Abstract
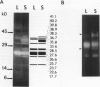
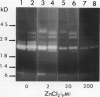
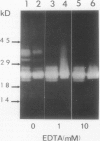
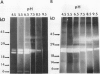
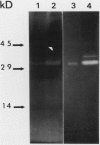
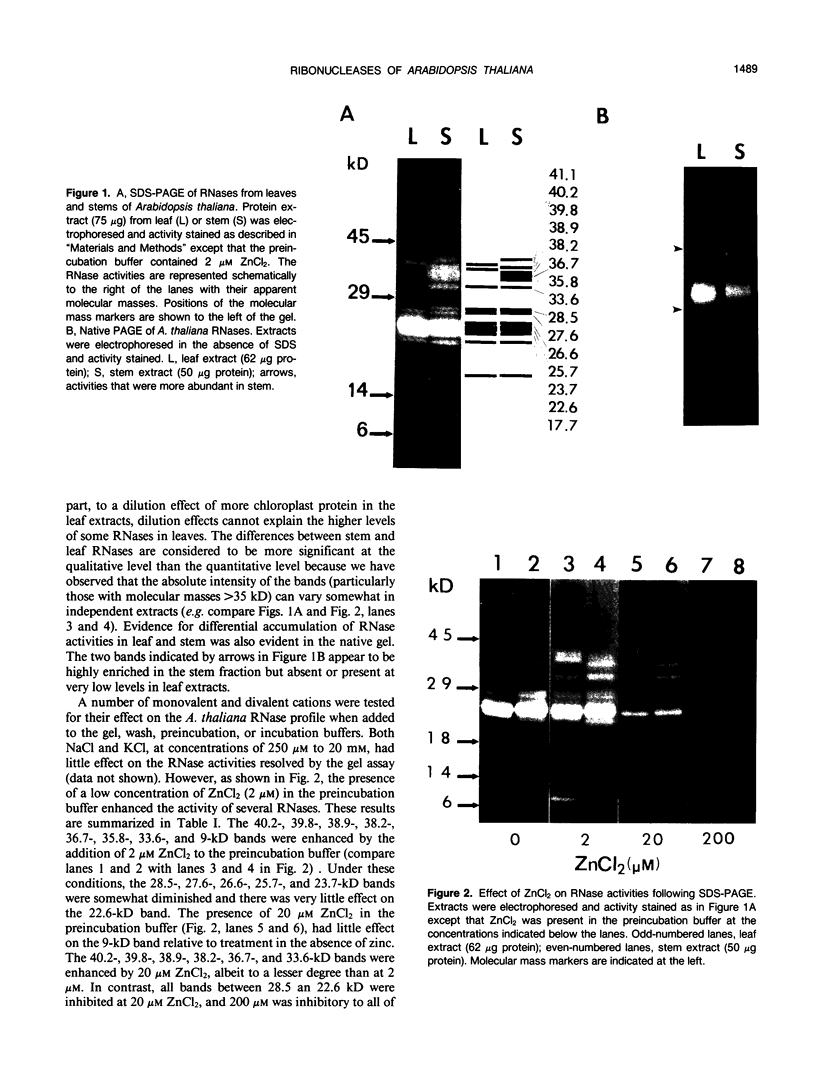
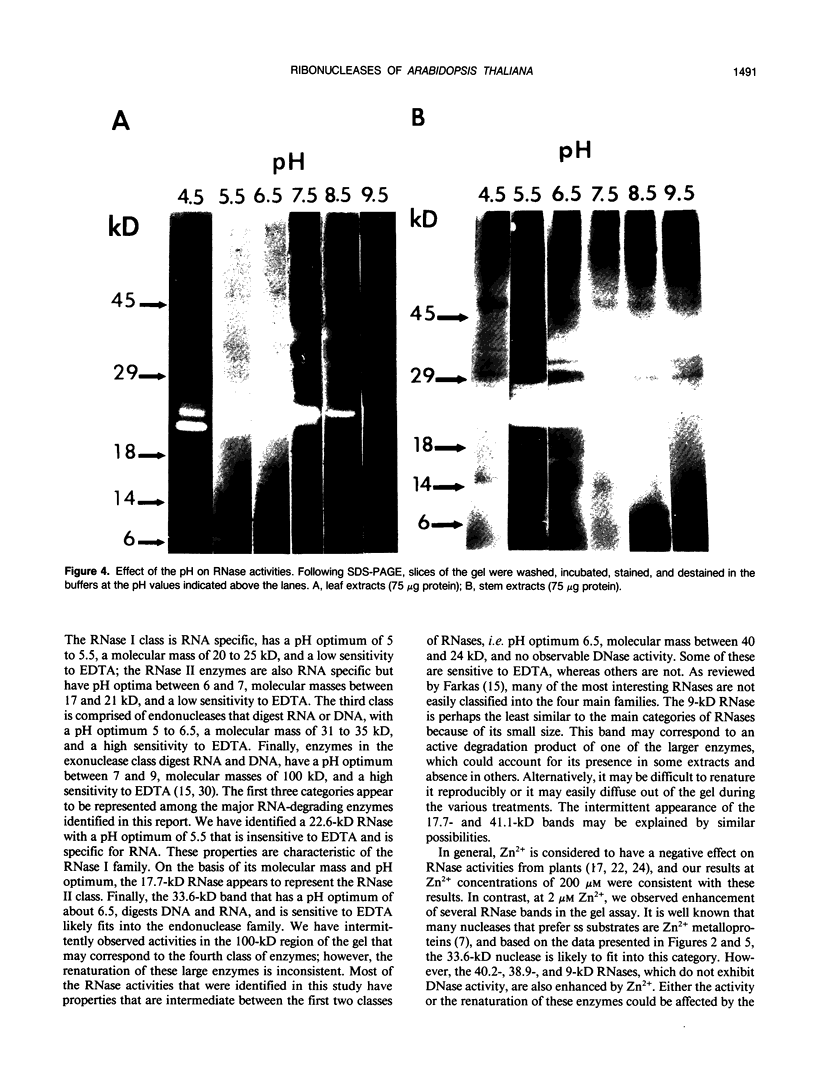
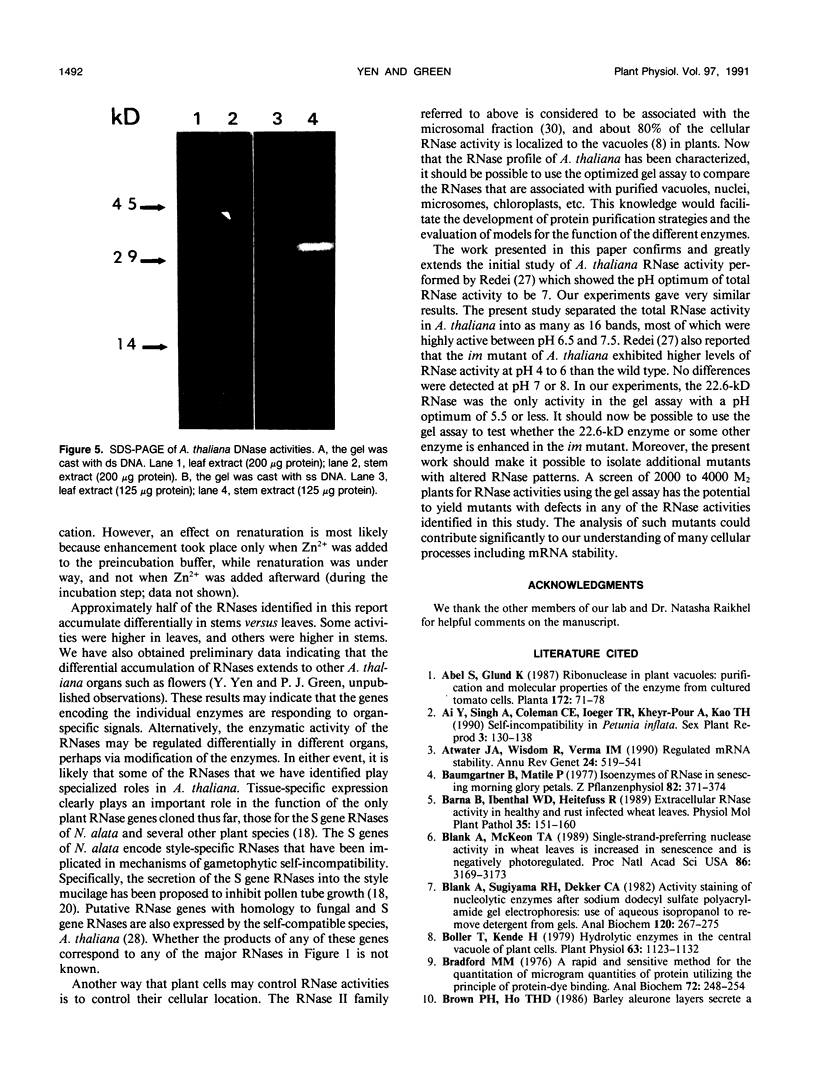
The profile of major ribonuclease (RNase) activities of Arabidopsis thaliana has been identified and characterized using a substrate-based gel assay. Following sodium dodecyl sulfatepolyacrylamide gel electrophoresis, as many as 16 RNases, varying in size from 9 to 41 kilodaltons can be detected. Most of the RNase activities exhibit a pH optimum of about 6.5; however, the activity of a 22.6-kilodalton RNase is greatly enhanced at low pH. A number of the RNases in the 30- to 41-kilodalton range are sensitive to ethylenediaminetetraacetic acid, and their activities are enhanced by the presence of a low concentration of zinc during renaturation. At least one RNase appears to comigrate with a major DNase activity. The differential accumulation of several RNases in stems versus leaves indicates that some RNases are controlled in an organ-specific manner in A. thaliana.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwater J. A., Wisdom R., Verma I. M. Regulated mRNA stability. Annu Rev Genet. 1990;24:519–541. doi: 10.1146/annurev.ge.24.120190.002511. [DOI] [PubMed] [Google Scholar]
- Blank A., McKeon T. A. Single-strand-preferring nuclease activity in wheat leaves is increased in senescence and is negatively photoregulated. Proc Natl Acad Sci U S A. 1989 May;86(9):3169–3173. doi: 10.1073/pnas.86.9.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank A., Sugiyama R. H., Dekker C. A. Activity staining of nucleolytic enzymes after sodium dodecyl sulfate-polyacrylamide gel electrophoresis: use of aqueous isopropanol to remove detergent from gels. Anal Biochem. 1982 Mar 1;120(2):267–275. doi: 10.1016/0003-2697(82)90347-5. [DOI] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown P. H., Ho T. H. Barley aleurone layers secrete a nuclease in response to gibberellic Acid : purification and partial characterization of the associated ribonuclease, deoxyribonuclease, and 3'-nucleotidase activities. Plant Physiol. 1986 Nov;82(3):801–806. doi: 10.1104/pp.82.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark K. R., Okuley J. J., Collins P. D., Sims T. L. Sequence variability and developmental expression of S-alleles in self-incompatible and pseudo-self-compatible petunia. Plant Cell. 1990 Aug;2(8):815–826. doi: 10.1105/tpc.2.8.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher M. P. The metabolic role of RNases. Trends Biochem Sci. 1988 Apr;13(4):136–139. doi: 10.1016/0968-0004(88)90070-9. [DOI] [PubMed] [Google Scholar]
- Donovan W. P., Kushner S. R. Polynucleotide phosphorylase and ribonuclease II are required for cell viability and mRNA turnover in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1986 Jan;83(1):120–124. doi: 10.1073/pnas.83.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D. M., Fairley J. L. Enzymes of nucleic acid metabolism from wheat seedlings. I. Purification and general properties of associated deoxyribonuclease, ribonuclease, and 3'-nucleotidase activities. J Biol Chem. 1969 May 10;244(9):2440–2449. [PubMed] [Google Scholar]
- Haring V., Gray J. E., McClure B. A., Anderson M. A., Clarke A. E. Self-incompatibility: a self-recognition system in plants. Science. 1990 Nov 16;250(4983):937–941. doi: 10.1126/science.2237440. [DOI] [PubMed] [Google Scholar]
- McClure B. A., Haring V., Ebert P. R., Anderson M. A., Simpson R. J., Sakiyama F., Clarke A. E. Style self-incompatibility gene products of Nicotiana alata are ribonucleases. Nature. 1989 Dec 21;342(6252):955–957. doi: 10.1038/342955a0. [DOI] [PubMed] [Google Scholar]
- POORT C., VAN VENROOYW DETECTION OF ENZYMES IN AGAR ELECTROPHEROGRAMS. Nature. 1964 Nov 14;204:684–684. doi: 10.1038/204684a0. [DOI] [PubMed] [Google Scholar]
- Randles J. W. Ribonuclease isozymes in Chinese cabbage systemically infected with turnip yellow mosaic virus. Virology. 1968 Dec;36(4):556–563. doi: 10.1016/0042-6822(68)90187-6. [DOI] [PubMed] [Google Scholar]
- Rédei G. P. Biochemical aspects of a genetically determined variegation in Arabidopsis. Genetics. 1967 Jul;56(3):431–443. doi: 10.1093/genetics/56.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. B., Green P. J. Genes with Homology to Fungal and S-Gene RNases Are Expressed in Arabidopsis thaliana. Plant Physiol. 1991 Jul;96(3):980–984. doi: 10.1104/pp.96.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M. Plant Nucleases: III. Polyacrylamide Gel Electrophoresis of Corn Ribonuclease Isoenzymes. Plant Physiol. 1971 Jul;48(1):64–68. doi: 10.1104/pp.48.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M. Plant nucleases: biochemistry and development of multiple molecular forms. Isozymes Curr Top Biol Med Res. 1982;6:33–54. [PubMed] [Google Scholar]
- Wolf G. Nachweis pflanzlicher Ribonukleasen in Polyacrylamid-Gelen nach Disk-Elektrophorese. Experientia. 1968 Sep 15;24(9):890–891. doi: 10.1007/BF02138632. [DOI] [PubMed] [Google Scholar]