Abstract
Legionella pneumophila expresses pili of variable lengths, either long (0.8 to 1.5 μm) or short (0.1 to 0.6 μm), that can be observed by transmission electron microscopy. We have identified a gene in L. pneumophila with homology to the type IV pilin genes (pilEL). An insertion mutation was constructed in pilEL and introduced into the L. pneumophila wild-type strain by allelic exchange. The pilin mutant is defective for expression of long pili. Reintroduction of the pilin locus on a cosmid vector restores expression of the long pili. The L. pneumophila pilEL mutant exhibited approximately a 50% decrease in adherence to human epithelial cells (HeLa and WI-26 cells), macrophages (U937 cells), and Acanthamoeba polyphaga but had a wild-type phenotype for intracellular replication within these cells. Southern hybridization analysis showed that the pilEL locus is present in L. pneumophila serogroups 1 through 13 but is variable in 16 other Legionella species. The presence of a type IV pilin gene and its expression by L. pneumophila may provide an advantage for colonization of lung tissues during Legionnaires’ disease and invasion of amoebas in the environment.
Legionella pneumophila replicates in protozoan hosts in an aquatic environment (17), which enhances bacterial survival in the environment (5, 8) and increases infectivity for mammalian cells (13). Once the bacteria have entered a human host through aerosolized droplets, the bacterium enters and replicates effectively within the phagosome of alveolar phagocytes and epithelial cells (12, 20, 36, 37). L. pneumophila is capable of intracellular survival and multiplication within similar specialized vacuoles in macrophages (26) and protozoan cells (1). L. pneumophila also utilizes similar mechanisms to parasitize both evolutionarily distant hosts (18). The ability of L. pneumophila to replicate intracellularly in these hosts is thought to be integral to the pathogenesis of the organism. Additionally, the ability of L. pneumophila to attach, enter, and replicate within epithelial cells of the lung has also been described elsewhere (12, 14, 22, 36, 38). L. pneumophila is able to replicate within both type I and type II mammalian pulmonary epithelial cells (12, 36). The ability of L. pneumophila to replicate within pulmonary epithelial cells may be responsible for epithelial cell necrosis found in infected lung tissue (30, 40).
The molecular determinants for attachment and entry of L. pneumophila into epithelial cells have not been well described. Binding studies have shown that the molecular determinants required for attachment to macrophages and lung fibroblasts may include one or more bacterial protein adhesins (22). In the presence of complement, the L. pneumophila major outer membrane protein facilitates attachment to monocytes and macrophages (9). Opsonized bacteria may then be recognized by host cell complement receptors and internalized by a pseudopod coil (27). An opsonin-independent mechanism of attachment has also been described for macrophages and lung fibroblasts (22, 42). A potential protozoan receptor for L. pneumophila attachment and invasion has recently been described (24, 51). However, the bacterial adhesin(s) involved in complement-independent attachment and adherence to protozoan surfaces has not yet been described.
For various gram-negative pathogenic bacteria, proteins localized to the surface of the bacterium have been found to facilitate attachment and entry into cultured eukaryotic cells and to be required for virulence. One example of such proteins is pilins or fimbrial proteins which assemble into structures on the bacterial surface (28, 49). Specifically, type IV pilin genes are found in a number of pathogenic bacteria including Neisseria species (34, 39) and Pseudomonas aeruginosa (29). The type IV pili are characterized by a conserved hydrophobic amino-terminal domain (25, 49). In addition, a short, positively charged leader sequence is present prior to processing of the prepilin protein. After processing, a conserved modified amino acid (N-methylphenylalanine) is usually the first residue in the mature pilin protein.
Pili have been previously demonstrated on the surface of Legionella species by transmission electron microscopy (41). The genetic components required for pilus expression have not been previously described. Therefore, the involvement of L. pneumophila pili in adherence and intracellular replication has not been directly addressed. In this paper, we describe the expression of various lengths of pili on the surface of L. pneumophila, which may correspond to at least two different sets of pilin genes. One of these pilin genes has been isolated and characterized. The pilEL gene of L. pneumophila is related to the type IV pili of Neisseria and Pseudomonas. A mutation in the type IV pilin gene results in the disappearance of the subset of long pili expressed on the surface of L. pneumophila and a defect for attachment to cultured epithelial cells, macrophages, and protozoan cells.
Identification of L. pneumophila pilus expression.
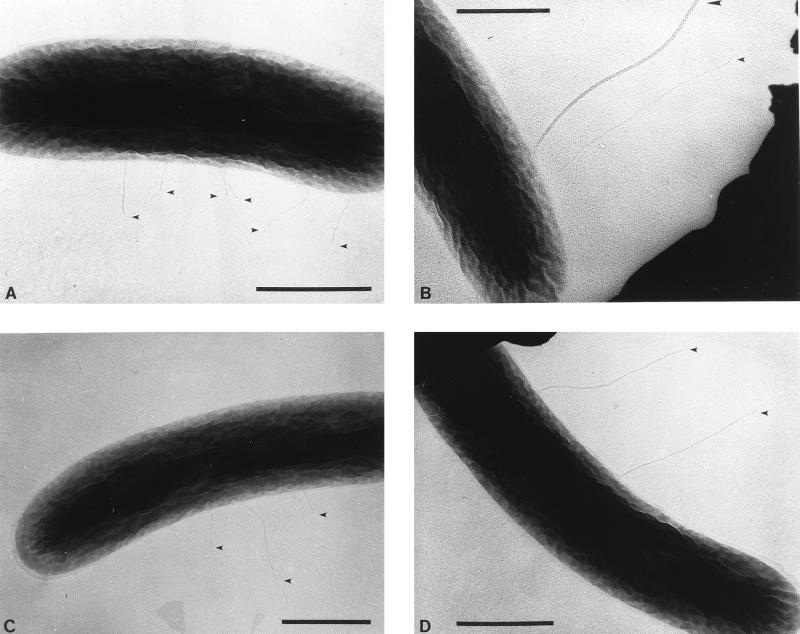
L. pneumophila AA100 was examined by transmission electron microscopy. The virulent L. pneumophila strain AA100 has been previously described (3, 15) and is a redesignation of strain 130b. L. pneumophila was grown at 37°C with buffered yeast extract broth (16) without shaking (to minimize possible shearing of pili) for 96 h. Bacteria were fixed by addition (1:1) of 2% formaldehyde-saline. Samples were applied to copper Formvar grids (Electron Microscopy Sciences, Fort Washington, Pa.) for 2 min and negatively stained with 1% phosphotungstic acid (pH 6.5) for 2 to 4 min. Samples were examined with a Hitachi 7000 transmission electron microscope (Hitachi, Inc., Tokyo, Japan). The bacteria expressed pili of various lengths which were designated as short (0.1 to 0.6 μm) (Fig. 1A) or long (0.8 to 1.5 μm) (Fig. 1B). The longer pili were found only infrequently (on approximately 10% of bacteria) compared to the shorter pili (on approximately 40% of bacteria). A large number of bacteria (50%) expressed no pili. Pili found on the surface of L. pneumophila were generally either long or short; simultaneous expression was not apparent.
FIG. 1.
Electron microscopic examination of expression of pili by L. pneumophila AA100. L. pneumophila wild-type strain AA100 expresses short (0.1 to 0.6 μm) (A) and long (0.8 to 1.5 μm) (B) pili. Pili are indicated by small arrowheads. Flagella are noticeably thicker than the pili observed and are indicated by large arrowheads. Bars, 0.5 μm.
Isolation of a pilin gene from L. pneumophila.
An ∼800-bp fragment was amplified by PCR from L. pneumophila AA100 genomic DNA by using degenerate primers which were originally designed to amplify regions of homology to members of the family of two-component regulators such as PhoP/PhoQ and BvgA/BvgS (23). One primer, TCR6 (5′CGCNNNCNNGCGCGATGNA), was found to amplify an ∼800-bp fragment. PCRs were carried out in the presence of 4 mM MgCl2 and Taq polymerase (Boehringer Mannheim, Indianapolis, Ind.) with standard concentrations of other reagents according to the manufacturer’s suggestions, with a DNA Thermal Cycler 480 (Perkin-Elmer, Norwalk, Conn.). Oligonucleotide primers were synthesized commercially (Gibco BRL, Gaithersburg, Md.). Chromosomal DNA preparations, restriction enzyme digestion, DNA ligation reactions, and in situ colony hybridization were performed as described previously (44). Restriction and modifying enzymes were obtained from Promega (Madison, Wis.) and New England Biolabs (Beverly, Mass.). Plasmid DNA preparations were made according to the manufacturer’s specifications with a Qiagen (Chatsworth, Calif.) Miniprep kit. Escherichia coli DH5α or HB101 was used for vector manipulations and grown in Luria-Bertani medium (35) with the appropriate antibiotic: chloramphenicol (40 μg/ml) or kanamycin (50 μg/ml). Electroporations were carried out with a Bio-Rad Gene Pulser (Hercules, Calif.) as recommended by the manufacturer.
The ∼800-bp fragment was cloned into the vector pCR2.1 with the TA cloning kit (Invitrogen, San Diego, Calif.). The resulting plasmid was designated pBJ110. Sequence analysis of the ∼800-bp insert identified an open reading frame. The translated sequence was similar to various type IV prepilin sequences including PilE from Neisseria gonorrhoeae (34), PilE and PilA from P. aeruginosa (29, 45), and fimbrial protein Q from Moraxella bovis (32).
In order to isolate a larger fragment of DNA containing the open reading frame, the ∼800-bp L. pneumophila fragment in pBJ110 was isolated from agarose gels and used to probe an L. pneumophila chromosomal DNA cosmid library. Cosmids containing the open reading frame were isolated from an L. pneumophila chromosomal DNA library constructed in the following manner. L. pneumophila AA100 chromosomal DNA was partially digested with Sau3A, and 35- to 50-kb sucrose gradient size-selected fragments were ligated into a BamHI site in the cosmid pTLP6, a derivative of pTLP5 (7, 33). The ligation mixture was packaged into λ phage with the use of a λ packaging mix (Stratagene, La Jolla, Calif.) and then transfected into E. coli HB101. Of six cosmids that hybridized to the probe, three were unique as assessed by restriction patterns generated during agarose gel electrophoresis (data not shown). Southern analysis of the cosmids identified a common 2-kb ClaI fragment which hybridized to the probe (data not shown). The fragment was isolated from one of the cosmids (pCC3) and cloned into pBC-RI to generate pBJ120. Plasmid pBC-RI is a derivative of pBC SK+ (Stratagene) from which the EcoRI restriction site has been removed. Thus, plasmid pBJ120 contains a 2-kb ClaI fragment (which includes pilEL) from pCC3 cloned into pBC-RI.
Sequence analysis of the pilin gene.
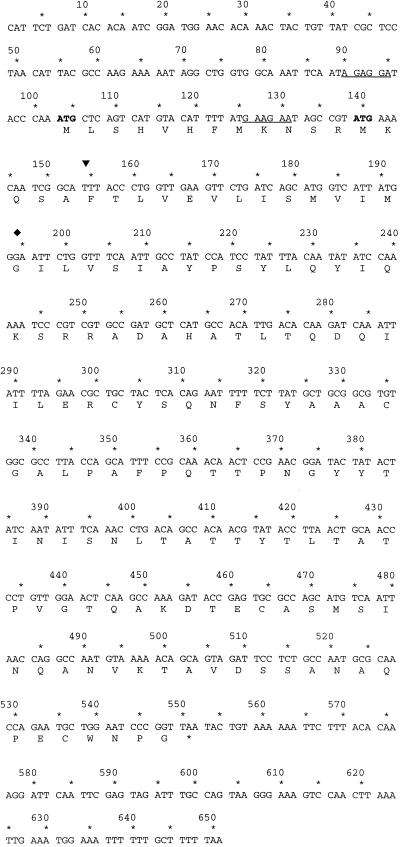
DNA sequence obtained from the 2-kb ClaI fragment of pBJ120 contained an open reading frame of 447 bases, beginning with an amino-terminal methionine (Fig. 2). Three methionines are present near the beginning of the open reading frame. However, only two potential ribosome binding sites are present: 7 bp from the first methionine and 7 bp from the third (Fig. 2). Translation of the longest possible open reading frame predicts a protein product of 149 amino acids in length with a molecular mass of 16.5 kDa.
FIG. 2.
Nucleotide and deduced amino acid sequences of L. pneumophila pilEL. Potential ribosome binding sites are underlined; potential sites for the start of translation are in boldface. The internal EcoRI site, which is the site of the kanamycin gene insertion in BS100, is indicated by the diamond. The position of cleavage of the leader peptide in type IV pilin genes is indicated by the arrowhead.
The open reading frame was compared to sequences deposited in GenBank for identification of similar translated sequences. An area of the amino-terminal region of the translated sequence was found to have 38% identity and 71% similarity to the amino-terminal region of PilE from N. gonorrhoeae and 33% identity and 54% similarity to PilA from P. aeruginosa (strain PAK) (Fig. 3). Comparable levels of similarity were seen for other members of the type IV pilin family of proteins as well (Fig. 3 and data not shown). The open reading frame was then designated pilEL in L. pneumophila.
FIG. 3.
Alignment of the deduced PilEL sequence with those of type IV pilin proteins. The arrowhead indicates the site of cleavage of the leader peptide between the conserved glycine and phenylalanine in type IV pilin genes. PilA is the type IV pilin gene in P. aeruginosa, PilE is in N. gonorrhoeae, and TfpQ is in M. bovis. Amino acids homologous among all four proteins are in boldface.
Type IV pili have been found in a number of pathogenic bacteria including N. gonorrhoeae, Neisseria meningitidis, M. bovis, P. aeruginosa, Dichelobacter nodosus, Vibrio cholerae, and enteropathogenic E. coli (49). Type IV pilins have a number of common characteristics including homology at the amino termini of the proteins. However, the pili can be separated into two distinct groups based upon variations in this same region (49). Group A prepilin proteins (found in N. gonorrhoeae, N. meningitidis, M. bovis, P. aeruginosa, and D. nodosus) are cleaved between a glycine and a phenylalanine residue to remove the prepilin leader sequence. The resulting mature pilin is N methylated prior to assembly of mature pili on the bacterial surface (25, 49). The group B pili (found in V. cholerae and enteropathogenic E. coli) are proteolytically cleaved between a glycine and a methionine or a leucine. The group B pili also contain a longer leader sequence than the group A pili. In all cases, an invariant glutamic acid is found five amino acid residues from the amino terminus of the mature protein. In pilEL of L. pneumophila, the phenylalanine is present at the appropriate position. However, the conserved glycine residue found at the cleavage site of many type IV pilins is not present in pilEL but has been replaced by alanine.
The amino-terminal domain of type IV pili is hydrophobic and contains an invariant glutamic acid residue five amino acids from the mature amino terminus of the protein. The L. pneumophila PilEL amino-terminal sequence is also hydrophobic and contains the invariant glutamic acid five amino acid residues from the putative cleavage site. The length of the pilin proteins with which PilEL has similarity is also conserved. The type IV pilin proteins are approximately 150 to 170 amino acids in length, while the longest possible PilEL open reading frame predicts translation of a protein product 149 amino acids in length.
Based upon sequence homology, the L. pneumophila pilEL gene is a member of the type IV prepilin genes. The putative leader sequence precedes a phenylalanine, as is found in the group A pili, but lacks the glycine present in all other members of both group A and group B. The glycine has been replaced with an alanine; both amino acids are neutral, polar, and similar in structure (alanine contains an extra methyl group). It is not known at this time whether the replacement has any effect on the potential processing of PilEL. However, replacement of the glycine at the leader sequence cleavage site of P. aeruginosa pilA has been studied in some detail (48). Among a number of replacements of glycine, only replacement with alanine resulted in processing of PilA into the mature pilin protein product. Therefore, on account of the degree of homology of pilEL to various members of the type IV prepilin gene family, including the presence of the phenylalanine, the conservative replacement of the glycine with an alanine at the putative cleavage site of the leader sequence, and the presence of the invariant glutamic acid in the sequence, we propose that the group A type IV prepilins be expanded to include pilEL.
In addition to the pilEL locus, an L. pneumophila locus which contains open reading frames encoding homologs of P. aeruginosa PilB, PilC, and PilD, which are required for type IV pilin processing and assembly and type II protein secretion, has been described (31). Southern analysis indicates that the two type IV pilin loci are unique, as hybridization under low-stringency conditions does not reveal binding of the pilBCD locus to cosmid or plasmid DNA containing pilEL (data not shown). Because multiple unique cosmids containing the pilEL locus were analyzed, this data also indicates that the loci are not located adjacent to each other. Whether the pilEL and pilBCD loci act together to assemble a pilus structure is not known at this time.
Generation of an L. pneumophila pilEL mutant.
A pilEL isogenic mutant was generated in L. pneumophila AA100 by the insertion of a Kanr cassette at the unique EcoRI site (Fig. 2) in the pilEL open reading frame. Plasmid pBJ114 is a derivative of pBJ120 in which a Kanr marker was inserted into an internal EcoRI site within pilEL. The Kanr gene was derived from pUC-4K (Pharmacia Biotech, Piscataway, N.J.). The plasmid pBOC20 contains a chloramphenicol resistance marker as well as a sucrose sensitivity marker and has been previously described (6). The plasmid pBJ115 (derived from pBOC20) is a shuttle vector designed to deliver the pilEL::Kan mutation to the L. pneumophila chromosome. Homologous recombination of the mutation onto the chromosome generated strain BS100, as confirmed by Southern analysis (data not shown).
Pilus expression in a pilEL mutant.
To confirm that pilEL was involved in expression of pili, the wild-type strain AA100 and the pilEL mutant BS100 were compared by transmission electron microscopy. As described above, AA100 was found to express pili of various lengths designated short or long. BS100 expressed only the shorter pili (Fig. 4C). Upon examination of BS100 harboring a cosmid containing the pilEL locus (pCC3), long pili were again displayed (Fig. 4D), at the same frequency as long pili expressed by AA100. No long pili were expressed from BS100 containing the cosmid vector alone (data not shown). The appearance of pili was documented for 50 to 100 bacteria for AA100, BS100, and BS100(pCC3). Thus, the pilEL locus is required for expression of the long pili on the surface of L. pneumophila.
FIG. 4.
Comparison of pili expressed in L. pneumophila AA100 and in the pilEL mutant. L. pneumophila wild-type strain AA100 expresses short (A) and long (B) pili. The pilEL mutant strain BS100 expresses only short pili (C). Cosmid pCC3, which contains the pilEL locus, is able to reintroduce expression of long pili to BS100 (D). Pili are indicated by small arrowheads; flagella are indicated by large arrowheads. Bars, 0.5 μm.
L. pneumophila AA100 expressed both long and short pili but not simultaneously. Expression of pili may be regulated by conditions which allow expression of only one subset of pili at a time, such as growth phase and/or phase variation. The pilEL mutant, however, exhibited expression of only the short pili. Expression of long pili by the mutant was complemented by a cosmid carrying pilEL. Because the pilin mutant was generated by insertion of a resistance marker, the mutation may have caused polar effects downstream of the pilEL gene. It is also possible that the mutation has interrupted a gene which affects the length of pili by either destabilizing the pilus structure or affecting secretion of pilus structural components. However, based upon sequence homology with other type IV pilin genes, pilEL is most likely a type IV pilin structural gene.
Intracellular replication of the pilEL mutant in mammalian and protozoan cells.
The phenotype of the pilEL mutant during an intracellular infection was determined in various cell culture systems and is summarized in Table 1. Intracellular replication assays for L. pneumophila within mammalian cells were performed. The macrophage-like cell line U937 was maintained, and infections were performed, as described elsewhere (18) with the following exceptions. Prior to infection, U937 cells were treated with phorbol 12-myristate 13-acetate for 48 h, as described previously (3), to differentiate the cells into nonreplicative, adherent, macrophage-like cells. For all infection assays, L. pneumophila strains were grown for 48 h on buffered charcoal yeast extract agar plates and resuspended in tissue culture medium prior to infection. Intracellular replication assays were performed in triplicate with a multiplicity of infection (MOI) of 10 for 1 h and allowed to proceed for 0, 2, 24, or 48 h. Assays measuring replication kinetics of infection within the macrophage-like cell line U937 revealed no defect for the pilEL mutant BS100 compared to the wild-type strain AA100.
TABLE 1.
Phenotypes of the pilEL mutant in vitro
| Cell type | Intracellular replication | Adherencea |
|---|---|---|
| U937 | Wild type | 33.9 ± 15.9 |
| HeLa | Wild type | 34.0 ± 20.1 |
| WI-26 VA4 | NDb | 48.7 ± 30.1 |
| A. polyphaga | Wild type | 53.4 ± 34.6 |
Adherence is expressed as percent adherence relative to wild-type levels of adherence (100%); percent adherence and standard deviation values are derived from at least three separate experiments performed in triplicate.
ND, not determined.
The cytopathogenicity of L. pneumophila BS100 was also determined. Cytopathogenicity assays were performed in triplicate with an MOI of 0.1. After the addition of bacteria to the U937 cell monolayer, the infection was allowed to proceed for 48 h. The supernatant from each well was removed, and Alamar Blue (AccuMed, Westlake, Ohio) was added according to the manufacturer’s suggestions to determine the viability of the monolayer relative to uninfected control wells, as previously described (6). The level of cytopathogenicity of BS100 was identical to that of AA100 for U937 cells (data not shown). BS100 was also able to replicate as efficiently as AA100 in HeLa cells, an epithelial cell-derived cervical carcinoma cell line. HeLa cells were maintained as described previously (20).
Additionally, the growth kinetics of an intracellular infection of BS100 within the protozoan Acanthamoeba polyphaga were determined as described elsewhere (18). Intracellular replication assays were performed in triplicate with 105 A. polyphaga cells/ml and an MOI of 0.01. After addition of bacteria to the monolayer, the infection was allowed to proceed for 0, 24, 48, and 72 h. Intracellular infections of the protozoan A. polyphaga with BS100 were determined to be identical to that with the wild-type strain AA100. Therefore, the pilEL locus was not required for intracellular survival or replication within mammalian macrophage cells, epithelial cells, or the protozoan host A. polyphaga.
Adherence of the pilEL mutant to mammalian and protozoan cells.
Because pili are known to be involved in adherence of bacteria to mammalian cells (49), the requirement of pilEL for attachment of L. pneumophila to mammalian and protozoan cells was investigated. Attachment to U937 macrophage-like cells and two different epithelial cell lines (HeLa cells and WI-26 cells) was tested. WI-26 VA4 cells (ATCC CCL-95.1) are derived from human embryonic epithelial lung tissue and are a type I pulmonary epithelial cell line. WI-26 cells were maintained in minimum essential medium with Earle’s salts, l-glutamine, and nonessential amino acids (Gibco BRL) with 10% heat inactivated fetal bovine serum at 37°C with 5% CO2. Attachment assays were performed with L. pneumophila strains grown as described above and also grown for 4 days without shaking to replicate growth conditions prior to examination by electron microscopy.
Attachment assays were performed in triplicate in 48-well tissue culture plates with 5 × 105 tissue culture cells/ml at an MOI of 20. Pretreatment of the monolayer with 1 μg of cytochalasin D (Calbiochem, La Jolla, Calif.) per ml was performed to inhibit bacterial uptake as previously described (18). Bacteria were centrifuged with the monolayer at 1,000 × g for 5 min and allowed to attach for 20 min at 37°C and 5% CO2. The monolayer was washed five times with tissue culture medium to remove nonadherent bacteria. To collect bacteria for enumeration, the monolayer was lysed by the addition of sterile water and bacteria were diluted in sterile water and plated on buffered charcoal yeast extract agar plates. The degree of attachment was expressed as percent attachment and was calculated as follows: (CFU of attached bacteria per milliliter ÷ CFU of added bacteria per milliliter) × 100. The percent wild-type attachment was calculated as follows: (percent attachment of the mutant strain ÷ percent attachment of the wild-type strain) × 100.
The pilEL mutant exhibited a decrease in attachment to both HeLa cells ([34.0 ± 20.1]% of wild-type attachment) and WI-26 cells ([48.7 ± 30.1]% of wild-type attachment) relative to that by AA100 (summarized in Table 1). Attachment assays were performed with L. pneumophila strains grown under two different growth conditions: those routinely used for growth of cultures prior to infection assays and those used for growth of L. pneumophila prior to electron microscopic examination for pili. Alteration of the growth conditions resulted in the same decrease in attachment for the pilEL mutant. Adherence to the macrophage-like cell line U937 was also decreased for the pilEL mutant ([33.9 ± 16.0]% of wild-type attachment) (Table 1).
The ability of the wild-type strain and mutant strain to adhere to protozoan cells was determined. Attachment assays were performed in assay buffer in triplicate at an MOI of 20 in 48-well tissue culture trays (18, 19). Bacteria were centrifuged at 1,000 × g for 5 min and allowed to attach for 20 min at 37°C. The monolayer was washed five times with assay buffer to remove nonadherent bacteria. The monolayer was lysed by the addition of 0.05% Triton X-100 (Sigma) as previously described (18). Bacterial enumeration was determined as described above. Adherence of L. pneumophila to A. polyphaga was assessed, and a decrease in attachment of the pilEL mutant was detected (Table 1). Pretreatment of the monolayer with 10 μM methylamine (Sigma) for 30 min to inhibit bacterial uptake by A. polyphaga had no effect on the difference between the wild type and the mutant for attachment. Therefore, since L. pneumophila is defective in attachment to mammalian and protozoan cells, the pili may represent an adherence factor that binds a common host cell component.
Prevalence of pilEL in Legionella.
The occurrence of pilEL among L. pneumophila serogroups and Legionella species was examined by Southern hybridization analysis (Table 2). L. pneumophila serogroup strains and Legionella species subjected to Southern analysis listed in Table 2 were kindly provided by R. Benson, B. Fields, and J. Pruckler from the Centers for Disease Control and Prevention. Digestion of chromosomal DNA was performed with ClaI, as the pilEL sequence in L. pneumophila AA100 does not contain an internal ClaI restriction site. The 2-kb ClaI fragment from pBJ120 was used as a probe to identify the pilEL locus. Labeling of DNA probes and Southern hybridizations were performed as described previously (5). High-stringency hybridization and washes were performed at 60°C; low-stringency hybridization and washes were performed at 45°C.
TABLE 2.
Legionella species and L. pneumophila serogroups and strains characterized by Southern analysis for the presence of the pilEL locus
| Legionella strain | pilEL locusa |
|---|---|
| L. pneumophila (serogroup 1) AA100 | + |
| L. pneumophila (serogroup 1) E1778 | + |
| L. pneumophila (serogroup 1) E1817 | + |
| L. pneumophila (serogroup 1) F286 | + |
| L. pneumophila (serogroup 1) F382 | + |
| L. pneumophila (serogroup 1) F395 | + |
| L. pneumophila (serogroup 1) F1056 | + |
| L. pneumophila (serogroup 1) F1283 | + |
| L. pneumophila (serogroup 1) Philadelphia 1 | + |
| L. pneumophila (serogroup 2) Togus 1 | + |
| L. pneumophila (serogroup 3) Bloomington 2 | + |
| L. pneumophila (serogroup 4) Los Angeles 1 | + |
| L. pneumophila (serogroup 5) Dallas 1 | + |
| L. pneumophila (serogroup 6) Chicago 2 | + |
| L. pneumophila (serogroup 7) Chicago 8 | + |
| L. pneumophila (serogroup 8) Concord 3 | + |
| L. pneumophila (serogroup 9) IN 23 | + |
| L. pneumophila (serogroup 10) Leiden 1 | + |
| L. pneumophila (serogroup 11) 797-PA-H | + |
| L. pneumophila (serogroup 12) 570-Co-H | + |
| L. pneumophila (serogroup 13) 82A3105 | + |
| L. dumoffi NY 23 | − |
| L. longbeachae Long Beach 4 | − |
| L. gormanii LS-13 | − |
| L. micdadei Rivera | − |
| L. wadsworthii 81-716 | + |
| L. oakridgensis OR-10 | + |
| L. feeleii WO-44C | − |
| L. sainthelensis Mount St. Helens 4 | − |
| L. spiritensis Mount St. Helens 9 | ± |
| L. jamestowniensis JA-26-G1-E2 | − |
| L. santicrucis SC-63-C7 | + |
| L. cherrii O RW | + |
| L. parisiensis PF-209C-C2 | + |
| L. erythra SE-32A-C8 | − |
| L. hackeliae Lansing 2 | − |
| L. israelensis Bercovier 4 | − |
The presence (+) or absence (−) of a pilEL locus as determined by Southern analysis is indicated.
Southern analysis confirmed the presence of one DNA fragment hybridizing to the pilEL locus under high-stringency conditions in each of the 13 serogroups tested (Table 2). Hybridizing bands from a ClaI digest of chromosomal DNA ranged in size from 2 to 6 kb (data not shown). Multiple strains of L. pneumophila serogroup 1 were also tested and found to be consistent with the serogroup data (Table 2 and data not shown). Southern hybridization was performed on chromosomal DNA from various Legionella species at low stringency to detect the presence of pilEL (Fig. 5). Hybridization was observed for L. wadsworthii, L. oakridgensis, L. santicrucis, L. cherrii, and L. parisiensis. Multiple bands were observed for each, which may be due to multiple copies of the locus or the introduction of an internal ClaI site within the pilEL locus in these species. Southern hybridization analysis of chromosomal DNA from Legionella species performed under high-stringency conditions detected no hybridization with pilEL; thus, the DNA sequences of the pilEL loci in different Legionella species are not identical.
FIG. 5.
Presence of pilEL in Legionella species. Chromosomal DNA from different Legionella species was subjected to Southern analysis under low-stringency conditions and probed with the pilEL locus. L. pneumophila AA100 chromosomal DNA hybridizes with a single 2-kb fragment (arrowhead) under high-stringency conditions. Lanes 1 to 17, L. pneumophila, L. dumoffi, L. longbeachae, L. gormanii, L. micdadei, L. wadsworthii, L. oakridgensis, L. feeleii, L. sainthelensis, L. spiritensis, L. jamestowniensis, L. santicrucis, L. cherrii, L. parisiensis, L. erythra, L. hackeliae, and L. israelensis, respectively.
In conclusion, the advantage that pilEL confers on L. pneumophila may be related to attachment of the bacteria to epithelial cells or macrophages during infection of a human host. The pilin mutant showed an ∼50% decrease in attachment to human macrophages and epithelial cells. As multiple adhesion factors are not uncommon in pathogenic bacteria and the role of pilEL in attachment is only moderate, the presence of other adhesion proteins required for attachment is likely. Recently, multiple mutants of L. pneumophila which are defective for attachment to both mammalian cells (18–20) and protozoa (24) have been described. As all the reported mutants are defective for cytopathogenicity or replication within macrophages, none are likely to have mutations in the pilEL locus. Whether the long pili are the ligand that allows bacterial attachment to the protozoan lectin receptor (24, 51) has yet to be determined.
Another possible role for pilEL may include enhanced survival in the environment. For example, expression of pilEL increases adhesion to protozoan host cells and may increase adherence to biofilms where L. pneumophila has been found in an aquatic environment (10). Additionally, expression under conditions encountered during intracellular growth, either within mammalian cells or within protozoa, has not been studied. However, L. pneumophila is more invasive of mammalian cells after intracellular replication within amoebae (13), and intracellular specific expression of L. pneumophila proteins has been observed during macrophage infection (2–6, 50). If increased expression of pilEL occurs during intracellular growth within a mammalian or protozoan host cell, an increase in attachment to or invasiveness of mammalian cells may be partially attributed to pilus expression.
The type IV pilin structural protein gene (pilE) and a gene required for pilus biogenesis (pilC) of N. gonorrhoeae are required for natural DNA transformation competence (11, 21, 43, 46, 52). Therefore, the potential role for pilEL in competence is being studied. The ability of L. pneumophila to become competent for transformation of DNA and the dependence of the competence phenotype upon pilEL have recently been discovered (47). Thus, we propose that the long pili described in this paper be termed CAP (competence- and adherence-associated pili).
Nucleotide sequence accession number.
The nucleotide sequence accession number is AF048690.
Acknowledgments
We thank M. R. Liles, V. K. Viswanathan, and N. P. Cianciotto for allowing use of the pilBCD clone prior to publication. We also thank Omar Harb, Lian-Yong Gao, and Matthew Nilles for critical review of the manuscript.
Y.A. is supported by Public Health Service grant 1R29AI38410. B.J.S. is a recipient of Public Health Service National Research Service Award TA-09509.
REFERENCES
- 1.Abu Kwaik Y. The phagosome containing Legionella pneumophila within the protozoan Hartmannella vermiformis is surrounded by the rough endoplasmic reticulum. Appl Environ Microbiol. 1996;62:2022–2028. doi: 10.1128/aem.62.6.2022-2028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Kwaik Y. Induced expression of the Legionella pneumophila gene encoding a 20-kilodalton protein during infection. Infect Immun. 1998;66:203–212. doi: 10.1128/iai.66.1.203-212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu Kwaik Y, Eisenstein B I, Engleberg N C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Kwaik Y, Engleberg N C. Cloning and molecular characterization of a Legionella pneumophila gene induced by intracellular infection and by various in vitro stress stimuli. Mol Microbiol. 1994;13:243–251. doi: 10.1111/j.1365-2958.1994.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 5.Abu Kwaik Y, Gao L, Harb O S, Stone B J. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol Microbiol. 1997;24:629–642. doi: 10.1046/j.1365-2958.1997.3661739.x. [DOI] [PubMed] [Google Scholar]
- 6.Abu Kwaik Y, Pederson L L. The use of differential display-PCR to isolate and characterize a Legionella pneumophila locus induced during the intracellular infection of macrophages. Mol Microbiol. 1996;21:543–556. doi: 10.1111/j.1365-2958.1996.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 7.Arroyo J, Hurley M C, Wolf M, McClain M S, Eisenstein B I, Engleberg N C. Shuttle mutagenesis of Legionella pneumophila: identification of a gene associated with host cell cytopathogenicity. Infect Immun. 1994;62:4075–4080. doi: 10.1128/iai.62.9.4075-4080.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker J, Lambert P A, Brown M R W. Influence of intra-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect Immun. 1993;61:3503–3510. doi: 10.1128/iai.61.8.3503-3510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellinger-Kawahara C, Horwitz M A. Complement component C3 fixes selectively to the major outer membrane protein (MOMP) of Legionella pneumophila and mediates phagocytosis of liposome-MOMP complexes by human monocytes. J Exp Med. 1990;172:1201–1210. doi: 10.1084/jem.172.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bentham R H, Broadbent C R, Marwood L N. The influence of the sessile population in the Legionella colonization of cooling towers. In: Barbaree J M, Breiman R F, Dufour A P, editors. Legionella: current status and emerging perspectives. Washington, D.C: American Society for Microbiology; 1993. pp. 267–269. [Google Scholar]
- 11.Biswas G D, Sox T, Blackman E, Sparling P F. Factors affecting genetic transformation of Neisseria gonorrhoeae. J Bacteriol. 1977;129:983–992. doi: 10.1128/jb.129.2.983-992.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cianciotto N P, Stamos J K, Kamp D W. Infectivity of Legionella pneumophila mip mutant for alveolar epithelial cells. Curr Microbiol. 1995;30:247–250. doi: 10.1007/BF00293641. [DOI] [PubMed] [Google Scholar]
- 13.Cirillo J D, Tompkins L S, Falkow S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daisy J A, Benson C E, McKitrick J, Friedman H M. Intracellular replication of Legionella pneumophila. J Infect Dis. 1981;143:460–464. doi: 10.1093/infdis/143.3.460. [DOI] [PubMed] [Google Scholar]
- 15.Edelstein P H, Brenner D J, Moss C W, Steigerwalt A G, Francis E M, George W L. Legionella wadsworthii species nova: a cause of human pneumonia. Ann Intern Med. 1982;97:809–813. doi: 10.7326/0003-4819-97-6-809. [DOI] [PubMed] [Google Scholar]
- 16.Feeley J C, Gorman G W, Weaver R E, Mackel D C, Smith H W. Primary isolation media for Legionnaires’ disease bacterium. J Clin Microbiol. 1978;8:320–325. doi: 10.1128/jcm.8.3.320-325.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fields B S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 18.Gao L, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao L-Y, Harb O S, Abu Kwaik Y. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect Immun. 1998;66:883–892. doi: 10.1128/iai.66.3.883-892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao, L.-Y., B. J. Stone, M. Guzman, J. K. Brieland, and Y. Abu Kwaik. Submitted for publication.
- 21.Gibbs C P, Reimann B Y, Schultz E, Kaufmann A, Haas R, Meyer T F. Reassortment of pilin genes in Neisseria gonorrhoeae occurs by two distinct mechanisms. Nature. 1989;338:651–652. doi: 10.1038/338651a0. [DOI] [PubMed] [Google Scholar]
- 22.Gibson F C, III, Tzianabos A O, Rodgers F C. Adherence of Legionella pneumophila to U-937 cells, guinea-pig alveolar macrophages, and MRC-5 cells by a novel, complement-independent binding mechanism. Can J Microbiol. 1994;40:865–872. doi: 10.1139/m94-137. [DOI] [PubMed] [Google Scholar]
- 23.Gross R. Signal transduction and virulence regulation in human and animal pathogens. FEMS Microbiol Rev. 1993;10:301–326. doi: 10.1111/j.1574-6968.1993.tb05873.x. [DOI] [PubMed] [Google Scholar]
- 24.Harb O S, Venkataraman C, Haack B J, Gao L-Y, Abu Kwaik Y. Heterogeneity in the attachment and uptake mechanisms of the Legionnaires’ disease bacterium, Legionella pneumophila, by protozoan hosts. Appl Environ Microbiol. 1998;64:126–132. doi: 10.1128/aem.64.1.126-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hobbs M, Mattick J S. Common components in the assembly of type 4 fimbriae, DNA transfer systems, filamentous phage and protein-secretion apparatus: a general system for the formation of surface-associated protein complexes. Mol Microbiol. 1993;10:233–243. doi: 10.1111/j.1365-2958.1993.tb01949.x. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz M A. The Legionnaires’ disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horwitz M A. Phagocytosis of the Legionnaires’ disease bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 28.Hultgren S J, Abraham S, Caparon M, Falk P, St. Geme III J W, Normark S. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell. 1993;73:887–901. doi: 10.1016/0092-8674(93)90269-v. [DOI] [PubMed] [Google Scholar]
- 29.Johnson K, Parker M L, Lory S. Nucleotide sequence and transcriptional initiation site of two Pseudomonas aeruginosa pilin genes. J Biol Chem. 1986;261:15703–15708. [PubMed] [Google Scholar]
- 30.Katz S M, Brodsky I, Kahn S B. Legionnaires’ disease: ultrastructural appearance of the agent in a lung biopsy specimen. Arch Pathol Lab Med. 1979;103:261–264. [PubMed] [Google Scholar]
- 31.Liles M R, Viswanathan V K, Cianciotto N P. Identification and temperature regulation of Legionella pneumophila genes involved in type IV pilus biogenesis and type II protein secretion. Infect Immun. 1998;66:1776–1782. doi: 10.1128/iai.66.4.1776-1782.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marrs C F, Schoolnik G, Koomey J M, Hardy J, Rothbard J, Falkow S. Cloning and sequencing of a Moraxella bovis pilin gene. J Bacteriol. 1985;163:132–139. doi: 10.1128/jb.163.1.132-139.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McClain M S, Hurley M C, Brieland J K, Engleberg N C. The Legionella pneumophila hel locus encodes intracellularly induced homologs of heavy-metal ion transporters of Alcaligenes spp. Infect Immun. 1996;64:1532–1540. doi: 10.1128/iai.64.5.1532-1540.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyer T F, Billyard E, Haas R, Storzbach S, So M. Pilus genes of Neisseria gonorrhoeae: chromosomal organization and DNA sequence. Proc Natl Acad Sci USA. 1984;81:6110–6114. doi: 10.1073/pnas.81.19.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 36.Mody C H, Paine III R, Shahrabadi M S, Simon R H, Pearlman E, Eisenstein B I, Toews G B. Legionella pneumophila replicates within rat alveolar epithelial cells. J Infect Dis. 1993;167:1138–1145. doi: 10.1093/infdis/167.5.1138. [DOI] [PubMed] [Google Scholar]
- 37.Nash T W, Libby D M, Horwitz M A. Interaction between the Legionnaires’ disease bacterium (Legionella pneumophila) and human alveolar macrophages. J Clin Invest. 1984;74:771–782. doi: 10.1172/JCI111493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oldham L J, Rogers F G. Adhesion, penetration and intracellular replication of Legionella pneumophila: an in vitro model of pathogenesis. J Gen Microbiol. 1985;131:697–706. doi: 10.1099/00221287-131-4-697. [DOI] [PubMed] [Google Scholar]
- 39.Potts W J, Saunders R J. Nucleotide sequence of the structural gene for class I pilin from Neisseria meningitidis: homologies with the pilE locus of Neisseria gonorrhoeae. Mol Microbiol. 1988;2:647–653. doi: 10.1111/j.1365-2958.1988.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 40.Rodgers F G. Ultrastructure of Legionella pneumophila. J Clin Pathol. 1979;32:1195–1202. doi: 10.1136/jcp.32.12.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodgers F G, Greaves P W, Macrae A D, Lewis M J. Electron microscopic evidence of flagella and pili on Legionella pneumophila. J Clin Pathol. 1980;33:1184–1188. doi: 10.1136/jcp.33.12.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodgers F G, Gibson F C., III Opsonin-independent adherence and intracellular development of Legionella pneumophila within U-937 cells. Can J Microbiol. 1993;39:718–722. doi: 10.1139/m93-103. [DOI] [PubMed] [Google Scholar]
- 43.Rudel T, Facius D, Barten R, Scheuerpflug I, Nonnenmacher E, Meyer T F. Role of pili and the phase-variable PilC protein in natural competence for transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci USA. 1995;92:7986–7990. doi: 10.1073/pnas.92.17.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Shaw C E, Taylor R K. Vibrio cholerae O395 tcpA pilin gene sequence and comparison of predicted protein structural features to those of type 4 pilins. Infect Immun. 1990;58:3042–3049. doi: 10.1128/iai.58.9.3042-3049.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sparling P F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966;92:1364–1370. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stone, B. J., and Y. Abu Kwaik. Submitted for publication.
- 48.Strom M S, Lory S. Amino acid substitutions in pilin of Pseudomonas aeruginosa. Effect on leader peptide cleavage, amino-terminal methylation, and pilus assembly. J Biol Chem. 1991;266:1656–1664. [PubMed] [Google Scholar]
- 49.Strom M S, Lory S. Structure-function and biogenesis of the type IV pili. Annu Rev Microbiol. 1993;47:565–596. doi: 10.1146/annurev.mi.47.100193.003025. [DOI] [PubMed] [Google Scholar]
- 50.Susa M, Hacker J, Marre R. De novo synthesis of Legionella pneumophila antigens during intracellular growth in phagocytic cells. Infect Immun. 1996;64:1679–1684. doi: 10.1128/iai.64.5.1679-1684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venkataraman C, Haack B J, Bondada S, Abu Kwaik Y. Identification of a Gal/GalNAc lectin in the protozoan Hartmannella vermiformis as a potential receptor for attachment and invasion by the Legionnaires’ disease bacterium. J Exp Med. 1997;186:537–547. doi: 10.1084/jem.186.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zang Q Y, DeRyckere D, Lauer P, Koomey M. Gene conversion in Neisseria gonorrhoeae: evidence for its role in pilus antigenic variation. Proc Natl Acad Sci USA. 1992;89:5366–5370. doi: 10.1073/pnas.89.12.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]