, and 12), were evaluated in T-47D human BC cells. Subsequently, tissue biodistribution and micro PET imaging were employed to evaluate the performance of 64Cu-labeled analogues in mice with T-47D xenografts. The results demonstrated that all analogues exhibited IC50 values below 100 nM and were effectively internalized into T-47D cells. Biodistribution studies revealed that BN analogues with 8-carbon connectors exhibited both the highest tumor uptake rate and increased uptake in normal liver tissue. Analogues with 6- or 8-carbon connectors demonstrated favorable tumor uptake, which was further confirmed by micro PET imaging. These findings established the feasibility of utilizing radiolabeled BN analogues for PET detection of GRPR-expressing BC [74].
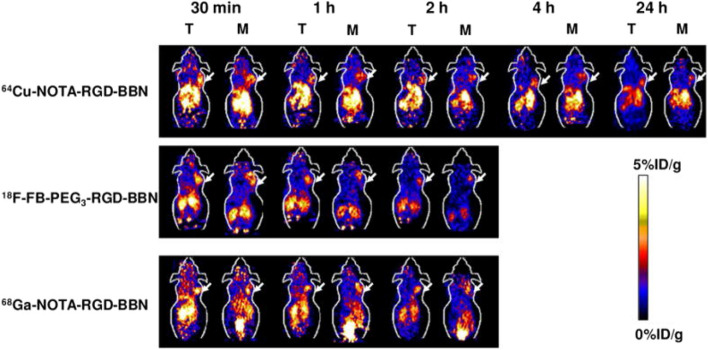
RGD and bombesin have shown promise as tumor imaging agents, targeting integrin αvβ3 and GRPR, respectively. In a study by Liu et al., a novel RGD-BBN heterodimer peptide was designed and synthesized, incorporating both RGD and BBN motifs into a single molecule. 18F-labeled RGD-BBN heterodimer demonstrated dual targeting capabilities for integrin αvβ3 and GRPR in a PC model (PC-3). The researchers also explored the potential of radiolabeled RGD-BBN tracers for BC detection using micro PET imaging. Cell binding analysis revealed that BC cells expressing high levels of GRPR typically exhibited low to moderate levels of integrin αvβ3, whereas those with high integrin αvβ3 expression displayed minimal GRPR expression. RGD-BBN heterodimers were labeled with three positron-emitting radionuclides, namely 18F, 64Cu, and 68Ga, and the PET studies with these three radiotracers were conducted in T-47D (GRPR+/low integrin αvβ3) and MDA-MB-435 (GRPR−/integrin αvβ3+) BC models. The results demonstrated that all three radiotracers exhibited dual binding affinity for integrin αvβ3 and GRPR in vitro. In MDA-MB-435 tumors models (GRPR−/integrin αvβ3+), the RGD-BBN radiotracer displayed notable advantages compared to the corresponding BBN analogues. Even though 18F-FB-PEG3-RGD-BBN exhibited lower tumor uptake than 64Cu-NOTA-RGD-BBN and 68Ga-NOTA-RGD-BBN, it provided enhanced contrast for BC visualization. 64Cu-NOTA-RGD-BBN exhibited longer tumor retention along with higher liver and kidney uptake, while 68Ga-NOTA-RGD-BBN displayed higher tumor uptake but also increased background accumulation. Overall, the labeling groups, chelating agents, and isotopes exerted profound effects on tumor targeting and in vivo kinetics of RGD-BBN tracers that simultaneously recognize dual integrin αvβ3 and GRPR. Further development of radiolabeled RGD-BBN tracers for PET imaging of tumors is warranted (Fig. 5) [75].
Fig. 5.
Decay-corrected whole-body coronal microPET images of T47D (T) and MDA-MB-435 (M) tumor-bearing mice at 30 min, 1 h, 2 h, 4 h, and 24 h after injecting 3.7 ~ 5.5 MBq (100 ~ 150 μCi) of 64Cu-NOTA-RGD-BBN, 18F-FB-PEG3-RGD-BBN or 68Ga-NOTA-RGD-BBN. Images shown are static scans of a single mouse, which is representative of the 4 mice tested in each group. Arrows indicate the presence of T47D (T) or MDA-MB-435 (M) tumors. Reprinted (adapted) with permission from {Liu, Z., et al., (18)F, (64)Cu, and (68)Ga labeled RGD-bombesin heterodimeric peptides for PET imaging of breast cancer. Bioconjug Chem, 2009. 20(5): p. 1016–25.}. Copyright {2009} American Chemical Society
In a study conducted by Christophe Van de Wiele et al., immunohistochemistry (IHC) was employed to investigate the uptake of 99mTc-RP527 and its association with GRPR expression in human BC. Nine patients with clinically diagnosed BC and 5 patients with tamoxifen-resistant metastatic BC underwent SPECT imaging using 99mTc-RP527. The results were compared with routine staging examinations of all patients, as well as routine histology and IHC staining in the initial 9 patients. All 9 patients with suspected breast lesions displayed positive tumor uptake. Among these patients, 8 exhibited significant uptake of 99mTc-RP527 in the primary lesions, involved lymph nodes, and certain distant metastases. Conversely, no uptake of 99mTc-RP527 was observed in the tamoxifen-resistant patients. These findings indicated that 99mTc-RP527 possessed a high affinity and binding specificity for primary BC. However, due to the limited samples, further experiments are necessary to establish the clinical significance and potential application of radiolabeled GRPR antagonists in BC imaging [76].
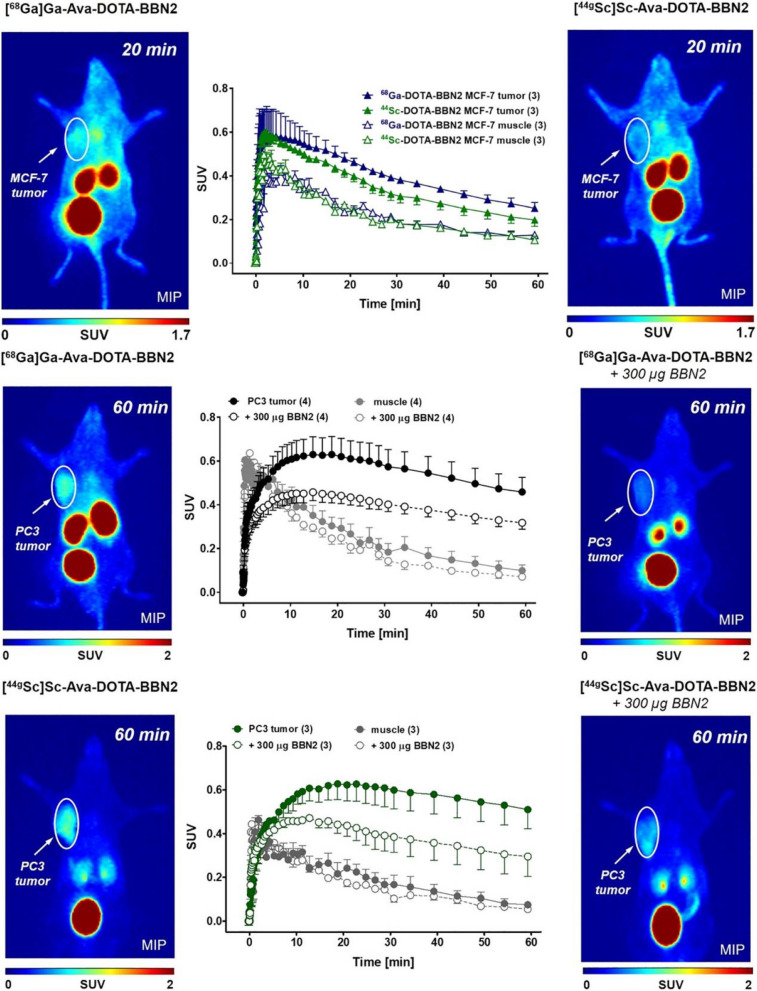
Radiolabeled peptides play a crucial role in targeted imaging and therapy of tumors. In a recent study by Simon Ferguson et al., the metabolically stable GRPR antagonist BBN2 was proposed for labeling with 18F and 68Ga, enabling PET imaging of GRPR in PC. They focused on the impact of combining 44gSc and 68 Ga-labeled DOTA complexes with the GRPR antagonist BBN2 on GRPR affinity in vitro, as well as their biodistribution and tumor uptake in MCF7 and PC-3 models. The DOTA-Ava-BBN2 peptide was labeled with the radionuclides 68Ga and 44gSc. The GRPR affinity was assessed in PC-3 cells, and the expression profile of GRPR was studied in human BC tissue samples and MCF7 cells. PET imaging was performed using the 68Ga and 44gSc-labeled peptides in xenograft models of MCF7 and PC-3 tumors (Fig. 6). The results demonstrated a high binding affinity for both 68Ga-DOTA-Ava-BBN2 and 44gSc-DOTA-Ava-BBN2. Gene expression microarray analysis revealed higher expression of GRPR mRNA in estrogen receptor (ER)-positive BC tissues, which was confirmed by western blotting and immunohistochemistry. However, PET imaging showed lower uptake of these two tracers in MCF7 tumors, while higher tumor uptake and retention were observed in PC-3 tumors models Comparing the biological distribution of DOTA-Ava-BBN2 peptides labeled with 68Ga and 44gSc, no differences were observed in MCF7 and PC-3 xenografts in vivo. Both tumor models exhibited similar patterns of tumor uptake and retention, as well as rapid clearance from the blood and kidneys [77].
Fig. 6.
Representative PET images (MIP) of MCF7 tumor-bearing mice at 20 min after injection of 68 Ga-DOTA-Ava-BBN2 (left) and 44gSc-DOTA-Ava-BBN2 (right). Corresponding time-activity curves (middle) show the radioactivity levels in the tumor and muscle for both radiopeptides over time as SUV values (mean ± SEM from n = 3 experiments). Representative PET images (MIP) of PC-3 tumor-bearing mice at 60 min after injection of 68 Ga-DOTA-Ava-BBN2 (top) and 44gSc-DOTA-Ava-BBN2 (bottom) under control (right) and blocking conditions (left). Corresponding time-activity curves (middle) show the radioactivity levels in the tumor and muscle for both radiopeptides over time as SUV values (mean ± SEM from n = 3 experiments). With permission, from Ref. [77]
Christian Stoykow et al. investigated the application of 68Ga-RM2 for PET imaging of breast cancer. Prior to 68Ga-RM2 PET/CT staging, 15 female BC patients with confirmed biopsies were included. A significant increase in 68 Ga-RM2 uptake was observed in 13 out of 18 tumor tissues compared to normal breast tissue (defined as PET positive). All PET-positive primary tumors were found to be ER- and PR-positive (13 out of 13), whereas only one PET-negative tumor was ER- and PR-positive. Normal breast tissue exhibited moderate inter- and intra-individual variability in GRPR binding, while physiological uptake in other organs, except the pancreas, showed a significant decrease. The uptake of 68Ga-RM2 in BC was associated with the expression of ER, PR, HER2/neu status, and the MIB-1 proliferation index. Importantly, 68Ga-RM2 PET/CT successfully detected intramammary lymph nodes with high 68Ga-RM2 uptake, contralateral axillary lymph node metastasis, and bone metastasis. These findings highlighted the potential of 68Ga-RM2 PET/CT as a promising imaging modality for the diagnosis of ER-positive BC [78].
The GRPR antagonist radioligand 67/68Ga/111In/177Lu-NeoBOMB1 has demonstrated favorable diagnostic characteristics in preclinical PC models, with 68Ga-NeoBOMB1 being particularly effective in detecting PC lesions in patients. To further investigate the diagnostic potential of NeoBOMB1 in GRPR-positive BC, Aikaterini Kaloudi et al. conducted a study using 67Ga-NeoBOMB1 in a BC model. They examined the distribution of 67Ga-NeoBOMB1, serving as a substitute for 68Ga-NeoBOMB1, in GRPR-expressing T-47D cells and animal models. They found that both NeoBOMB1 and natGa-NeoBOMB1 exhibited high affinity for GRPR. 67Ga-NeoBOMB1 demonstrated strong binding to the cell membrane of T-47D cells but displayed limited internalization, behaving like a radioligand antagonist. Importantly, 67Ga-NeoBOMB1 specifically localized in the tumors of mice bearing T-47D xenograft tumors. These findings indicated that 67Ga-NeoBOMB1 can effectively target GRPR-positive BC in animal models and holds promising potential for future clinical applications [79].
To identify potential candidates for GRPR-based imaging or targeted therapy, Clément Morgat et al. conducted immunohistochemistry screening of invasive BC to assess the presence and intensity of GRPR expression. The study examined tissue samples from 1432 patients with primary breast tumors who underwent surgery at the Bergonié Institute between 2000 and 2005, prior to receiving neoadjuvant therapy. The researchers investigated the correlation between GRPR expression and various clinical, pathological, and biological parameters, as well as its association with the absence of distant metastasis. The findings revealed that among the 1432 tumor cases, the incidence of GRPR overexpression was 75.8%, which was closely linked to a positive ER status. When considering different molecular subtypes of BC, GRPR was highly expressed in 86.2% of luminal A-like tumors, 70.5% of luminal B-like HER2-negative tumors, 82.8% of luminal B-likeB-like HER2-positive tumors, 21.3% of HER2-enriched tumors, and 7.8% of triple-negative tumors. Notably, in cases where GRPR was overexpressed in breast tumors, 94.6% of metastatic lymph nodes also exhibited high levels of GRPR expression. Given the substantial overexpression of GRPR in a significant proportion of ER-positive tumors, the use of radiolabeled GRPR ligands for imaging and treatment holds promising clinical prospects for patients with ER-positive BC [31].
High expression of GRPR and somatostatin receptor 2 (SSTR2) in BC makes them attractive targets for receptor-mediated nuclear imaging and therapy. Simone U. Dalm et al. conducted a study to assess the suitability of these receptors as targets for metastatic BC. The researchers utilized autoradiography to detect ribonucleic acid expression in human peripheral blood and correlated it with radioligand binding. Furthermore, they quantitatively analyzed the mRNA levels of GRPR and SSTR2 in 60 pairs of primary tumors and corresponding metastatic tumors using reverse transcription polymerase chain reaction (RT-PCR). The expression of receptor mRNA was examined in relation to clinicopathological factors, and a comparison was made between primary tumors and their respective metastatic tumors. The study revealed a significant correlation between radioligand binding of GRPR and SSTR and the expression of receptor mRNA in tumor tissue. Notably, high levels of GRPR and SSTR2 were observed in ER-positive tumors, which aligns with their previous findings and the results reported by Kumar et al. [80, 81]. In Kumar's clinical study, the successful imaging of BC lesions using the GRPR radioligand 68Ga-RM2 was positively correlated with ER and PR status. Additionally, Prignon et al. demonstrated that the GRPR agonist 68Ga-AMBA was more suitable for monitoring the response to hormone therapy in ER-positive BC models compared to 18F-FDG PET. Regarding SSTR2 expression, there were no significant differences in most cases, except for a notably lower expression of the SSTR2 gene in liver and ovarian metastatic tumors compared to corresponding primary tumors [82]. Consequently, nuclear imaging and/or targeted therapy to GRPR and SSTR2 hold promise for improving the care of both primary and metastatic BC [83].
Zhang et al. conducted a study on the potential application of the dual-targeting tracer 68Ga-BBN-RGD, which targets both GRPR and integrin αvβ3, in PET/CT imaging of BC and metastatic tumors. The study included 22 female patients with BC, of which 16 were diagnosed with BC using molybdenum target X-ray examination, and the remaining 6 underwent radical mastectomy. All 22 patients underwent PET/CT imaging for 30–45 min following intravenous injection of 68Ga-BBN-RGD. Additionally, 11 patients underwent 68Ga-BBN PET/CT within 2 weeks. The final diagnosis was based on histopathological examination of surgical resection or biopsy samples. The imaging results demonstrated positive accumulation of 68Ga-BBN-RGD in both primary and metastatic lesions. The maximum standardized uptake value (SUVmax) of 68Ga-BBN-RGD PET was found to be higher in the ER-positive group compared to the ER-negative group. Moreover, the mean SUV values of 68Ga-BBN-RGD showed a strong correlation with the expression of both GRPR and integrin αvβ3 in both primary and metastatic lesions. This dual-targeting radiotracer exhibited significant uptake in primary and metastatic lesions of BC. 68Ga-BBN-RGD PET/CT hold great potential in the differential diagnosis of BC, axillary lymph node metastasis, and distant metastasis [84].
Morgat et al. reviewed and compared the differences between 68Ga-RM2 PET and 18F-FDG PET in different phenotypic BC. The binding of 68Ga-RM2 in GRPR-expressing tumors was significantly higher than that in GRPR-negative tumors (P = 0.022). In ER+ tumors, the binding of 68Ga-RM2 was significantly higher than that of 18F-FDG (P = 0.015). In tumors with low expression of Ki-67, the binding of 68Ga-RM2 was also significantly higher than that of 18F-FDG (P = 0.029). In general, the binding of 68Ga-RM2 and 18F-FDG in tumor specimens showed the opposite pattern, and the binding of 68Ga-RM2 in low 18F-FDG-bound tumors was significantly higher than that in high 18F-FDG-bound tumors (P = 0.021). This negative correlation was also recorded in a small number of patients who underwent 18F-FDG PET/CT before surgery. The results of this study showed that GRPR-targeted radiotracers can act as an alternative in PET/CT imaging of ER+ breast tumors [85].
Other cancers
Mattei et al. conducted an analysis of 238 lung cancer specimens, encompassing both SCLC and NSCLC, and investigated the relation between immunohistochemical results and clinical stage, cell type, sex, and survival rate [33]. The study revealed that GRPR expression was more abundant in advanced stages of the disease, indicating a significant correlation between clinical stage and GRPR expression. While the expression of GRPR is generally similar in SCLC and NSCLC, NSCLC exhibited higher GRPR intensity. Traditional 18F-FDG lacks specificity and is difficult to distinguish lung tumors from inflammation. However, radioligands targeting GRPR are more specific and can solve this problem much better. Carroll et al. performed an immunohistochemical study on 50 human colon cancer specimens [86]. They observed high expression of both GRP and GRPR in the majority of cancers (62%), while normal adjacent tissues did not exhibit significant expression. An interesting finding was that the co-expression of these two proteins consistently occurred in highly differentiated tumor regions, but not in poorly differentiated tumor regions, suggesting a close association between GRP/GRPR expression and tumor differentiation. GRPR overexpression on the cell surface has also been observed in various other tumors, including head and neck cancer, renal cancer, and intestinal and bronchial carcinoid [87], although limited clinical studies have been conducted in these areas.
Conclusion
GRPR is commonly overexpressed in multiple cancer types. Some GRPR ligands exhibit favorable binding ability to the receptor and can effectively facilitate in vivo imaging when combined with radionuclides. The utilization of radionuclide-labeled GRPR ligands holds promise for early detection, clinical diagnosis, and treatment of prostate cancer and breast cancer. This review summurized the advances of GRPR-targeted radiotracers for the diagnosis and treatment of cancers, paving the way for significant transformations in clinical practice.
| Name | Sequence | Binding affinity & binding capacity | Human study | Ref |
|---|---|---|---|---|
| BBN | pGlu-Gln-Arg-Leu-Gly-Asn-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2 | IC50 = 1.36 nM (BBN), 1.8 ± 0.2 nM ([Tyr4]BBN), 3.3 ± 0.4 nM ([Lys3]-BBN), 20.8 ± 0.3(Aca-BBN(7–14)) | No | [56–58] |
| AMBA | Gly-4-aminobenzoyl-Gln-Trp-Ala-Val-Gly-His-Leu-Met-NH2 | Kd = 0.7 ± 0.1 nM (111In-DOTA-AMBA) | No | [62, 88] |
| RM1 | Gly-4-aminobenzoyl-H–D-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2 | IC50 = 14 ± 3.4 nM ( natIn-DOTA-RM1) Kd = 2.4 ± 0.2 nM (111In-DOTA-RM1) | No | [62, 88] |
| RM2 | 4-amino-1-carboxymethylpiperidine-D-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2 | IC50 = 7.7 ± 3.3 nM (RM2), 9.3 ± 3.3 nM (natIn-DOTA-RM2), Kd = 2.9 ± 0.4 nM (111In-DOTA-RM2) | Yes | [63, 88] |
| RM26 | D-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2 | IC50 = 5.5 ± 0.4 nM (natCo-NOTA-PEG3-RM26) | Yes | [70, 88] |
| JMV5132 | MPAA-βAla-βAla-[H–D-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2] | IC50 = 6.8 nM (JMV5132), 3.0 nM (natGa-NODA-JMV5132), 10.0 nM (AlnatF-NODA-JMV5132) | No | [67] |
| JMV4168 | βAla-βAla-[H–D-Phe-Gln-Trp-Ala-Val-Gly-His-Sta-Leu-NH2] | IC50 = 13.2 nM (JMV4168), 3.2 nM (natGa-DOTA-JMV4168) | No | [67] |
| JMV594 | H–D-Phe-Gln-Trp-Ala-Val-GlyHis-Sta-Leu-NH2 | IC50 = 2.2 ± 0.1 nM (JMV594) | No | [66] |
| SB3 | p-aminomethylaniline-diglycolicacid-D-Phe-Gln-Trp-Ala-Val-Gly-His-Leu-NHEt | IC50 = 4.6 ± 0.5 nM (SB3), 1.5 ± 0.3 nM (natGa-DOTA-SB3) | No | [69, 88] |
Acknowledgements
Not applicable.
Abbreviations
- AMP
Adenosine monophosphate
- BBN
Bombesin
- BC
Breast cancer
- CAS
Caspase
- CT
Computed tomography
- DAG
Diacylglycerol
- EGFR
Epidermal growth factor receptor
- ERK
Extracellular regulated protein kinases
- ER
Estrogen receptor
- ESR1
Estrogen receptor 1
- FAK
Focal Adhesion Kinase
- 18F-FCH
18F-methylcholine
- GPCR
G-protein-coupled receptor
- GRP
Gastrin releasing peptide
- GRPR
Gastrin realizing peptide receptor
- HER2
Human epidermal growth factor receptor-2
- ICH
Immunohistochemistry
- IC50
Half maximal inhibitory concentration
- IP3
Inositol triphosphate
- ISUP
International Society of Urological Pathology
- MAPK
Mitogen-activated protein kinase
- MIP
Representative PET images
- MRI
Magnetic Resonance Imaging
- mRNA
Messenger RNA
- NSCLC
Non-small cell lung cancer
- PC
Prostate cancer
- PET
Positron emission tomography
- PIP2
Phosphatidylinositol-4,5-bisphosphate
- PKC
Protein kinase C
- PKD
Protein kinase D
- PLC
Phospholipase C
- PR
Progesterone receptor
- pro-GRP
Produce gastrin-releasing peptide precursor
- PSMA
Prostate-specific membrane antigen
- Rho
Ras homologue
- RT-PCR
Reverse Transcription-Polymerase Chain Reaction
- SCLC
Small-cell lung carcinoma
- SPECT
Single photon emission computed tomography
- SSTR2
Somatostatin receptor 2
- SUV
Standard uptake value
- TKI
Tyrosine kinase inhibitors
Authors’ contributions
Mr. Yuze Ma read the literatures and wrote the draft. Prof. Dr Feng Gao supervised him how to write the manuscript and corrected the draft.
Funding
This research was funded by grants from Natural Science Foundation of Shandong Province (ZR2019BA015, ZR2023MH004).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their consent for publication.
Competing interests
There is no conflict of interests regarding this manuscript.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Spindel ER, Giladi E, Brehm P, Goodman RH, Segerson TP. Cloning and functional characterization of a complementary DNA encoding the murine fibroblast bombesin/gastrin-releasing peptide receptor. Mol endocrinol (Baltimore, Md.). 1990;4:1956–1963. doi: 10.1210/mend-4-12-1956. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez N, Moody TW, Igarashi H, Ito T, Jensen RT. Bombesin-related peptides and their receptors: recent advances in their role in physiology and disease states. Curr Opin Endocrinol Diabetes Obes. 2008;15:58–64. doi: 10.1097/MED.0b013e3282f3709b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rozengurt E. Bombesin stimulation of mitogenesis Specific receptors, signal transduction, and early events. Am rev respir dis. 1990;142:S11–15. doi: 10.1164/ajrccm/142.6_Pt_2.S11. [DOI] [PubMed] [Google Scholar]
- 4.Rozengurt E, Fabregat I, Coffer A, Gil J, Sinnett-Smith J. Mitogenic signalling through the bombesin receptor: role of a guanine nucleotide regulatory protein. J Cell Sci Suppl. 1990;13:43–56. doi: 10.1242/jcs.1990.Supplement_13.6. [DOI] [PubMed] [Google Scholar]
- 5.Jensen RT, Battey JF, Spindel ER, Benya RV. International Union of Pharmacology LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol rev. 2008;60:1–42. doi: 10.1124/pr.107.07108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rouissi N, Rhaleb NE, Nantel F, Dion S, Drapeau G, Regoli D. Characterization of bombesin receptors in peripheral contractile organs. Br J Pharmacol. 1991;103:1141–1147. doi: 10.1111/j.1476-5381.1991.tb12314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safavy A, Raisch KP, Matusiak D, Bhatnagar S, Helson L. Single-drug multiligand conjugates: synthesis and preliminary cytotoxicity evaluation of a paclitaxel-dipeptide "scorpion" molecule. Bioconjug Chem. 2006;17:565–570. doi: 10.1021/bc050224c. [DOI] [PubMed] [Google Scholar]
- 8.Weber HC. Regulation and signaling of human bombesin receptors and their biological effects. Curr Opin Endocrinol Diabetes Obes. 2009;16:66–71. doi: 10.1097/MED.0b013e32831cf5aa. [DOI] [PubMed] [Google Scholar]
- 9.Patel M, Kawano T, Suzuki N, Hamakubo T, Karginov AV, Kozasa T. Gα13/PDZ-RhoGEF/RhoA signaling is essential for gastrin-releasing peptide receptor-mediated colon cancer cell migration. Mol Pharmacol. 2014;86:252–262. doi: 10.1124/mol.114.093914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.E. Rozengurt, S. Guha, J. Sinnett-Smith, Gastrointestinal peptide signalling in health and disease. Eur J Surg Suppl. 2002:(587):23-38. [PubMed]
- 11.Patel O, Dumesny C, Giraud AS, Baldwin GS, Shulkes A. Stimulation of proliferation and migration of a colorectal cancer cell line by amidated and glycine-extended gastrin-releasing peptide via the same receptor. Biochem Pharmacol. 2004;68:2129–2142. doi: 10.1016/j.bcp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Rozengurt E. Neuropeptides as growth factors for normal and cancerous cells. Trends Endocrinol Metab. 2002;13:128–134. doi: 10.1016/S1043-2760(01)00544-6. [DOI] [PubMed] [Google Scholar]
- 13.Qu X, Xiao D, Weber HC. Human gastrin-releasing peptide receptor mediates sustained CREB phosphorylation and transactivation in HuTu 80 duodenal cancer cells. FEBS Lett. 2002;527:109–113. doi: 10.1016/S0014-5793(02)03177-0. [DOI] [PubMed] [Google Scholar]
- 14.Degen LP, Peng F, Collet A, Rossi L, Ketterer S, Serrano Y, Larsen F, Beglinger C, Hildebrand P. Blockade of GRP receptors inhibits gastric emptying and gallbladder contraction but accelerates small intestinal transit. Gastroenterology. 2001;120:361–368. doi: 10.1053/gast.2001.21174. [DOI] [PubMed] [Google Scholar]
- 15.Schubert ML, Hightower J, Coy DH, Makhlouf GM. Regulation of acid secretion by bombesin/GRP neurons of the gastric fundus. Am J Physiol. 1991;260:G156–160. doi: 10.1152/ajpgi.1991.260.1.G156. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrand P, Lehmann FS, Ketterer S, Christ AD, Stingelin T, Beltinger J, Gibbons AH, Coy DH, Calam J, Larsen F, Beglinger C. Regulation of gastric function by endogenous gastrin releasing peptide in humans: studies with a specific gastrin releasing peptide receptor antagonist. Gut. 2001;49:23–28. doi: 10.1136/gut.49.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettersson M, Ahrén B. Gastrin releasing peptide (GRP): effects on basal and stimulated insulin and glucagon secretion in the mouse. Peptides. 1987;8:55–60. doi: 10.1016/0196-9781(87)90165-3. [DOI] [PubMed] [Google Scholar]
- 18.De la Fuente M, Del Rio M, Ferrandez MD, Hernanz A. Modulation of phagocytic function in murine peritoneal macrophages by bombesin, gastrin-releasing peptide and neuromedin C. Immunology. 1991;73:205–211. [PMC free article] [PubMed] [Google Scholar]
- 19.Del Rio M, De la Fuente M. Chemoattractant capacity of bombesin, gastrin-releasing peptide and neuromedin C is mediated through PKC activation in murine peritoneal leukocytes. Regul Pept. 1994;49:185–193. doi: 10.1016/0167-0115(94)90140-6. [DOI] [PubMed] [Google Scholar]
- 20.Levine AS, Morley JE. Stress-induced eating in rats. Am J Physiol. 1981;241:R72–76. doi: 10.1152/ajpregu.1981.241.1.R72. [DOI] [PubMed] [Google Scholar]
- 21.Kraly FS, Miller LA, Gibbs J. Diurnal variation for inhibition of eating by bombesin in the rat. Physiol Behav. 1983;31:395–399. doi: 10.1016/0031-9384(83)90208-1. [DOI] [PubMed] [Google Scholar]
- 22.Roesler R, Luft T, Oliveira SH, Farias CB, Almeida VR, Quevedo J, Dal Pizzol F, Schröder N, Izquierdo I, Schwartsmann G. Molecular mechanisms mediating gastrin-releasing peptide receptor modulation of memory consolidation in the hippocampus. Neuropharmacology. 2006;51:350–357. doi: 10.1016/j.neuropharm.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 23.Merali Z, McIntosh J, Kent P, Michaud D, Anisman H. Aversive and appetitive events evoke the release of corticotropin-releasing hormone and bombesin-like peptides at the central nucleus of the amygdala, The Journal of neuroscience : the official journal of the Society for. Neuroscience. 1998;18:4758–4766. doi: 10.1523/JNEUROSCI.18-12-04758.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown MR, Gray TS. Peptide injections into the amygdala of conscious rats: effects on blood pressure, heart rate and plasma catecholamines. Regul Pept. 1988;21:95–106. doi: 10.1016/0167-0115(88)90094-8. [DOI] [PubMed] [Google Scholar]
- 25.Shumyatsky GP, Tsvetkov E, Malleret G, Vronskaya S, Hatton M, Hampton L, Battey JF, Dulac C, Kandel ER, Bolshakov VY. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell. 2002;111:905–918. doi: 10.1016/S0092-8674(02)01116-9. [DOI] [PubMed] [Google Scholar]
- 26.Markwalder R, Reubi JC. Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Can Res. 1999;59:1152–1159. [PubMed] [Google Scholar]
- 27.Körner M, Waser B, Rehmann R, Reubi JC. Early over-expression of GRP receptors in prostatic carcinogenesis. Prostate. 2014;74:217–224. doi: 10.1002/pros.22743. [DOI] [PubMed] [Google Scholar]
- 28.Beer M, Montani M, Gerhardt J, Wild PJ, Hany TF, Hermanns T, Müntener M, Kristiansen G. Profiling gastrin-releasing peptide receptor in prostate tissues: clinical implications and molecular correlates. Prostate. 2012;72:318–325. doi: 10.1002/pros.21434. [DOI] [PubMed] [Google Scholar]
- 29.Gugger M, Reubi JC. Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast. Am J Pathol. 1999;155:2067–2076. doi: 10.1016/S0002-9440(10)65525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reubi C, Gugger M, Waser B. Co-expressed peptide receptors in breast cancer as a molecular basis for in vivo multireceptor tumour targeting. Eur J Nucl Med Mol Imaging. 2002;29:855–862. doi: 10.1007/s00259-002-0794-5. [DOI] [PubMed] [Google Scholar]
- 31.Morgat C, MacGrogan G, Brouste V, Vélasco V, Sévenet N, Bonnefoi H, Fernandez P, Debled M, Hindié E. Expression of Gastrin-Releasing Peptide Receptor in Breast Cancer and Its Association with Pathologic, Biologic, and Clinical Parameters: A Study of 1,432 Primary Tumors, Journal of nuclear medicine : official publication, Society of. Nuclear Medicine. 2017;58:1401–1407. doi: 10.2967/jnumed.116.188011. [DOI] [PubMed] [Google Scholar]
- 32.Dalm SU, Martens JW, Sieuwerts AM, van Deurzen CH, Koelewijn SJ, de Blois E, Maina T, Nock BA, Brunel L, Fehrentz JA, Martinez J, de Jong M, Melis M. In vitro and in vivo application of radiolabeled gastrin-releasing peptide receptor ligands in breast cancer, Journal of nuclear medicine : official publication, Society of. Nuclear Medicine. 2015;56:752–757. doi: 10.2967/jnumed.114.153023. [DOI] [PubMed] [Google Scholar]
- 33.Mattei J, Achcar RD, Cano CH, Macedo BR, Meurer L, Batlle BS, Groshong SD, Kulczynski JM, Roesler R, Dal Lago L, Brunetto AT, Schwartsmann G. Gastrin-releasing peptide receptor expression in lung cancer. Arch Pathol Lab Med. 2014;138:98–104. doi: 10.5858/arpa.2012-0679-OA. [DOI] [PubMed] [Google Scholar]
- 34.Reubi JC, Körner M, Waser B, Mazzucchelli L, Guillou L. High expression of peptide receptors as a novel target in gastrointestinal stromal tumours. Eur J Nucl Med Mol Imaging. 2004;31:803–810. doi: 10.1007/s00259-004-1476-2. [DOI] [PubMed] [Google Scholar]
- 35.Reubi JC. Peptide receptor expression in GEP-NET. Virchows Archiv : an international journal of pathology. 2007;451(Suppl 1):S47–50. doi: 10.1007/s00428-007-0443-2. [DOI] [PubMed] [Google Scholar]
- 36.Reubi JC, Wenger S, Schmuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: detection with the universal radioligand (125)I-[D-TYR(6), beta-ALA(11), PHE(13), NLE(14)] bombesin(6–14) Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8:1139–1146. [PubMed] [Google Scholar]
- 37.Bitar KN, Zhu XX. Expression of bombesin-receptor subtypes and their differential regulation of colonic smooth muscle contraction. Gastroenterology. 1993;105:1672–1680. doi: 10.1016/0016-5085(93)91062-M. [DOI] [PubMed] [Google Scholar]
- 38.Bartholdi MF, Wu JM, Pu H, Troncoso P, Eden PA, Feldman RI. In situ hybridization for gastrin-releasing peptide receptor (GRP receptor) expression in prostatic carcinoma. Int J Cancer. 1998;79:82–90. doi: 10.1002/(SICI)1097-0215(19980220)79:1<82::AID-IJC16>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 39.Sun B, Halmos G, Schally AV, Wang X, Martinez M. Presence of receptors for bombesin/gastrin-releasing peptide and mRNA for three receptor subtypes in human prostate cancers. Prostate. 2000;42:295–303. doi: 10.1002/(SICI)1097-0045(20000301)42:4<295::AID-PROS7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 40.Bologna M, Festuccia C, Muzi P, Biordi L, Ciomei M. Bombesin stimulates growth of human prostatic cancer cells in vitro. Cancer. 1989;63:1714–1720. doi: 10.1002/1097-0142(19900501)63:9<1714::AID-CNCR2820630912>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 41.Reile H, Armatis PE, Schally AV. Characterization of high-affinity receptors for bombesin/gastrin releasing peptide on the human prostate cancer cell lines PC-3 and DU-145: internalization of receptor bound 125I-(Tyr4) bombesin by tumor cells. Prostate. 1994;25:29–38. doi: 10.1002/pros.2990250105. [DOI] [PubMed] [Google Scholar]
- 42.Ehlers RA, Kim S, Zhang Y, Ethridge RT, Murrilo C, Hellmich MR, Evans DB, Townsend Jr CM, Mark Evers B. Gut peptide receptor expression in human pancreatic cancers. Ann Surg. 2000;231(6):838–48. doi: 10.1097/00000658-200006000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giacchetti S, Gauvillé C, de Crémoux P, Bertin L, Berthon P, Abita JP, Cuttitta F, Calvo F. Characterization, in some human breast cancer cell lines, of gastrin-releasing peptide-like receptors which are absent in normal breast epithelial cells. Int J Cancer. 1990;46:293–298. doi: 10.1002/ijc.2910460226. [DOI] [PubMed] [Google Scholar]
- 44.Halmos G, Wittliff JL, Schally AV. Characterization of bombesin/gastrin-releasing peptide receptors in human breast cancer and their relationship to steroid receptor expression. Can Res. 1995;55:280–287. [PubMed] [Google Scholar]
- 45.Uchida K, Kojima A, Morokawa N, Tanabe O, Anzai C, Kawakami M, Eto Y, Yoshimura K. Expression of progastrin-releasing peptide and gastrin-releasing peptide receptor mRNA transcripts in tumor cells of patients with small cell lung cancer. J Cancer Res Clin Oncol. 2002;128:633–640. doi: 10.1007/s00432-002-0392-8. [DOI] [PubMed] [Google Scholar]
- 46.Zelefsky MJ, Fuks Z, Happersett L, Lee HJ, Ling CC, Burman CM, Hunt M, Wolfe T, Venkatraman ES, Jackson A, Skwarchuk M, Leibel SA. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2000;55:241–249. doi: 10.1016/S0167-8140(99)00100-0. [DOI] [PubMed] [Google Scholar]
- 47.Ashman JB, Zelefsky MJ, Hunt MS, Leibel SA, Fuks Z. Whole pelvic radiotherapy for prostate cancer using 3D conformal and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:765–771. doi: 10.1016/j.ijrobp.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 48.Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, Gropler RJ, Knuuti J, Schelbert HR, Travin MI. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures, Journal of nuclear cardiology : official publication of the American Society of Nuclear. Cardiology. 2016;23:1187–1226. doi: 10.1007/s12350-016-0522-3. [DOI] [PubMed] [Google Scholar]
- 49.Berg E, Cherry SR. Innovations in Instrumentation for Positron Emission Tomography. Semin Nucl Med. 2018;48:311–331. doi: 10.1053/j.semnuclmed.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satoh Y, Motosugi U, Imai M, Onishi H. Comparison of dedicated breast positron emission tomography and whole-body positron emission tomography/computed tomography images: a common phantom study. Ann Nucl Med. 2020;34:119–127. doi: 10.1007/s12149-019-01422-0. [DOI] [PubMed] [Google Scholar]
- 51.Pichler V, Berroterán-Infante N, Philippe C, Vraka C, Klebermass EM, Balber T, Pfaff S, Nics L, Mitterhauser M, Wadsak W. An Overview of PET Radiochemistry, Part 1: The Covalent Labels (18)F, (11)C, and (13)N, Journal of nuclear medicine : official publication, Society of. Nuclear Medicine. 2018;59:1350–1354. doi: 10.2967/jnumed.117.190793. [DOI] [PubMed] [Google Scholar]
- 52.Zhou Y, Baidoo KE, Brechbiel MW. Mapping biological behaviors by application of longer-lived positron emitting radionuclides. Adv Drug Deliv Rev. 2013;65:1098–1111. doi: 10.1016/j.addr.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 54.Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002;167:2435–2439. doi: 10.1016/S0022-5347(05)64999-3. [DOI] [PubMed] [Google Scholar]
- 55.Elter M, Schulz-Wendtland R, Wittenberg T. The prediction of breast cancer biopsy outcomes using two CAD approaches that both emphasize an intelligible decision process. Med Phys. 2007;34:4164–4172. doi: 10.1118/1.2786864. [DOI] [PubMed] [Google Scholar]
- 56.Marsouvanidis PJ, Nock BA, Hajjaj B, Fehrentz JA, Brunel L, M'Kadmi C, van der Graaf L, Krenning EP, Maina T, Martinez J, de Jong M. Gastrin releasing peptide receptor-directed radioligands based on a bombesin antagonist: synthesis, (111)in-labeling, and preclinical profile. J Med Chem. 2013;56:2374–2384. doi: 10.1021/jm301692p. [DOI] [PubMed] [Google Scholar]
- 57.Lucente E, Liu H, Liu Y, Hu X, Lacivita E, Leopoldo M, Cheng Z. Novel (64)Cu Labeled RGD(2)-BBN Heterotrimers for PET Imaging of Prostate Cancer. Bioconjug Chem. 2018;29:1595–1604. doi: 10.1021/acs.bioconjchem.8b00113. [DOI] [PubMed] [Google Scholar]
- 58.Yang YS, Zhang X, Xiong Z, Chen X. Comparative in vitro and in vivo evaluation of two 64Cu-labeled bombesin analogs in a mouse model of human prostate adenocarcinoma. Nucl Med Biol. 2006;33:371–380. doi: 10.1016/j.nucmedbio.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Maina T, Nock B, Mather S. Targeting prostate cancer with radiolabelled bombesins. Cancer imaging : the official publication of the International Cancer Imaging Society. 2006;6:153–157. doi: 10.1102/1470-7330.2006.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu Z, Ananias HJ, Carlucci G, Hoving HD, Helfrich W, Dierckx RA, Wang F, de Jong IJ, Elsinga PH. An update of radiolabeled bombesin analogs for gastrin-releasing peptide receptor targeting. Curr Pharm Des. 2013;19:3329–3341. doi: 10.2174/1381612811319180015. [DOI] [PubMed] [Google Scholar]
- 61.Cescato R, Maina T, Nock B, Nikolopoulou A, Charalambidis D, Piccand V, Reubi JC. Bombesin receptor antagonists may be preferable to agonists for tumor targeting, Journal of nuclear medicine : official publication, Society of. Nuclear Medicine. 2008;49:318–326. doi: 10.2967/jnumed.107.045054. [DOI] [PubMed] [Google Scholar]
- 62.Mansi R, Wang X, Forrer F, Kneifel S, Tamma ML, Waser B, Cescato R, Reubi JC, Maecke HR. Evaluation of a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-conjugated bombesin-based radioantagonist for the labeling with single-photon emission computed tomography, positron emission tomography, and therapeutic radionuclides, Clinical cancer research : an official journal of the American Association for. Can Res. 2009;15:5240–5249. doi: 10.1158/1078-0432.CCR-08-3145. [DOI] [PubMed] [Google Scholar]
- 63.Mansi R, Wang X, Forrer F, Waser B, Cescato R, Graham K, Borkowski S, Reubi JC, Maecke HR. Development of a potent DOTA-conjugated bombesin antagonist for targeting GRPr-positive tumours. Eur J Nucl Med Mol Imaging. 2011;38:97–107. doi: 10.1007/s00259-010-1596-9. [DOI] [PubMed] [Google Scholar]
- 64.Schroeder RP, van Weerden WM, Krenning EP, Bangma CH, Berndsen S, Grievink-de Ligt CH, Groen HC, Reneman S, de Blois E, Breeman WA, de Jong M. Gastrin-releasing peptide receptor-based targeting using bombesin analogues is superior to metabolism-based targeting using choline for in vivo imaging of human prostate cancer xenografts. Eur J Nucl Med Mol Imaging. 2011;38(7):1257–66. doi: 10.1007/s00259-011-1775-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lears KA, Ferdani R, Liang K, Zheleznyak A, Andrews R, Sherman CD, Achilefu S, Anderson CJ, Rogers BE. In vitro and in vivo evaluation of 64Cu-labeled SarAr-bombesin analogs in gastrin-releasing peptide receptor-expressing prostate cancer, Journal of nuclear medicine : official publication, Society of. Nuclear Medicine. 2011;52:470–477. doi: 10.2967/jnumed.110.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tokita K, Katsuno T, Hocart SJ, Coy DH, Llinares M, Martinez J, Jensen RT. Molecular basis for selectivity of high affinity peptide antagonists for the gastrin-releasing peptide receptor. J Biol Chem. 2001;276:36652–36663. doi: 10.1074/jbc.M104566200. [DOI] [PubMed] [Google Scholar]
- 67.Chatalic KL, Franssen GM, van Weerden WM, McBride WJ, Laverman P, de Blois E, Hajjaj B, Brunel L, Goldenberg DM, Fehrentz JA, Martinez J, Boerman OC, de Jong M. Preclinical comparison of Al18F- and 68Ga-labeled gastrin-releasing peptide receptor antagonists for PET imaging of prostate cancer, Journal of nuclear medicine : official publication, Society of. Nuclear Medicine. 2014;55:2050–2056. doi: 10.2967/jnumed.114.141143. [DOI] [PubMed] [Google Scholar]
- 68.Maina T, Bergsma H, Kulkarni HR, Mueller D, Charalambidis D, Krenning EP, Nock BA, de Jong M, Baum RP. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [68Ga]SB3 and PET/CT. Eur J Nucl Med Mol Imaging. 2016;43:964–973. doi: 10.1007/s00259-015-3232-1. [DOI] [PubMed] [Google Scholar]
- 69.Bakker IL, Fröberg AC, Busstra MB, Verzijlbergen JF, Konijnenberg M, van Leenders G, Schoots IG, de Blois E, van Weerden WM, Dalm SU, Maina T, Nock BA, de Jong M. GRPr Antagonist (68)Ga-SB3 PET/CT Imaging of Primary Prostate Cancer in Therapy-Naïve Patients, Journal of nuclear medicine : official publication, Society of. Nuclear Medicine. 2021;62:1517–1523. doi: 10.2967/jnumed.120.258814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitran B, Thisgaard H, Rosenström U, Dam JH, Larhed M, Tolmachev V, Orlova A. High Contrast PET Imaging of GRPR Expression in Prostate Cancer Using Cobalt-Labeled Bombesin Antagonist RM26. Contrast Media Mol Imaging. 2017;2017:6873684. doi: 10.1155/2017/6873684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang J, Niu G, Fan X, Lang L, Hou G, Chen L, Wu H, Zhu Z, Li F, Chen X. PET Using a GRPR Antagonist (68)Ga-RM26 in Healthy Volunteers and Prostate Cancer Patients, Journal of nuclear medicine : official publication, Society of. Nuclear Medicine. 2018;59:922–928. doi: 10.2967/jnumed.117.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L, Zhang Z, Merkens H, Zeisler J, Zhang C, Roxin A, Tan R, Bénard F, Lin KS. (68)Ga-Labeled [Leu(13)ψThz(14)]Bombesin(7–14) Derivatives: Promising GRPR-Targeting PET Tracers with Low Pancreas Uptake. Molecules (Basel, Switzerland). 2022;27:3777. doi: 10.3390/molecules27123777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schollhammer R, Robert G, Asselineau J, Yacoub M, Vimont D, Balamoutoff N, Bladou F, Benard A, Hindie E, Gallerande HC, Morgat C. Comparison of (68)Ga-PSMA-617 PET/CT and (68)Ga-RM2 PET/CT in Patients with Localized Prostate Cancer Who Are Candidates for Radical Prostatectomy: A Prospective, Single-Arm, Single-Center, Phase II Study, Journal of nuclear medicine : official publication, Society of. Nuclear Medicine. 2023;64:379–385. doi: 10.2967/jnumed.122.263889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Parry JJ, Andrews R, Rogers BE. MicroPET imaging of breast cancer using radiolabeled bombesin analogs targeting the gastrin-releasing peptide receptor. Breast Cancer Res Treat. 2007;101:175–183. doi: 10.1007/s10549-006-9287-8. [DOI] [PubMed] [Google Scholar]
- 75.Liu Z, Yan Y, Liu S, Wang F, Chen X. (18)F, (64)Cu, and (68)Ga labeled RGD-bombesin heterodimeric peptides for PET imaging of breast cancer. Bioconjug Chem. 2009;20:1016–1025. doi: 10.1021/bc9000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van de Wiele C, Phonteyne P, Pauwels P, Goethals I, Van den Broecke R, Cocquyt V, Dierckx RA. Gastrin-releasing peptide receptor imaging in human breast carcinoma versus immunohistochemistry, Journal of nuclear medicine : official publication, Society of. Nuclear Medicine. 2008;49:260–264. doi: 10.2967/jnumed.107.047167. [DOI] [PubMed] [Google Scholar]
- 77.Ferguson S, Wuest M, Richter S, Bergman C, Dufour J, Krys D, Simone J, Jans HS, Riauka T, Wuest F. A comparative PET imaging study of (44g)Sc- and (68)Ga-labeled bombesin antagonist BBN2 derivatives in breast and prostate cancer models. Nucl Med Biol. 2020;90–91:74–83. doi: 10.1016/j.nucmedbio.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Stoykow C, Erbes T, Maecke HR, Bulla S, Bartholomä M, Mayer S, Drendel V, Bronsert P, Werner M, Gitsch G, Weber WA, Stickeler E, Meyer PT. Gastrin-releasing Peptide Receptor Imaging in Breast Cancer Using the Receptor Antagonist (68)Ga-RM2 And PET. Theranostics. 2016;6:1641–1650. doi: 10.7150/thno.14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaloudi A, Lymperis E, Giarika A, Dalm S, Orlandi F, Barbato D, Tedesco M, Maina T, de Jong M, Nock BA. NeoBOMB1, a GRPR-Antagonist for Breast Cancer Theragnostics: First Results of a Preclinical Study with [(67)Ga]NeoBOMB1 in T-47D Cells and Tumor-Bearing Mice. Molecules. 2017;22(11):1950. doi: 10.3390/molecules22111950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dalm SU, Sieuwerts AM, Look MP, Melis M, van Deurzen CH, Foekens JA, de Jong M, Martens JW. Clinical Relevance of Targeting the Gastrin-Releasing Peptide Receptor, Somatostatin Receptor 2, or Chemokine C-X-C Motif Receptor 4 in Breast Cancer for Imaging and Therapy, Journal of nuclear medicine : official publication, Society of. Nuclear Medicine. 2015;56:1487–1493. doi: 10.2967/jnumed.115.160739. [DOI] [PubMed] [Google Scholar]
- 81.Kumar U, Grigorakis SI, Watt HL, Sasi R, Snell L, Watson P, Chaudhari S. Somatostatin receptors in primary human breast cancer: quantitative analysis of mRNA for subtypes 1–5 and correlation with receptor protein expression and tumor pathology. Breast Cancer Res Treat. 2005;92:175–186. doi: 10.1007/s10549-005-2414-0. [DOI] [PubMed] [Google Scholar]
- 82.Prignon A, Nataf V, Provost C, Cagnolini A, Montravers F, Gruaz-Guyon A, Lantry LE, Talbot JN, Nunn AD. (68)Ga-AMBA and (18)F-FDG for preclinical PET imaging of breast cancer: effect of tamoxifen treatment on tracer uptake by tumor. Nucl Med Biol. 2015;42:92–98. doi: 10.1016/j.nucmedbio.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 83.Dalm SU, Schrijver WA, Sieuwerts AM, Look MP, Ziel-van der Made AC, de Weerd V, Martens JW, van Diest PJ, de Jong M, van Deurzen CH. Prospects of Targeting the Gastrin Releasing Peptide Receptor and Somatostatin Receptor 2 for Nuclear Imaging and Therapy in Metastatic Breast Cancer. PLoS ONE. 2017;12:e0170536. doi: 10.1371/journal.pone.0170536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J, Mao F, Niu G, Peng L, Lang L, Li F, Ying H, Wu H, Pan B, Zhu Z, Chen X. (68)Ga-BBN-RGD PET/CT for GRPR and Integrin α(v)β(3) Imaging in Patients with Breast Cancer. Theranostics. 2018;8:1121–1130. doi: 10.7150/thno.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Morgat C, Schollhammer R, Macgrogan G, Barthe N, Velasco V, Vimont D, Cazeau AL, Fernandez P, Hindie E. Comparison of the binding of the gastrin-releasing peptide receptor (GRP-R) antagonist 68Ga-RM2 and 18F-FDG in breast cancer samples. PLoS ONE. 2019;14:e0210905. doi: 10.1371/journal.pone.0210905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carroll RE, Matkowskyj KA, Chakrabarti S, McDonald TJ, Benya RV. Aberrant expression of gastrin-releasing peptide and its receptor by well-differentiated colon cancers in humans. Am J Physiol. 1999;276:G655–665. doi: 10.1152/ajpgi.1999.276.3.G655. [DOI] [PubMed] [Google Scholar]
- 87.Lango MN, Dyer KF, Lui VW, Gooding WE, Gubish C, Siegfried JM, Grandis JR. Gastrin-releasing peptide receptor-mediated autocrine growth in squamous cell carcinoma of the head and neck. J Natl Cancer Inst. 2002;94:375–383. doi: 10.1093/jnci/94.5.375. [DOI] [PubMed] [Google Scholar]
- 88.Mansi R, Nock BA, Dalm SU, Busstra MB, van Weerden WM, Maina T. Radiolabeled Bombesin Analogs. Cancers (Basel). 2021;13(22):5766. doi: 10.3390/cancers13225766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.